 Found 954 hits of ki for UniProtKB: P07711
Found 954 hits of ki for UniProtKB: P07711 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Homo sapiens (Human)) | BDBM50167289
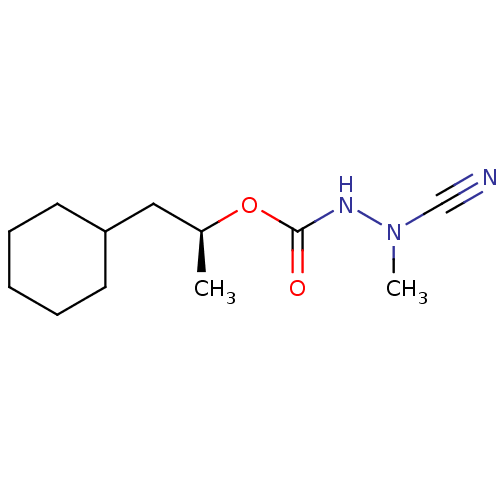
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...)Show InChI InChI=1S/C12H21N3O2/c1-10(8-11-6-4-3-5-7-11)17-12(16)14-15(2)9-13/h10-11H,3-8H2,1-2H3,(H,14,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50167288

((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...)Show InChI InChI=1S/C13H17N3O2/c1-3-12(9-11-7-5-4-6-8-11)18-13(17)15-16(2)10-14/h4-8,12H,3,9H2,1-2H3,(H,15,17)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19770
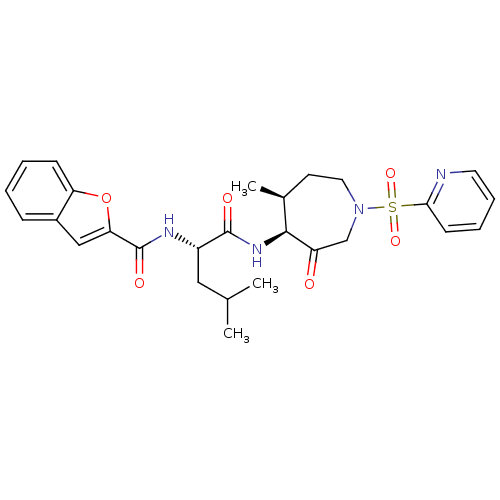
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1[C@@H](C)CCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-20(29-27(34)23-15-19-8-4-5-9-22(19)37-23)26(33)30-25-18(3)11-13-31(16-21(25)32)38(35,36)24-10-6-7-12-28-24/h4-10,12,15,17-18,20,25H,11,13-14,16H2,1-3H3,(H,29,34)(H,30,33)/t18-,20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | -14.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50335281

(CHEMBL1651355 | N-(Phenylcarbamoyl)-leucyl-methyla...)Show SMILES CC(C)C[C@H](NC(=O)Nc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C16H23N5O2/c1-12(2)10-14(15(22)21(4)20(3)11-17)19-16(23)18-13-8-6-5-7-9-13/h5-9,12,14H,10H2,1-4H3,(H2,18,19,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0680 | -13.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50451631

(CHEMBL4212454)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc(nn1C)C(C)(C)C |r| Show InChI InChI=1S/C21H28N6O2/c1-21(2,3)18-13-17(26(5)24-18)19(28)23-16(12-15-10-8-7-9-11-15)20(29)27(6)25(4)14-22/h7-11,13,16H,12H2,1-6H3,(H,23,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50304793
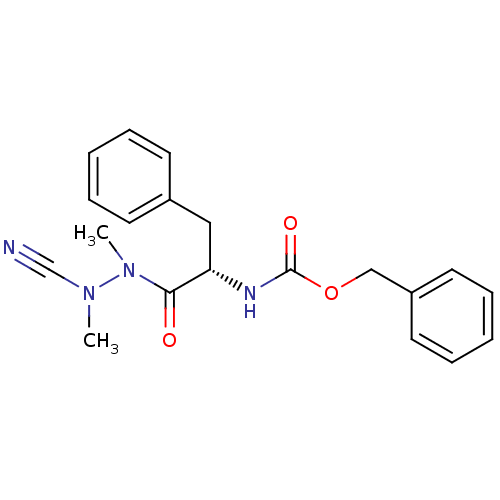
((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-1-ox...)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H22N4O3/c1-23(15-21)24(2)19(25)18(13-16-9-5-3-6-10-16)22-20(26)27-14-17-11-7-4-8-12-17/h3-12,18H,13-14H2,1-2H3,(H,22,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50335289
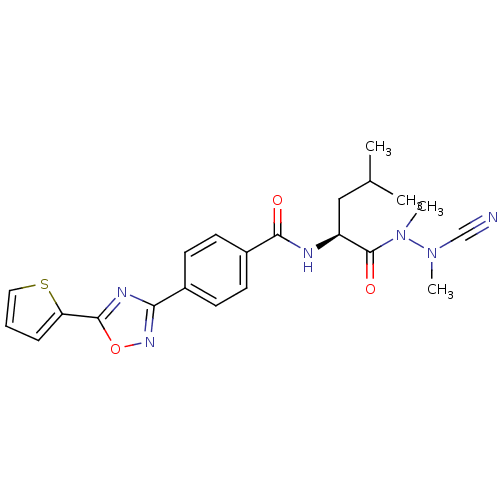
(CHEMBL1651361 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C22H24N6O3S/c1-14(2)12-17(22(30)28(4)27(3)13-23)24-20(29)16-9-7-15(8-10-16)19-25-21(31-26-19)18-6-5-11-32-18/h5-11,14,17H,12H2,1-4H3,(H,24,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549366

(CHEMBL4755248)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NS(=O)(=O)c1cccc(Br)c1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50335279
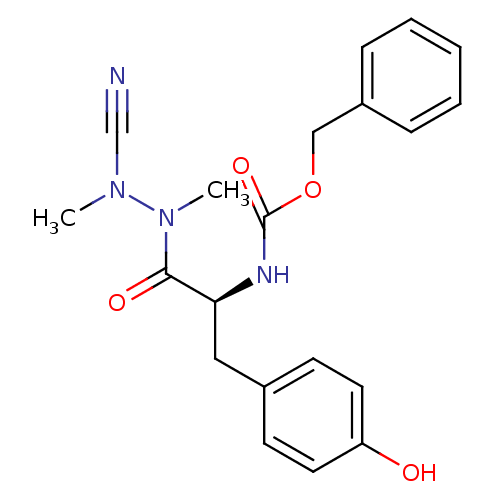
(CHEMBL1651353 | N-(Benzyloxycarbonyl)-tyrosyl-meth...)Show SMILES CN(C#N)N(C)C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H22N4O4/c1-23(14-21)24(2)19(26)18(12-15-8-10-17(25)11-9-15)22-20(27)28-13-16-6-4-3-5-7-16/h3-11,18,25H,12-13H2,1-2H3,(H,22,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50335280

(CHEMBL1651354 | N-(Benzylcarbamoyl)-leucyl-methyla...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H25N5O2/c1-13(2)10-15(16(23)22(4)21(3)12-18)20-17(24)19-11-14-8-6-5-7-9-14/h5-9,13,15H,10-11H2,1-4H3,(H2,19,20,24)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50258507

(CHEMBL4078345)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C30H32N2O4/c1-23(33)17-19-27(20-18-24-11-5-2-6-12-24)31-29(34)28(21-25-13-7-3-8-14-25)32-30(35)36-22-26-15-9-4-10-16-26/h2-17,19,27-28H,18,20-22H2,1H3,(H,31,34)(H,32,35)/b19-17+/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay |
J Med Chem 60: 6911-6923 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00405
BindingDB Entry DOI: 10.7270/Q2FJ2K73 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50335278

(CHEMBL1651352 | N-(Benzyloxycarbonyl)-cyclohexylal...)Show SMILES CN(C#N)N(C)C(=O)[C@H](CC1CCCCC1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C20H28N4O3/c1-23(15-21)24(2)19(25)18(13-16-9-5-3-6-10-16)22-20(26)27-14-17-11-7-4-8-12-17/h4,7-8,11-12,16,18H,3,5-6,9-10,13-14H2,1-2H3,(H,22,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002402
(CHEMBL194643)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cccc2ccccc12 Show InChI InChI=1S/C35H32N4O5S/c40-32-23-39(45(43,44)33-16-5-6-19-36-33)20-8-15-30(32)37-35(42)31(22-24-17-18-25-9-1-2-11-27(25)21-24)38-34(41)29-14-7-12-26-10-3-4-13-28(26)29/h1-7,9-14,16-19,21,30-31H,8,15,20,22-23H2,(H,37,42)(H,38,41)/t30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50084650
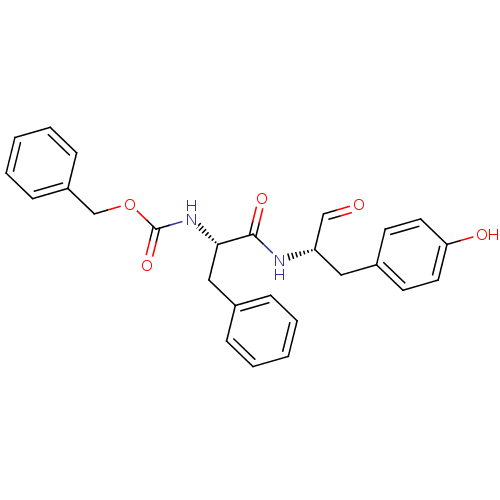
(CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...)Show SMILES Oc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C=O)cc1 Show InChI InChI=1S/C26H26N2O5/c29-17-22(15-20-11-13-23(30)14-12-20)27-25(31)24(16-19-7-3-1-4-8-19)28-26(32)33-18-21-9-5-2-6-10-21/h1-14,17,22,24,30H,15-16,18H2,(H,27,31)(H,28,32)/t22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50451625

(CHEMBL4217414)Show SMILES CC(C)C[C@H](NC(=O)c1cc(nn1C)C(C)(C)C)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C18H30N6O2/c1-12(2)9-13(17(26)24(8)22(6)11-19)20-16(25)14-10-15(18(3,4)5)21-23(14)7/h10,12-13H,9H2,1-8H3,(H,20,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549370

(CHEMBL4746022)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Br)c1)NC(=O)c1cc(nn1C)C(C)(C)C)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002374
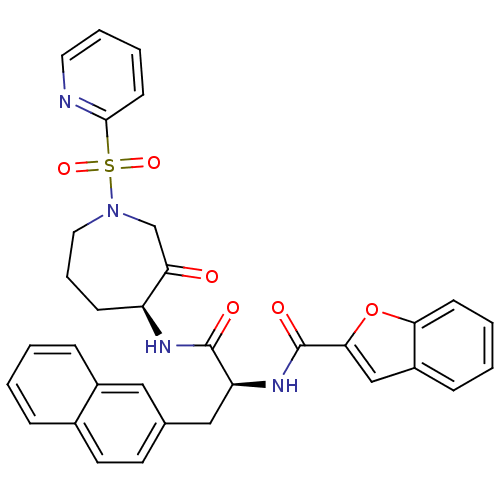
(CHEMBL194861)Show SMILES O=C(N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1)[C@H](Cc1ccc2ccccc2c1)NC(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C33H30N4O6S/c38-28-21-37(44(41,42)31-13-5-6-16-34-31)17-7-11-26(28)35-32(39)27(19-22-14-15-23-8-1-2-9-24(23)18-22)36-33(40)30-20-25-10-3-4-12-29(25)43-30/h1-6,8-10,12-16,18,20,26-27H,7,11,17,19,21H2,(H,35,39)(H,36,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50090644

(CHEMBL48837 | {(S)-1-[(S)-1-(4-Butoxy-benzyl)-2,3-...)Show SMILES CCCCOc1ccc(C[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C(=O)C=O)cc1 Show InChI InChI=1S/C31H34N2O6/c1-2-3-18-38-26-16-14-24(15-17-26)19-27(29(35)21-34)32-30(36)28(20-23-10-6-4-7-11-23)33-31(37)39-22-25-12-8-5-9-13-25/h4-17,21,27-28H,2-3,18-20,22H2,1H3,(H,32,36)(H,33,37)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Queen's University Belfast
Curated by ChEMBL
| Assay Description
Ability to block cathepsin L-catalyzed hydrolysis of the fluorogenic substrate Z-Phe-Arg-NHMec |
Bioorg Med Chem Lett 10: 1771-3 (2000)
BindingDB Entry DOI: 10.7270/Q2TQ60SW |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509394

(CHEMBL4584291)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H29ClN2O3/c1-21(33)12-18-26(19-13-22-8-4-2-5-9-22)31-29(35)27(20-23-10-6-3-7-11-23)32-28(34)24-14-16-25(30)17-15-24/h2-12,14-18,26-27H,13,19-20H2,1H3,(H,31,35)(H,32,34)/b18-12+/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50258515

(CHEMBL4083754)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)\C=C\S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C31H36N2O5S/c1-24(2)22-29(33-31(35)38-23-26-14-8-4-9-15-26)30(34)32-27(19-18-25-12-6-3-7-13-25)20-21-39(36,37)28-16-10-5-11-17-28/h3-17,20-21,24,27,29H,18-19,22-23H2,1-2H3,(H,32,34)(H,33,35)/b21-20+/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina , Viale Annunziata, 98168 Messina, Italy.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L using Cbz-Phe-Arg-AMC as substrate after 10 mins by fluorescence assay |
J Med Chem 60: 6911-6923 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00405
BindingDB Entry DOI: 10.7270/Q2FJ2K73 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19775
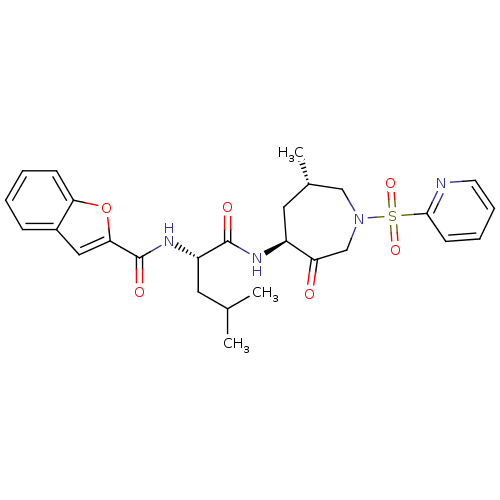
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1C[C@H](C)CN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)12-21(30-27(34)24-14-19-8-4-5-9-23(19)37-24)26(33)29-20-13-18(3)15-31(16-22(20)32)38(35,36)25-10-6-7-11-28-25/h4-11,14,17-18,20-21H,12-13,15-16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | -12.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549372
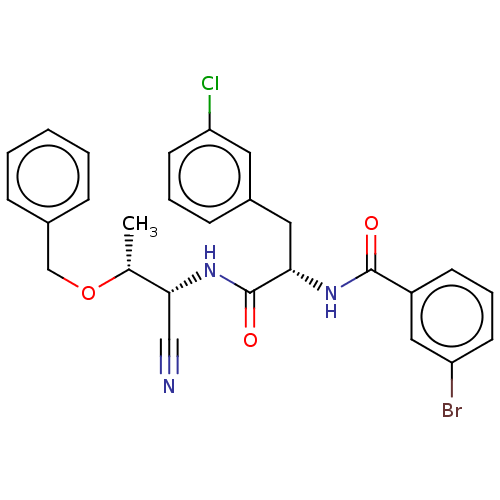
(CHEMBL4744431)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)c1cccc(Br)c1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50167290
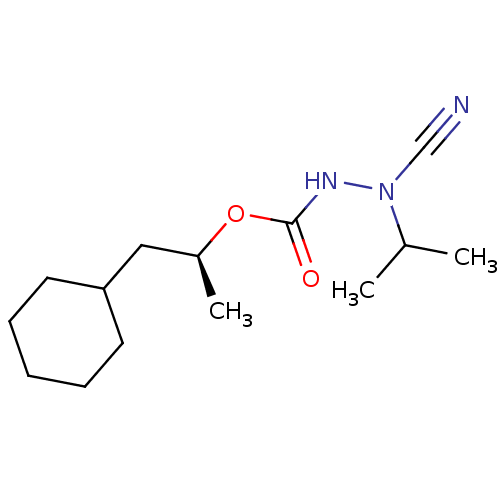
((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)17(10-15)16-14(18)19-12(3)9-13-7-5-4-6-8-13/h11-13H,4-9H2,1-3H3,(H,16,18)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 3039-43 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.032
BindingDB Entry DOI: 10.7270/Q2MS3S8C |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002399
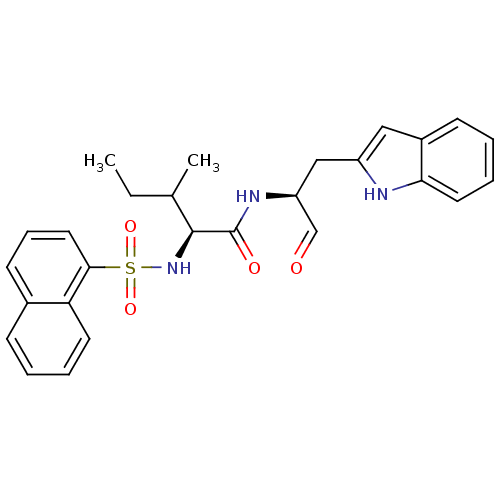
(CHEMBL197958)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1cc2ccccc2[nH]1)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-11-19-9-4-6-12-23(19)25)27(32)29-22(17-31)16-21-15-20-10-5-7-13-24(20)28-21/h4-15,17-18,22,26,28,30H,3,16H2,1-2H3,(H,29,32)/t18?,22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50304794

((S)-benzyl 1-(2-cyano-1,2-dimethylhydrazinyl)-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C17H24N4O3/c1-13(2)10-15(16(22)21(4)20(3)12-18)19-17(23)24-11-14-8-6-5-7-9-14/h5-9,13,15H,10-11H2,1-4H3,(H,19,23)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50374367
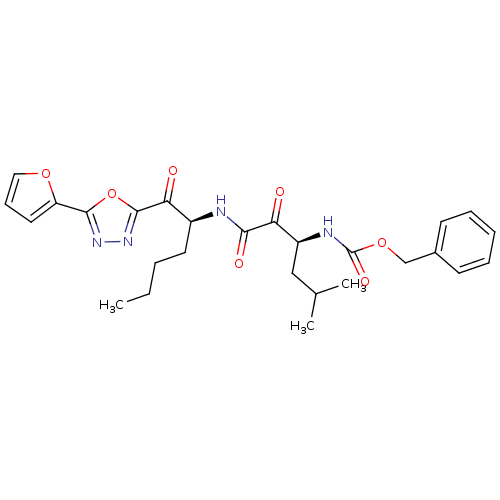
(CHEMBL271004)Show SMILES CCCC[C@H](NC(=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C27H32N4O7/c1-4-5-12-19(23(33)26-31-30-25(38-26)21-13-9-14-36-21)28-24(34)22(32)20(15-17(2)3)29-27(35)37-16-18-10-7-6-8-11-18/h6-11,13-14,17,19-20H,4-5,12,15-16H2,1-3H3,(H,28,34)(H,29,35)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L |
Bioorg Med Chem 16: 1562-95 (2008)
Article DOI: 10.1016/j.bmc.2007.11.015
BindingDB Entry DOI: 10.7270/Q21J9BNH |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50410979
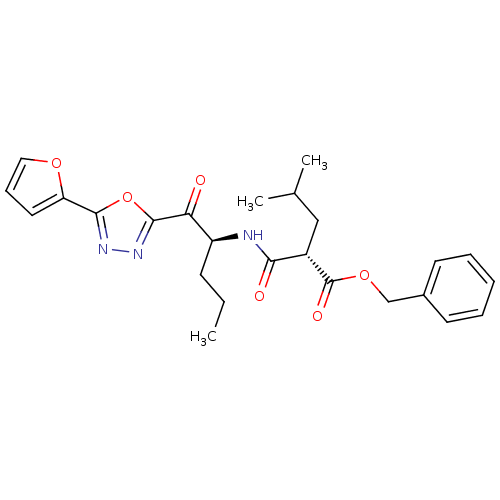
(CHEMBL207347)Show SMILES CCC[C@H](NC(=O)[C@H](CC(C)C)C(=O)OCc1ccccc1)C(=O)c1nnc(o1)-c1ccco1 Show InChI InChI=1S/C25H29N3O6/c1-4-9-19(21(29)24-28-27-23(34-24)20-12-8-13-32-20)26-22(30)18(14-16(2)3)25(31)33-15-17-10-6-5-7-11-17/h5-8,10-13,16,18-19H,4,9,14-15H2,1-3H3,(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
Bioorg Med Chem Lett 16: 2909-14 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.001
BindingDB Entry DOI: 10.7270/Q2QN6808 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137388
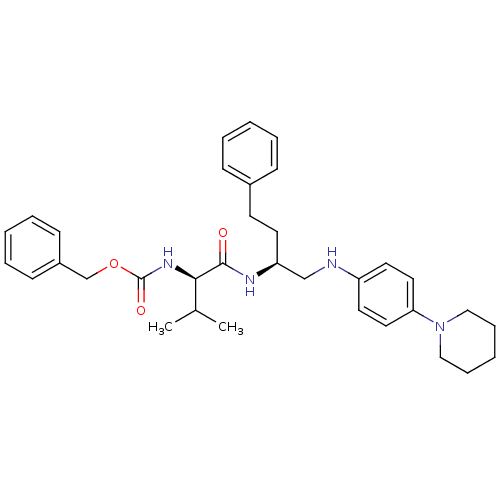
(((R)-2-Methyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES CC(C)[C@@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C34H44N4O3/c1-26(2)32(37-34(40)41-25-28-14-8-4-9-15-28)33(39)36-30(17-16-27-12-6-3-7-13-27)24-35-29-18-20-31(21-19-29)38-22-10-5-11-23-38/h3-4,6-9,12-15,18-21,26,30,32,35H,5,10-11,16-17,22-25H2,1-2H3,(H,36,39)(H,37,40)/t30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137398
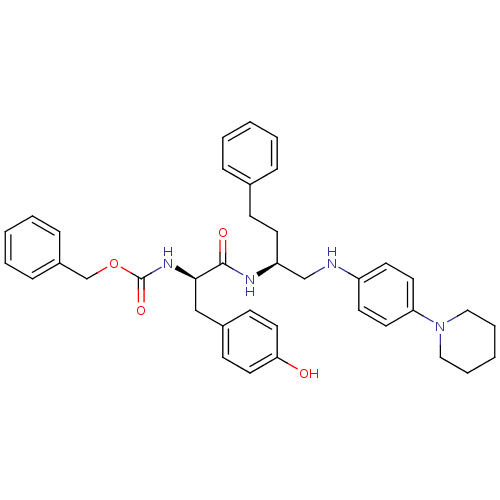
(((R)-2-(4-Hydroxy-phenyl)-1-{3-phenyl-1-[(4-piperi...)Show SMILES Oc1ccc(C[C@@H](NC(=O)OCc2ccccc2)C(=O)N[C@@H](CCc2ccccc2)CNc2ccc(cc2)N2CCCCC2)cc1 Show InChI InChI=1S/C38H44N4O4/c43-35-22-15-30(16-23-35)26-36(41-38(45)46-28-31-12-6-2-7-13-31)37(44)40-33(17-14-29-10-4-1-5-11-29)27-39-32-18-20-34(21-19-32)42-24-8-3-9-25-42/h1-2,4-7,10-13,15-16,18-23,33,36,39,43H,3,8-9,14,17,24-28H2,(H,40,44)(H,41,45)/t33-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
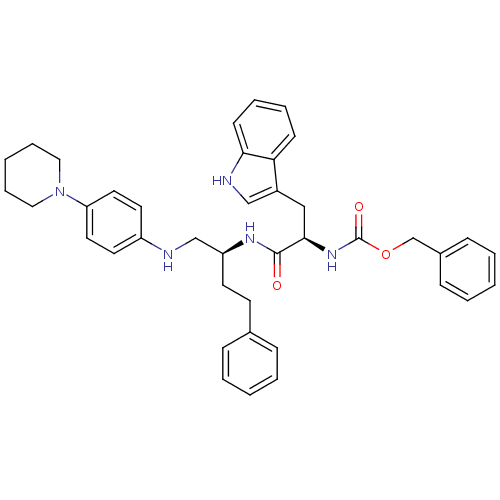
Procathepsin L
(Homo sapiens (Human)) | BDBM50137394
(((R)-2-(3H-Indol-3-yl)-1-{3-phenyl-1-[(4-piperidin...)Show SMILES O=C(N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1)OCc1ccccc1 Show InChI InChI=1S/C40H45N5O3/c46-39(38(26-32-27-42-37-17-9-8-16-36(32)37)44-40(47)48-29-31-14-6-2-7-15-31)43-34(19-18-30-12-4-1-5-13-30)28-41-33-20-22-35(23-21-33)45-24-10-3-11-25-45/h1-2,4-9,12-17,20-23,27,34,38,41-42H,3,10-11,18-19,24-26,28-29H2,(H,43,46)(H,44,47)/t34-,38+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
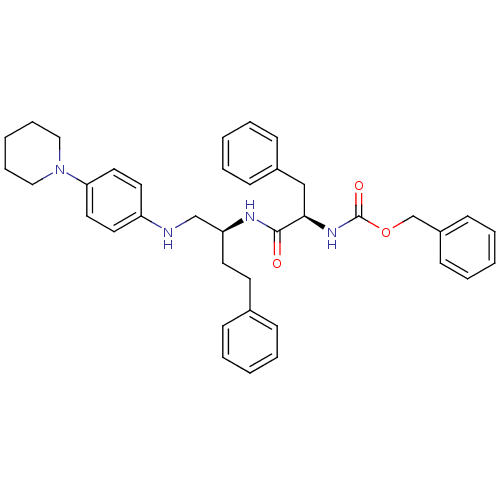
Procathepsin L
(Homo sapiens (Human)) | BDBM50137400
(((R)-2-Phenyl-1-{3-phenyl-1-[(4-piperidin-1-yl-phe...)Show SMILES O=C(N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)CNc1ccc(cc1)N1CCCCC1)OCc1ccccc1 Show InChI InChI=1S/C38H44N4O3/c43-37(36(27-31-15-7-2-8-16-31)41-38(44)45-29-32-17-9-3-10-18-32)40-34(20-19-30-13-5-1-6-14-30)28-39-33-21-23-35(24-22-33)42-25-11-4-12-26-42/h1-3,5-10,13-18,21-24,34,36,39H,4,11-12,19-20,25-29H2,(H,40,43)(H,41,44)/t34-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50549374

(CHEMBL4746374)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)[C@H](Cc1cccc(Cl)c1)NC(=O)c1cccc(c1)-c1cccnc1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115827
BindingDB Entry DOI: 10.7270/Q2CR5XZ7 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50335283

(CHEMBL1651357 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazo...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C23H27N7O3S/c1-15(2)12-18(22(31)30(4)29(3)14-24)26-23(32)25-13-16-7-9-17(10-8-16)20-27-21(33-28-20)19-6-5-11-34-19/h5-11,15,18H,12-13H2,1-4H3,(H2,25,26,32)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
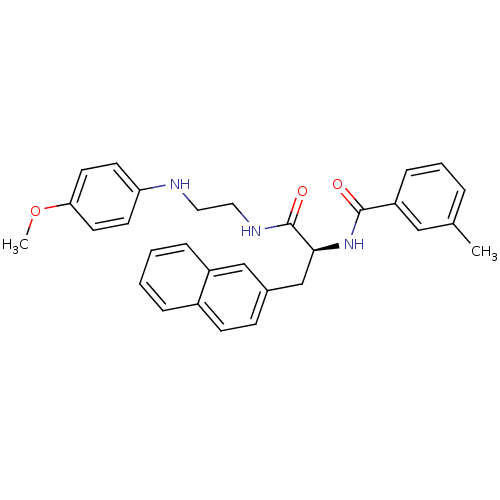
Procathepsin L
(Homo sapiens (Human)) | BDBM19564
((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-2-[(3-met...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C30H31N3O3/c1-21-6-5-9-25(18-21)29(34)33-28(20-22-10-11-23-7-3-4-8-24(23)19-22)30(35)32-17-16-31-26-12-14-27(36-2)15-13-26/h3-15,18-19,28,31H,16-17,20H2,1-2H3,(H,32,35)(H,33,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
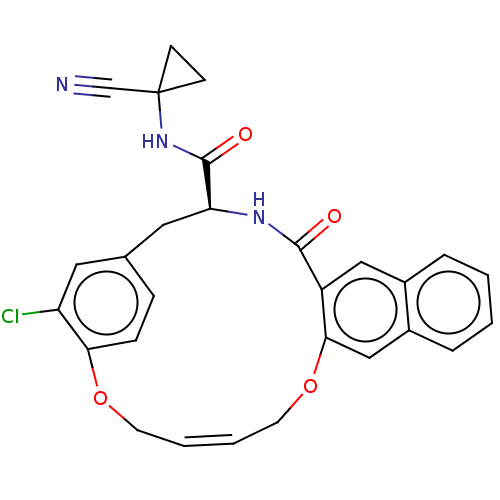
Procathepsin L
(Homo sapiens (Human)) | BDBM50263570
(CHEMBL4066422)Show SMILES Clc1cc2C[C@H](NC(=O)c3cc4ccccc4cc3OC\C=C\COc1cc2)C(=O)NC1(CC1)C#N |r,t:23| Show InChI InChI=1S/C28H24ClN3O4/c29-22-13-18-7-8-24(22)35-11-3-4-12-36-25-16-20-6-2-1-5-19(20)15-21(25)26(33)31-23(14-18)27(34)32-28(17-30)9-10-28/h1-8,13,15-16,23H,9-12,14H2,(H,31,33)(H,32,34)/b4-3+/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland.
Curated by ChEMBL
| Assay Description
Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method |
J Med Chem 61: 3350-3369 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01869
BindingDB Entry DOI: 10.7270/Q2R49T79 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19566
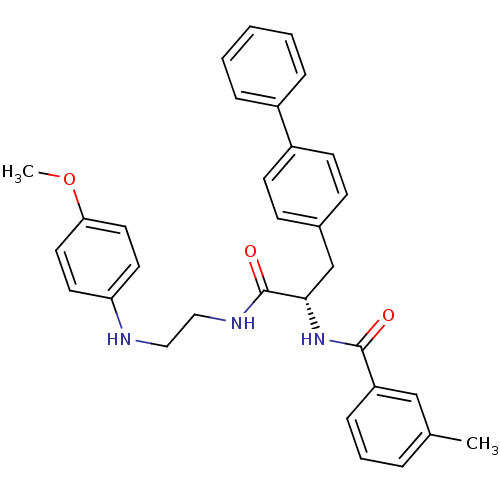
((2S)-N-{2-[(4-methoxyphenyl)amino]ethyl}-2-[(3-met...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2ccc(cc2)-c2ccccc2)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C32H33N3O3/c1-23-7-6-10-27(21-23)31(36)35-30(22-24-11-13-26(14-12-24)25-8-4-3-5-9-25)32(37)34-20-19-33-28-15-17-29(38-2)18-16-28/h3-18,21,30,33H,19-20,22H2,1-2H3,(H,34,37)(H,35,36)/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50168941

(CHEMBL3804928)Show SMILES OC(=O)CCn1cc(COC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C#N)nn1 |r| Show InChI InChI=1S/C26H28N6O6/c27-14-21(17-37-18-22-15-32(31-30-22)12-11-24(33)34)28-25(35)23(13-19-7-3-1-4-8-19)29-26(36)38-16-20-9-5-2-6-10-20/h1-10,15,21,23H,11-13,16-18H2,(H,28,35)(H,29,36)(H,33,34)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin L using Cbz-Phe-Arg-pNA as substrate incubated for 30 mins measured for 20 mins by photometrical analysis |
ACS Med Chem Lett 7: 211-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00474
BindingDB Entry DOI: 10.7270/Q2TH8PMM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137392
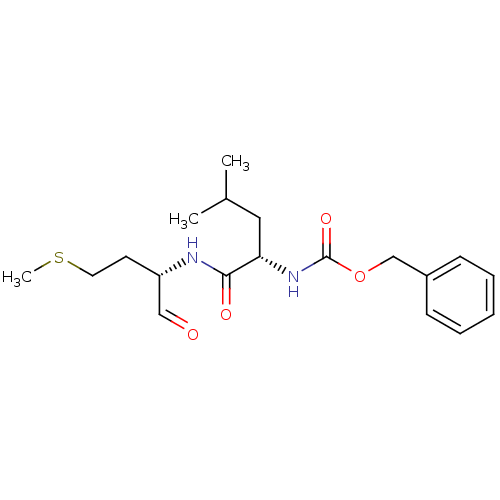
(CHEMBL99195 | [(S)-1-((S)-1-Formyl-3-methylsulfany...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O Show InChI InChI=1S/C19H28N2O4S/c1-14(2)11-17(18(23)20-16(12-22)9-10-26-3)21-19(24)25-13-15-7-5-4-6-8-15/h4-8,12,14,16-17H,9-11,13H2,1-3H3,(H,20,23)(H,21,24)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cathepsin L |
Bioorg Med Chem Lett 14: 87-90 (2003)
BindingDB Entry DOI: 10.7270/Q2930SKK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM31992
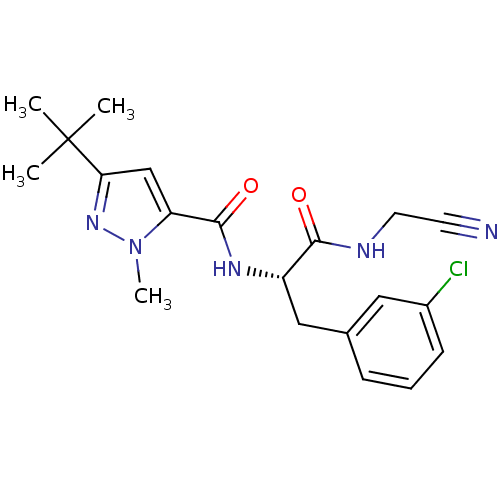
(Dipeptidyl nitrile inhibitor, 25)Show SMILES Cn1nc(cc1C(=O)N[C@@H](Cc1cccc(Cl)c1)C(=O)NCC#N)C(C)(C)C |r| Show InChI InChI=1S/C20H24ClN5O2/c1-20(2,3)17-12-16(26(4)25-17)19(28)24-15(18(27)23-9-8-22)11-13-6-5-7-14(21)10-13/h5-7,10,12,15H,9,11H2,1-4H3,(H,23,27)(H,24,28)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50335282
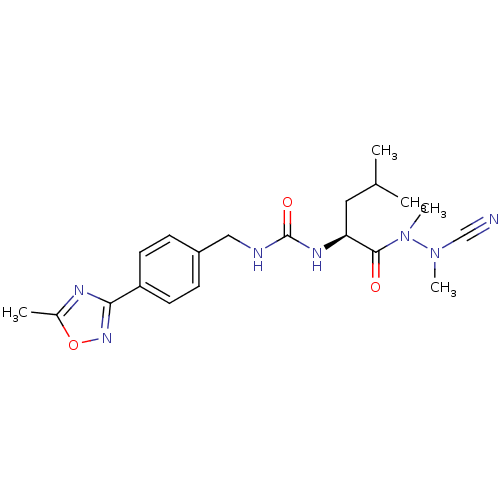
(CHEMBL1651356 | N-[4-(5-Methyl-1,2,4-oxadiazol-3-y...)Show SMILES CC(C)C[C@H](NC(=O)NCc1ccc(cc1)-c1noc(C)n1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C20H27N7O3/c1-13(2)10-17(19(28)27(5)26(4)12-21)24-20(29)22-11-15-6-8-16(9-7-15)18-23-14(3)30-25-18/h6-9,13,17H,10-11H2,1-5H3,(H2,22,24,29)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50002400
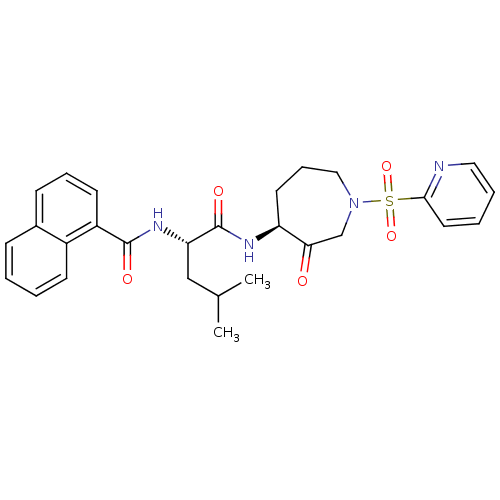
(CHEMBL382286)Show SMILES CC(C)C[C@H](NC(=O)c1cccc2ccccc12)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C28H32N4O5S/c1-19(2)17-24(31-27(34)22-12-7-10-20-9-3-4-11-21(20)22)28(35)30-23-13-8-16-32(18-25(23)33)38(36,37)26-14-5-6-15-29-26/h3-7,9-12,14-15,19,23-24H,8,13,16-18H2,1-2H3,(H,30,35)(H,31,34)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant against human cathepsin L |
J Med Chem 48: 6870-8 (2005)
Article DOI: 10.1021/jm0502079
BindingDB Entry DOI: 10.7270/Q2WM1CZC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19553
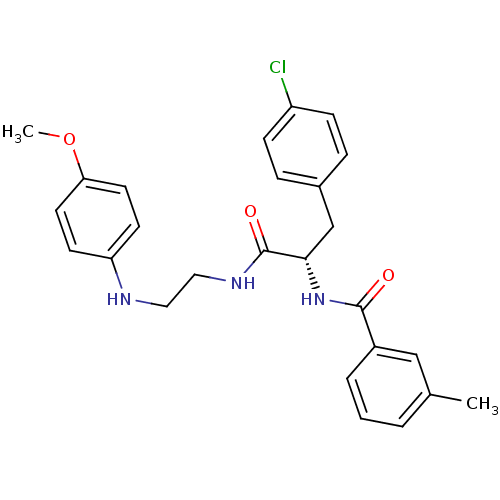
((2S)-3-(4-chlorophenyl)-N-{2-[(4-methoxyphenyl)ami...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2ccc(Cl)cc2)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C26H28ClN3O3/c1-18-4-3-5-20(16-18)25(31)30-24(17-19-6-8-21(27)9-7-19)26(32)29-15-14-28-22-10-12-23(33-2)13-11-22/h3-13,16,24,28H,14-15,17H2,1-2H3,(H,29,32)(H,30,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509387

(CHEMBL4475066)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C29H29FN2O3/c1-21(33)12-18-26(19-13-22-8-4-2-5-9-22)31-29(35)27(20-23-10-6-3-7-11-23)32-28(34)24-14-16-25(30)17-15-24/h2-12,14-18,26-27H,13,19-20H2,1H3,(H,31,35)(H,32,34)/b18-12+/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50108840
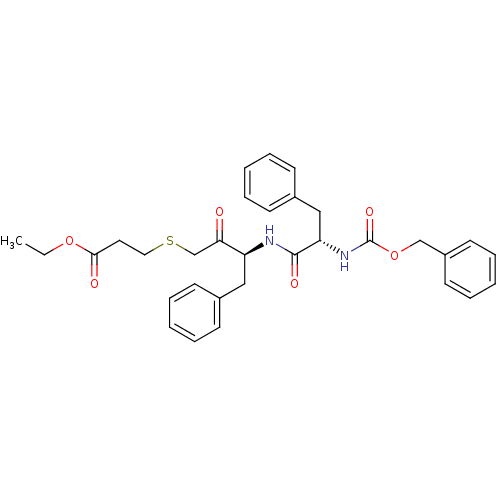
(3-[3-(2-Benzyloxycarbonylamino-3-phenyl-propionyla...)Show SMILES CCOC(=O)CCSCC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C32H36N2O6S/c1-2-39-30(36)18-19-41-23-29(35)27(20-24-12-6-3-7-13-24)33-31(37)28(21-25-14-8-4-9-15-25)34-32(38)40-22-26-16-10-5-11-17-26/h3-17,27-28H,2,18-23H2,1H3,(H,33,37)(H,34,38)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human Cathepsin L |
J Med Chem 45: 676-84 (2002)
BindingDB Entry DOI: 10.7270/Q2ZK5G0V |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50335276
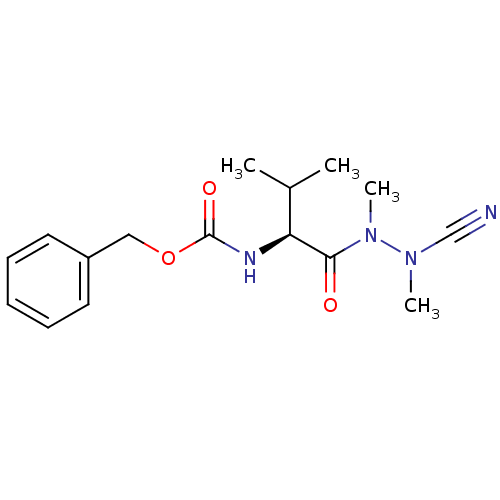
(CHEMBL1651242 | N-(Benzyloxycarbonyl)-valyl-methyl...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N(C)N(C)C#N |r| Show InChI InChI=1S/C16H22N4O3/c1-12(2)14(15(21)20(4)19(3)11-17)18-16(22)23-10-13-8-6-5-7-9-13/h5-9,12,14H,10H2,1-4H3,(H,18,22)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin L after 30 mins by spectrophotometric assay |
J Med Chem 54: 396-400 (2011)
Article DOI: 10.1021/jm101272p
BindingDB Entry DOI: 10.7270/Q2PV6MC3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19554
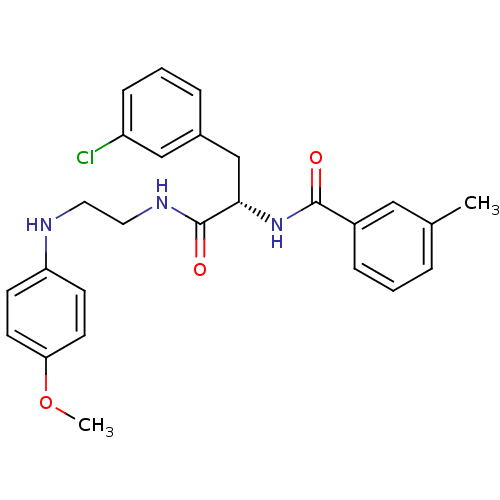
((2S)-3-(3-chlorophenyl)-N-{2-[(4-methoxyphenyl)ami...)Show SMILES COc1ccc(NCCNC(=O)[C@H](Cc2cccc(Cl)c2)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C26H28ClN3O3/c1-18-5-3-7-20(15-18)25(31)30-24(17-19-6-4-8-21(27)16-19)26(32)29-14-13-28-22-9-11-23(33-2)12-10-22/h3-12,15-16,24,28H,13-14,17H2,1-2H3,(H,29,32)(H,30,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 15: 4979-84 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.017
BindingDB Entry DOI: 10.7270/Q2ZG6QJ8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19610
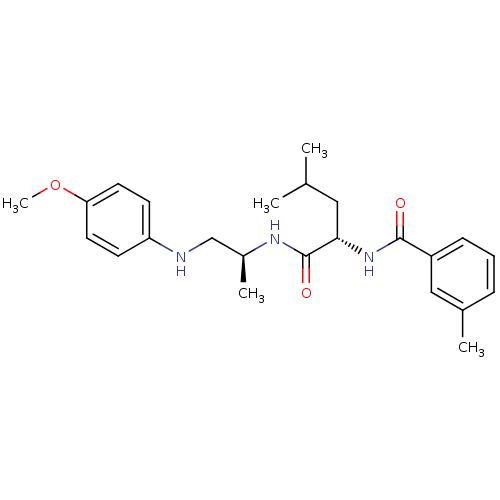
((2S)-N-[(2S)-1-[(4-methoxyphenyl)amino]propan-2-yl...)Show SMILES COc1ccc(NC[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)c2cccc(C)c2)cc1 |r| Show InChI InChI=1S/C24H33N3O3/c1-16(2)13-22(27-23(28)19-8-6-7-17(3)14-19)24(29)26-18(4)15-25-20-9-11-21(30-5)12-10-20/h6-12,14,16,18,22,25H,13,15H2,1-5H3,(H,26,29)(H,27,28)/t18-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... |
Bioorg Med Chem Lett 16: 1486-90 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.056
BindingDB Entry DOI: 10.7270/Q2TQ5ZTJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50108862
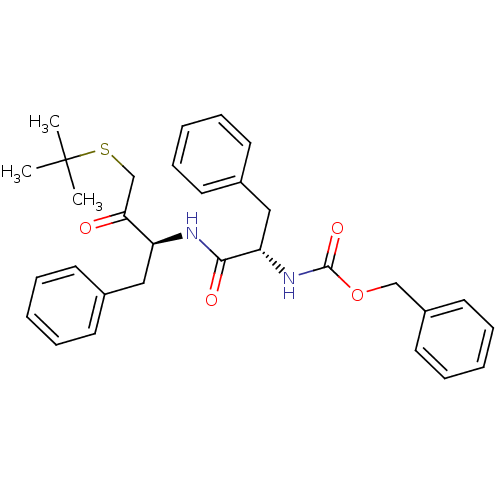
(CHEMBL161651 | [1-(1-Benzyl-3-tert-butylsulfanyl-2...)Show SMILES CC(C)(C)SCC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H36N2O4S/c1-31(2,3)38-22-28(34)26(19-23-13-7-4-8-14-23)32-29(35)27(20-24-15-9-5-10-16-24)33-30(36)37-21-25-17-11-6-12-18-25/h4-18,26-27H,19-22H2,1-3H3,(H,32,35)(H,33,36)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human Cathepsin L |
J Med Chem 45: 676-84 (2002)
BindingDB Entry DOI: 10.7270/Q2ZK5G0V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data