

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20079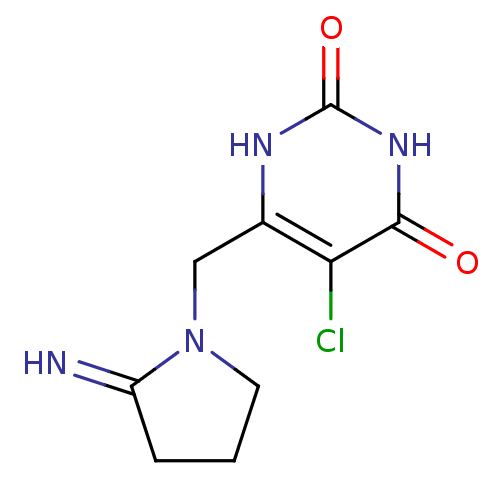 (5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.30 | -12.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50121753 (1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, L.L.C. Curated by ChEMBL | Assay Description Binding affinity towards recombinant thymidine phosphorylase TP | Bioorg Med Chem Lett 13: 107-10 (2002) BindingDB Entry DOI: 10.7270/Q2GM87V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50313094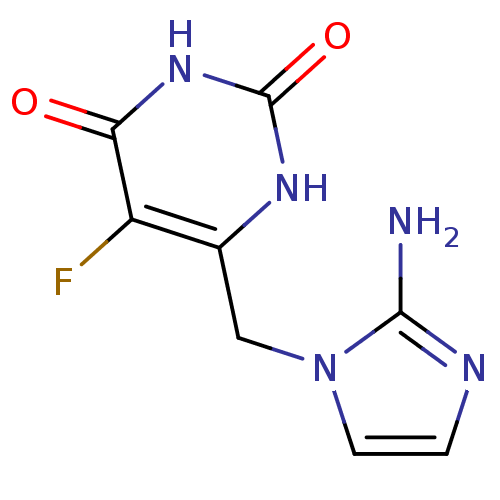 (5-fluoro-6-[(2-aminoimidazol-1-yl)methyl]uracil | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University at Buffalo Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase | Bioorg Med Chem Lett 20: 1648-51 (2010) Article DOI: 10.1016/j.bmcl.2010.01.076 BindingDB Entry DOI: 10.7270/Q26T0MSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50121753 (1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against human thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20079 (5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531739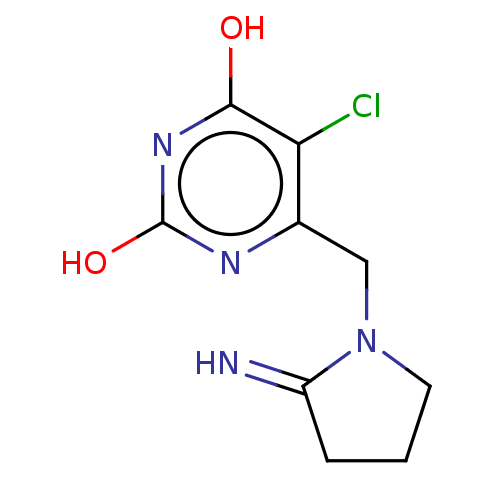 (MA-1 | TPI (freebase) | Tipiracil) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Competitive inhibition of thymidine phosphorylase (unknown origin) | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20079 (5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against thymidine phosphorylase | J Med Chem 48: 392-402 (2005) Article DOI: 10.1021/jm049494r BindingDB Entry DOI: 10.7270/Q2445N8K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20079 (5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase by [3H]thymidine incorporation assay | Eur J Med Chem 43: 1248-60 (2008) Article DOI: 10.1016/j.ejmech.2007.07.015 BindingDB Entry DOI: 10.7270/Q2Z31ZDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20061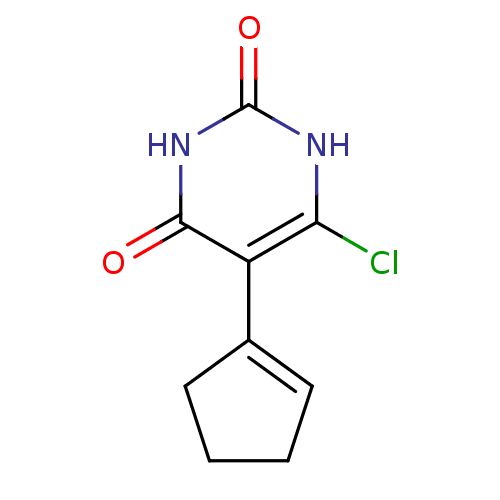 (5-Substituted-6-chlorouracil, 7a | 6-chloro-5-(cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -9.50 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50310199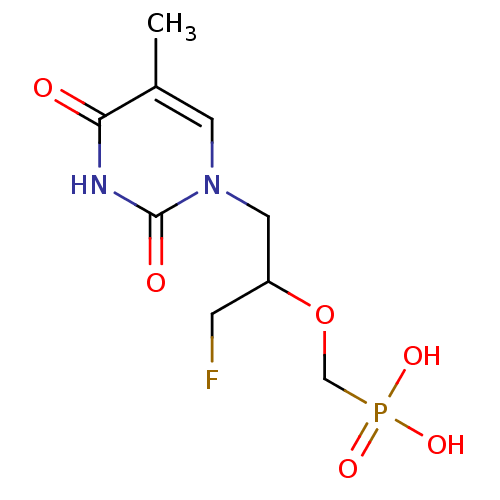 ((1-fluoro-3-(5-methyl-2,4-dioxo-3,4-dihydropyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50310200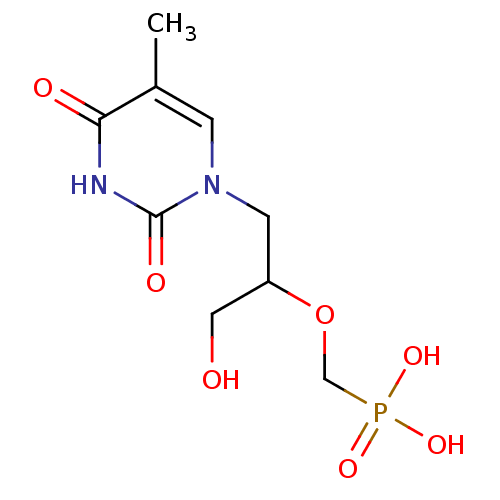 ((1-hydroxy-3-(5-methyl-2,4-dioxo-3,4-dihydropyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50310201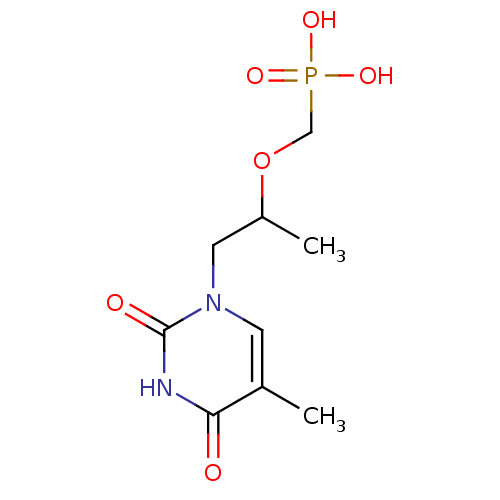 ((1-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50310202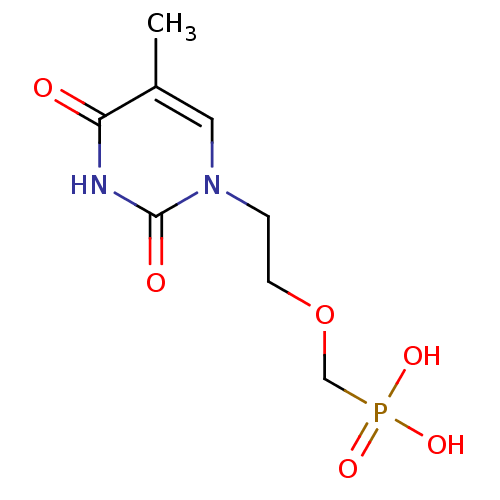 ((2-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201010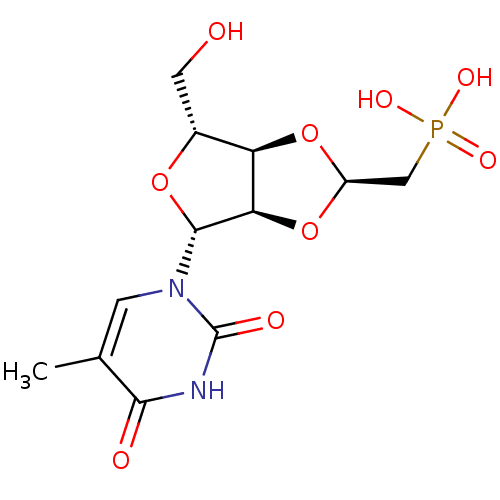 (((2R,3aR,4R,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50310203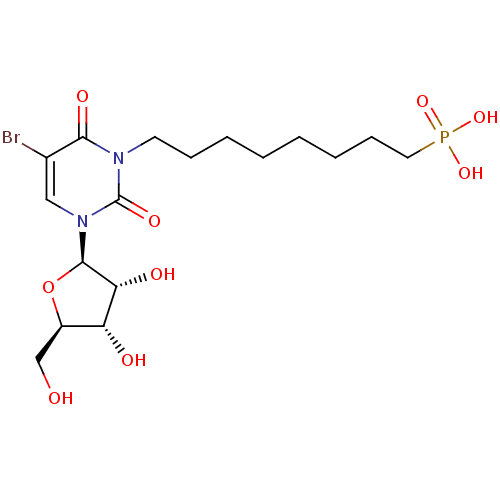 (8-(5-bromo-3-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201010 (((2R,3aR,4R,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20069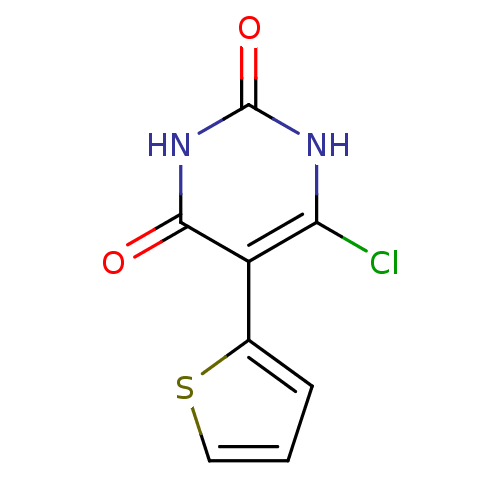 (5-Substituted-6-chlorouracil, 10e | 6-chloro-5-(th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 280 | -9.29 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20065 (5-Substituted-6-chlorouracil, 10a | 6-chloro-5-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -9.07 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20073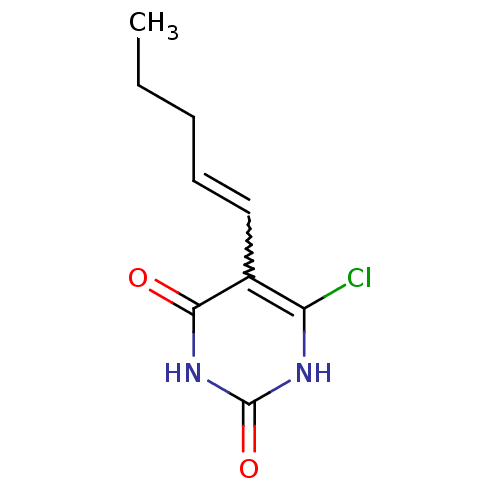 (5-Substituted-6-chlorouracil, 13c | 6-chloro-5-[(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 410 | -9.06 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20062 (5-Substituted-6-chlorouracil, 7b | 6-chloro-5-(cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 420 | -9.04 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20064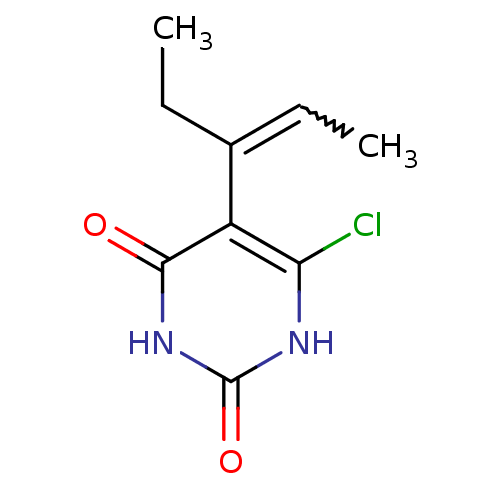 (5-Substituted-6-chlorouracil, 7d | 6-chloro-5-[(2E...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 490 | -8.95 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20075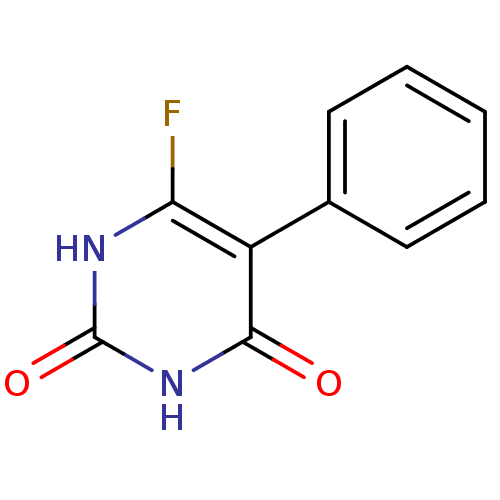 (6-Fluoro-5-phenylpyrimidine-2,4(1H,3H)-dione, 21 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | -8.94 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20072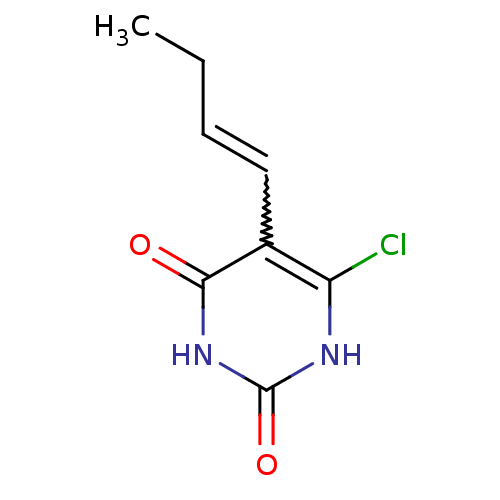 (5-Substituted-6-chlorouracil, 13b | 5-[(1E)-but-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 630 | -8.79 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20068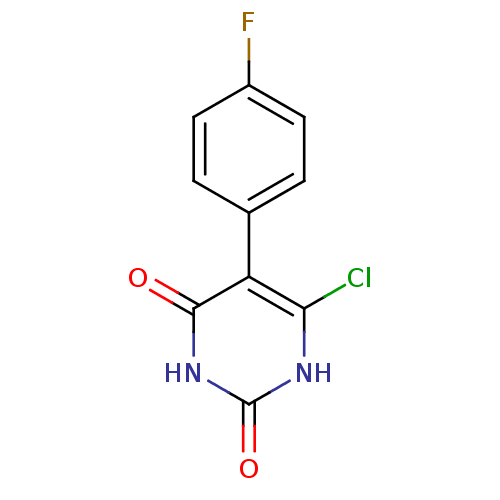 (5-Substituted-6-chlorouracil, 10d | 6-chloro-5-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 710 | -8.72 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50117689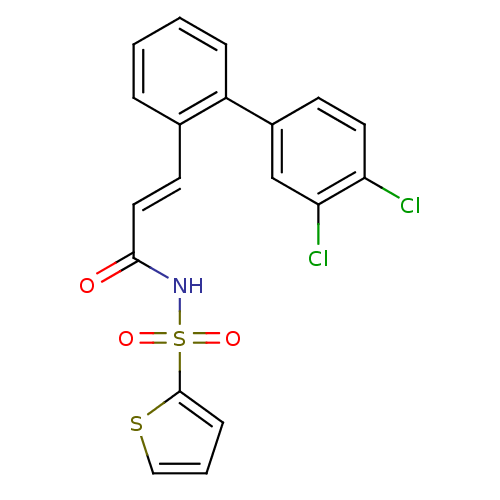 (CHEMBL87371 | Thiophene-2-sulfonic acid [(E)-3-(3'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound was determined against Prostanoid TP receptor | Bioorg Med Chem Lett 12: 2583-6 (2002) BindingDB Entry DOI: 10.7270/Q2QN663S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20056 (5-Substituted-6-chlorouracil, 5c | 5-butyl-6-chlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.03E+3 | -8.49 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201013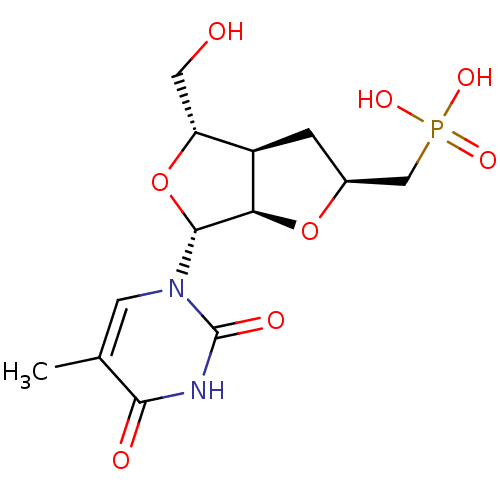 (((2S,3aR,4S,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20059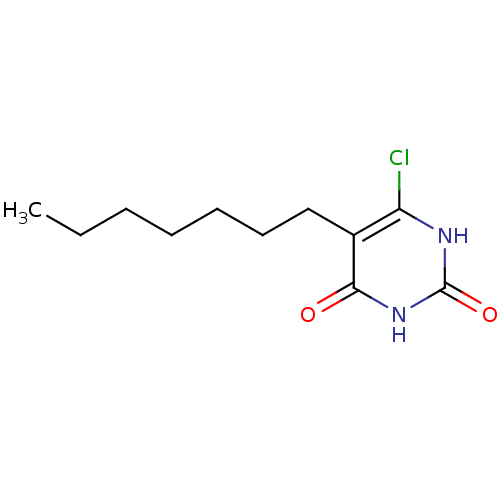 (5-Substituted-6-chlorouracil, 5f | 6-chloro-5-hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.06E+3 | -8.47 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20071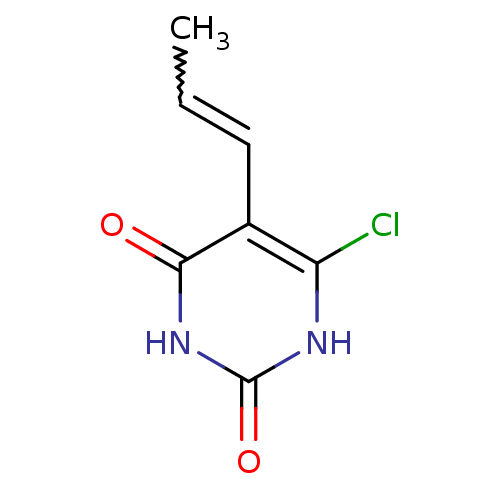 (5-Substituted-6-chlorouracil, 13a | 6-chloro-5-[(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.17E+3 | -8.41 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20076 (6-Bromo-5-phenylpyrimidine-2,4(1H,3H)-dione, 23 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.21E+3 | -8.39 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50004462 (CHEMBL3233329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore Curated by ChEMBL | Assay Description Mixed-type inhibition of human recombinant thymidine phosphorylase expressed in Escherichia coli assessed as conversion of thymidide to thymine by Li... | Eur J Med Chem 78: 294-303 (2014) Article DOI: 10.1016/j.ejmech.2014.03.063 BindingDB Entry DOI: 10.7270/Q2SF2XP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20066 (5-Substituted-6-chlorouracil, 10b | 6-chloro-5-(3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.74E+3 | -8.17 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50004460 (CHEMBL3233327) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore Curated by ChEMBL | Assay Description Mixed-type inhibition of human recombinant thymidine phosphorylase expressed in Escherichia coli assessed as conversion of thymidide to thymine by Li... | Eur J Med Chem 78: 294-303 (2014) Article DOI: 10.1016/j.ejmech.2014.03.063 BindingDB Entry DOI: 10.7270/Q2SF2XP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20058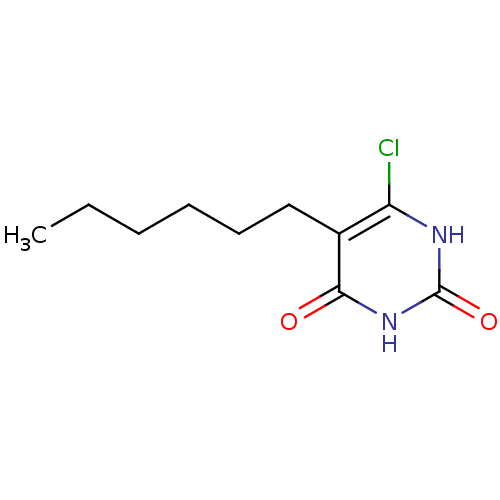 (5-Substituted-6-chlorouracil, 5e | 6-chloro-5-hexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.83E+3 | -8.14 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122770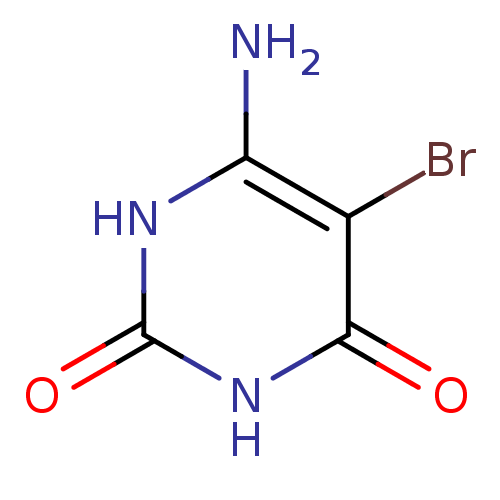 (6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20070 (5-Substituted-6-chlorouracil, 10f | 6-chloro-5-(py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.01E+3 | -7.83 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20063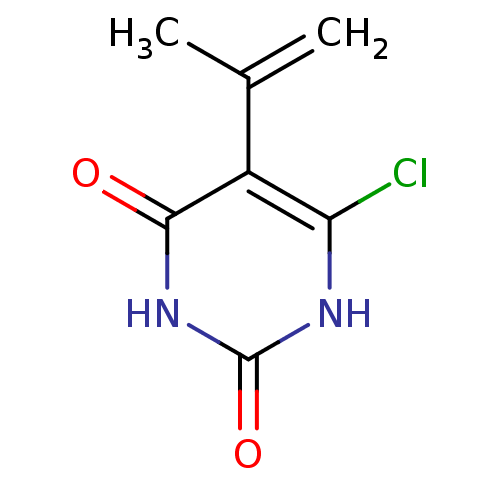 (5-Substituted-6-chlorouracil, 7c | 6-chloro-5-(pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.34E+3 | -7.77 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20057 (5-Substituted-6-chlorouracil, 5d | 6-chloro-5-pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.67E+3 | -7.71 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20067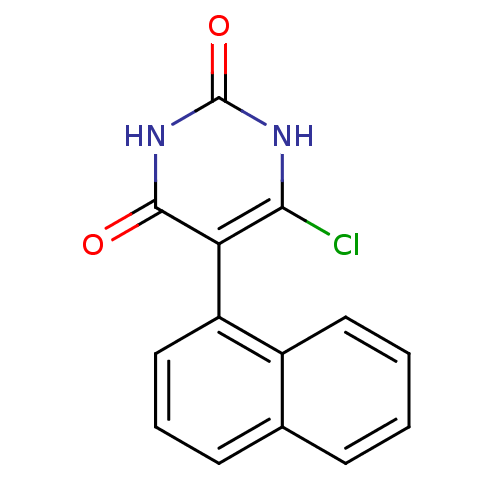 (5-Substituted-6-chlorouracil, 10c | 6-chloro-5-(na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.59E+3 | -7.57 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20060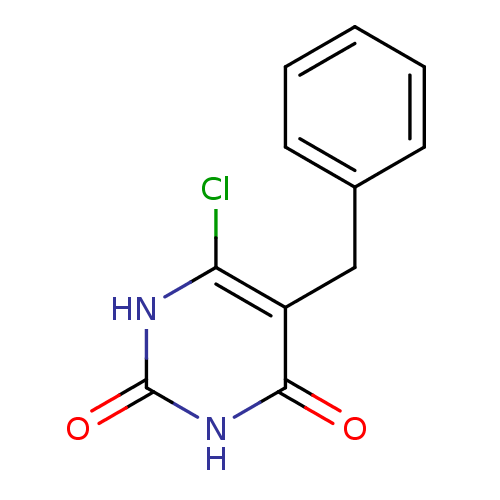 (5-Substituted-6-chlorouracil, 5g | 5-benzyl-6-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.65E+3 | -7.56 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20055 (5-Substituted-6-chlorouracil, 5b | 6-chloro-5-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.81E+3 | -7.43 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201015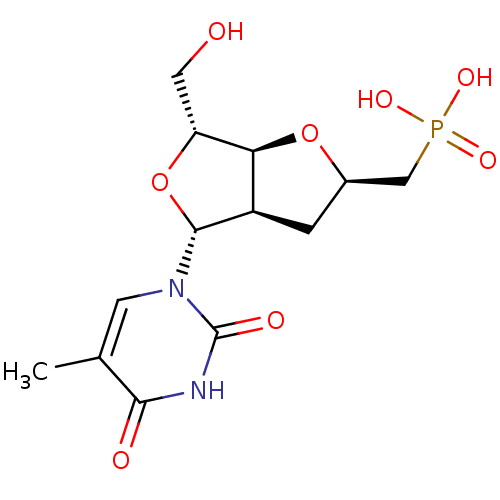 (((2R,3aR,4R,6R,6aS)-6-(hydroxymethyl)-4-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50439105 (CHEMBL2418061) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore Curated by ChEMBL | Assay Description Mixed inhibition of human recombinant thymidine phosphorylase expressed in Escherichia coli using thymidine as substrate by Lineweaver-Burk plot anal... | Eur J Med Chem 67: 325-34 (2013) Article DOI: 10.1016/j.ejmech.2013.06.051 BindingDB Entry DOI: 10.7270/Q2QR4ZJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20054 (5-Substituted-6-chlorouracil, 5a | 6-chloro-5-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+4 | >-6.66 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20078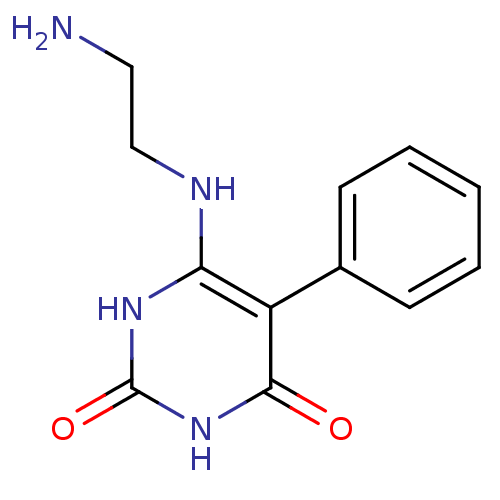 (6-[(2-Aminoethyl)amino]-5-phenylpyrimidine-2,4(1H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+4 | >-6.66 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20077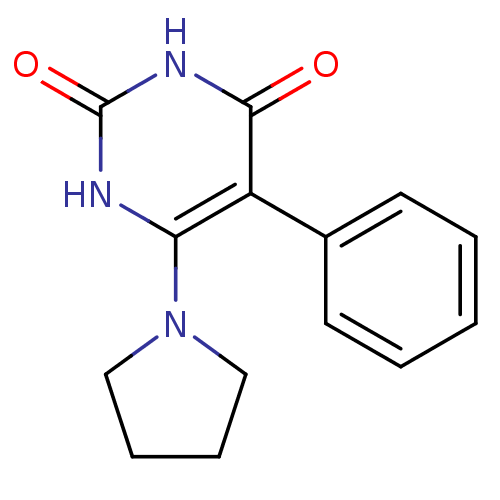 (5-Phenyl-6-pyrrolidin-1-ylpyrimidine-2,4(1H,3H)-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+4 | >-6.66 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20074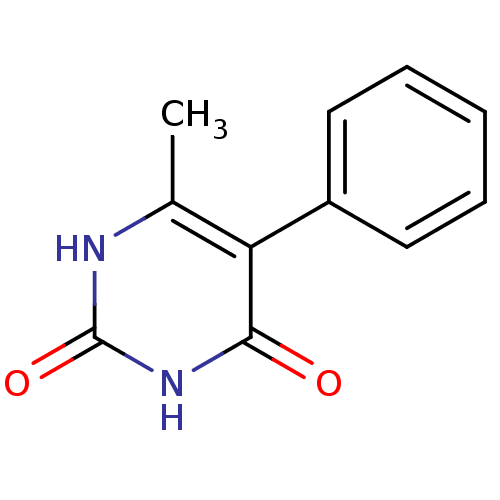 (6-Methyl-5-phenylpyrimidine-2,4(1H,3H)-dione, 16 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+4 | >-6.66 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50439106 (CHEMBL2418074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore Curated by ChEMBL | Assay Description Mixed inhibition of human recombinant thymidine phosphorylase expressed in Escherichia coli using thymidine as substrate by Lineweaver-Burk plot anal... | Eur J Med Chem 67: 325-34 (2013) Article DOI: 10.1016/j.ejmech.2013.06.051 BindingDB Entry DOI: 10.7270/Q2QR4ZJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201012 (((2R,3aR,4S,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201011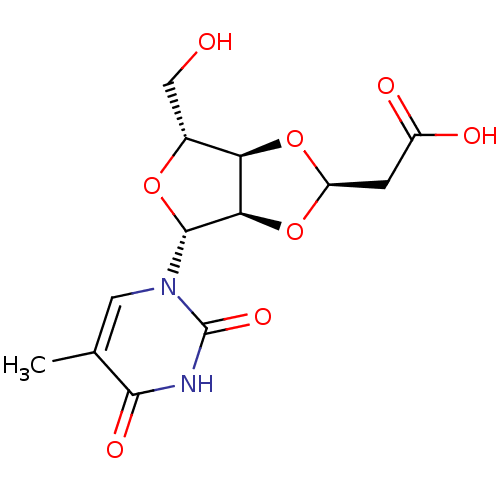 (2-((2R,3aR,4R,6R,6aR)-4-(hydroxymethyl)-6-(5-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |