

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274679 (CHEMBL4128530) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Tested for thromboxane antagonist potency (pA2) against U 44619 induced platelet aggregation using human platelet rich plasma | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274694 (CHEMBL4129901) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274686 (CHEMBL4129679) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274689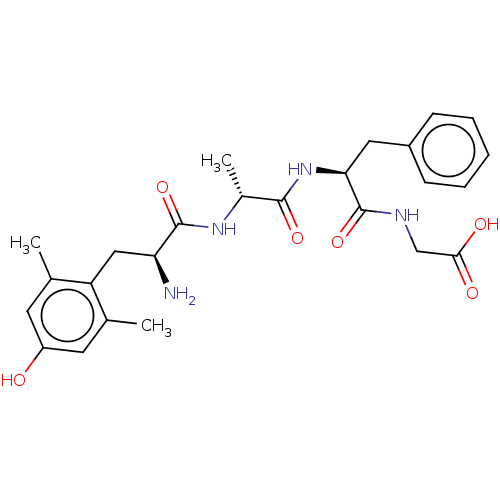 (CHEMBL4126050) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274696 (CHEMBL4126102) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0120 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274697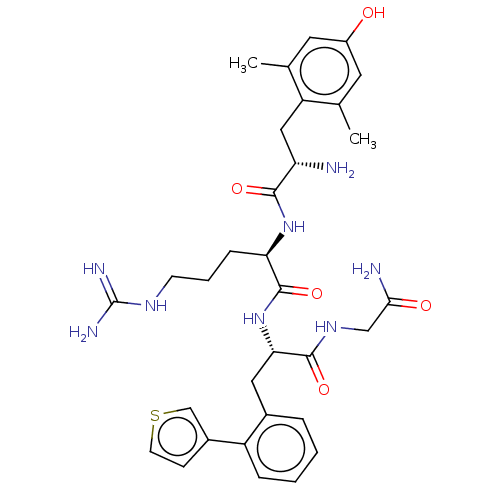 (CHEMBL4129689) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334990 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334990 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430610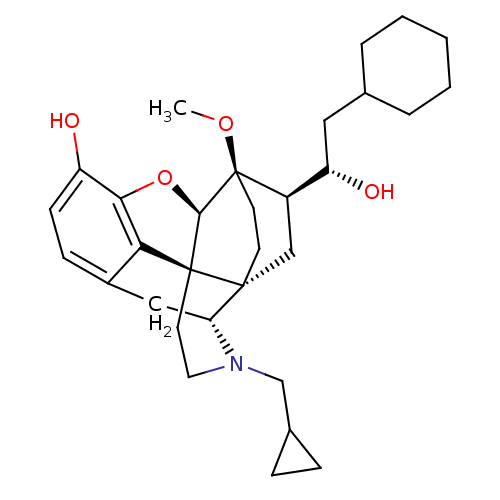 (CHEMBL2338726) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274731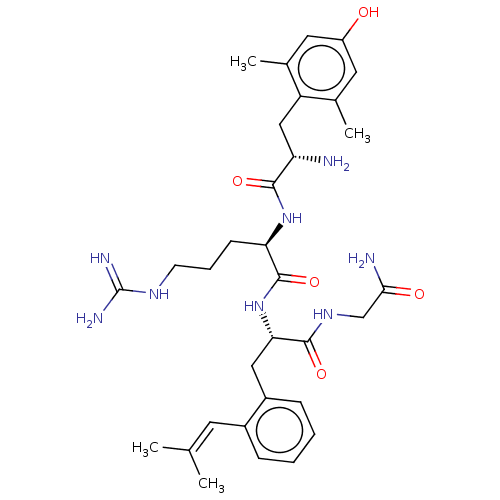 (CHEMBL4126322) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334991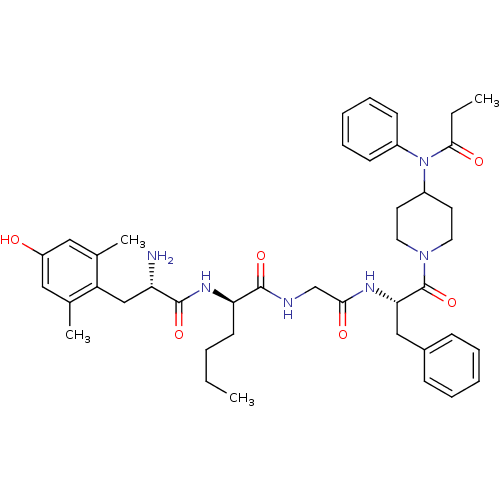 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334991 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274741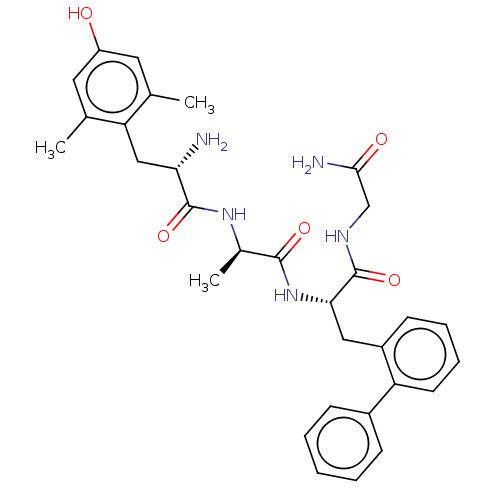 (CHEMBL4126470) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Tested for thromboxane antagonist potency (pA2) against U 44619 induced platelet aggregation using human platelet rich plasma | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50274729 (CHEMBL4128945) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in HEK293 cells co-expressing Rluc2-EPAC-GFP10 biosenser assessed as inhibition of forskolin-ind... | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430611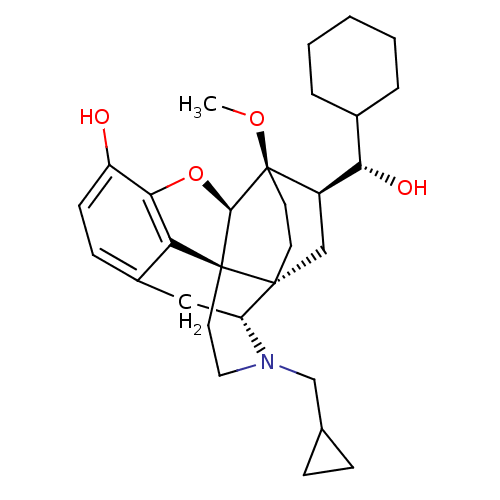 (CHEMBL2338725) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535355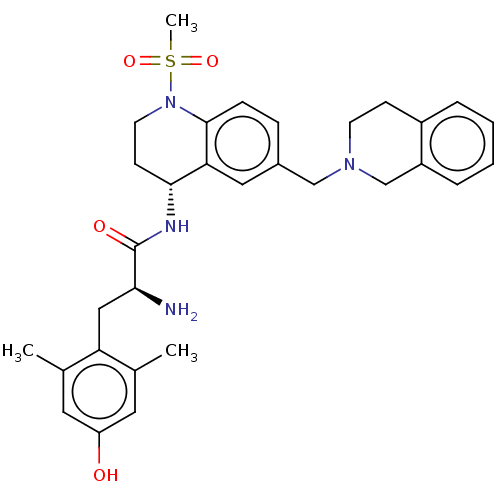 (CHEMBL4548498) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 hr | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334994 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334994 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430612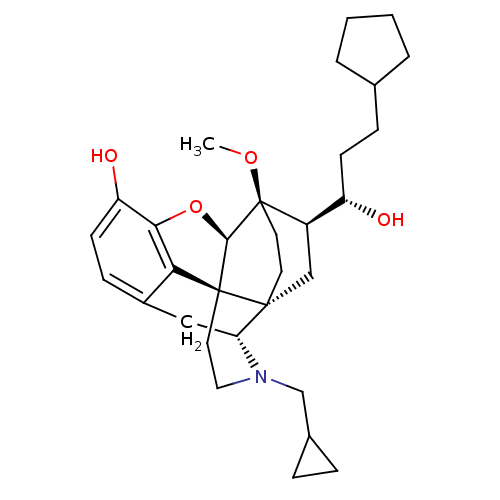 (CHEMBL2338724) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430586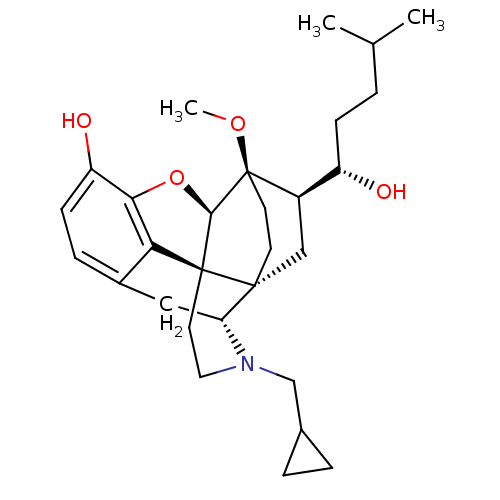 (CHEMBL2338721) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430613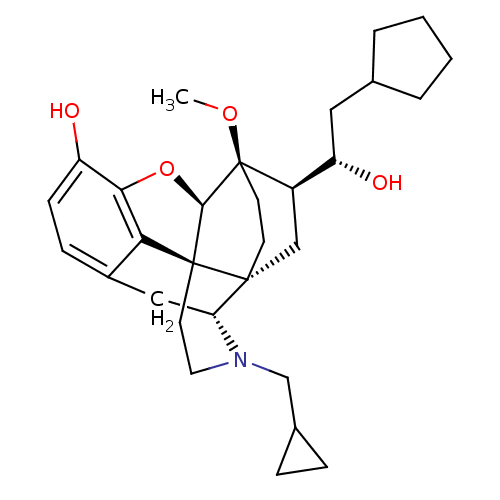 (CHEMBL2338723) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50248795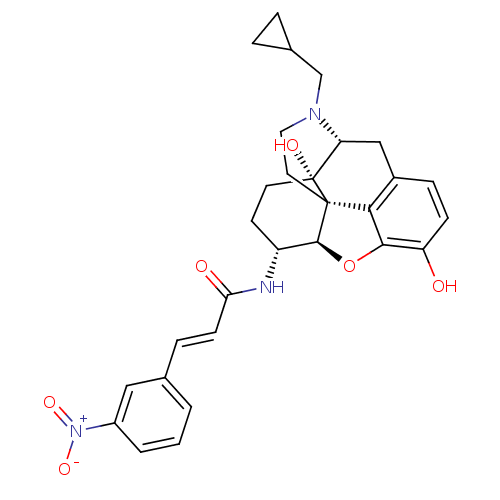 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membrane assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 1546-52 (2009) Article DOI: 10.1021/jm8015552 BindingDB Entry DOI: 10.7270/Q2513Z24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071865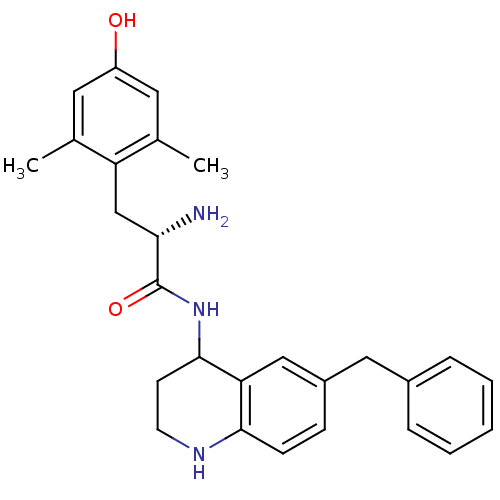 ((S)-2-Amino-N-(6-benzyl-1,2,3,4-tetrahydro-quinoli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Compound was tested for its ability to stimulate [35S]GTP-gamma-S, binding to membranes from C6 glioma cells stably expressing rat mu opioid receptor... | Bioorg Med Chem Lett 8: 2685-8 (1999) BindingDB Entry DOI: 10.7270/Q2154HJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071865 ((S)-2-Amino-N-(6-benzyl-1,2,3,4-tetrahydro-quinoli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 in guinea pig brain membranes | Bioorg Med Chem Lett 8: 2685-8 (1999) BindingDB Entry DOI: 10.7270/Q2154HJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430624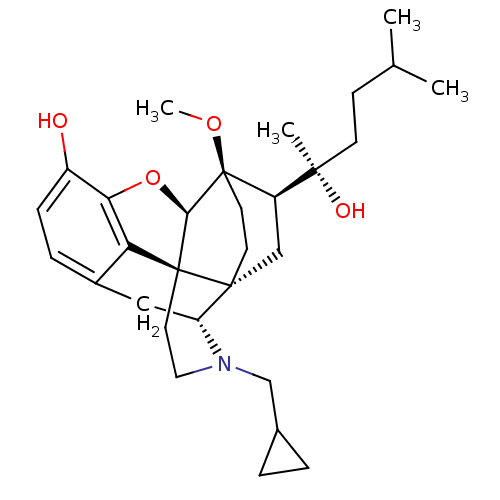 (CHEMBL2338745) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50491860 (CHEMBL2387215) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 23: 3434-7 (2013) Article DOI: 10.1016/j.bmcl.2013.03.065 BindingDB Entry DOI: 10.7270/Q2T72MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430609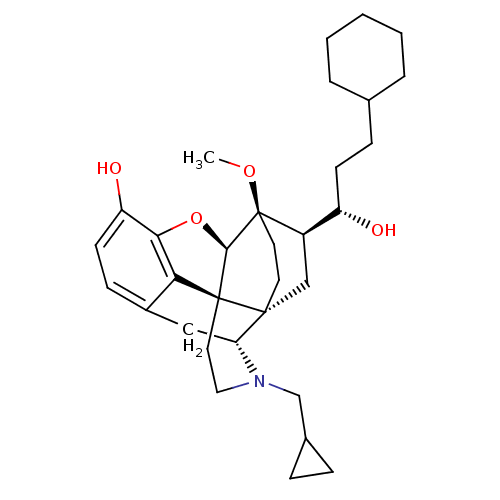 (CHEMBL2338727) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090755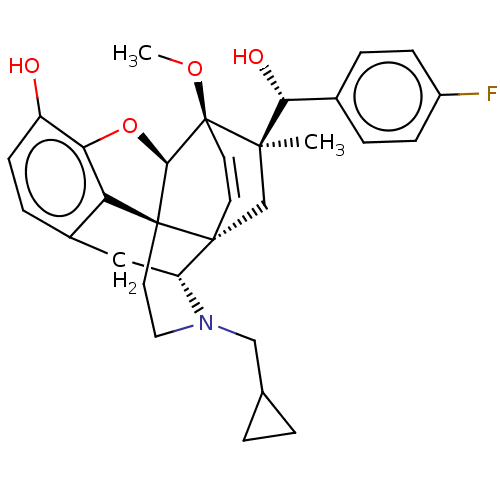 (CHEMBL3581743 | US9259422, 22, R = 4-FPh- BU10120 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535340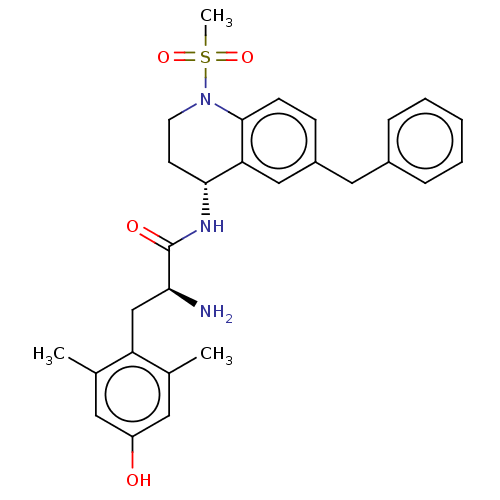 (CHEMBL4521319) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 hr | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430608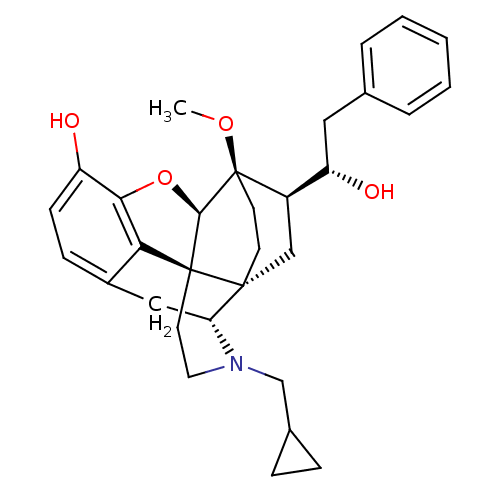 (CHEMBL2338728) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535333 (CHEMBL4588807) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 hr | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50427555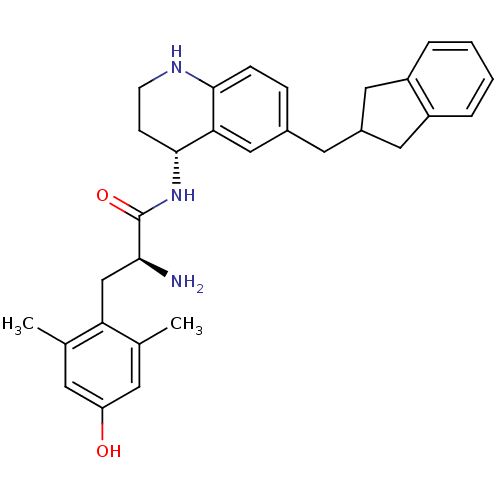 (CHEMBL2322563) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat mu-opioid receptor expressed in C6 cell membrane assessed as [35S]GTPgammaS binding for 1 hr by liquid scintillation counting... | J Med Chem 58: 8952-69 (2015) Article DOI: 10.1021/acs.jmedchem.5b01270 BindingDB Entry DOI: 10.7270/Q2RR213J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090906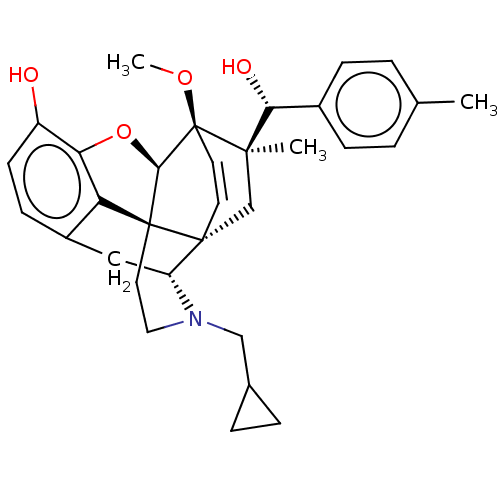 (CHEMBL3581742) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50427555 (CHEMBL2322563) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr by liquid scintillation counting a... | J Med Chem 56: 2139-49 (2013) Article DOI: 10.1021/jm400050y BindingDB Entry DOI: 10.7270/Q2K075MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Tested for thromboxane antagonist potency (pA2) against U 44619 induced platelet aggregation using human platelet rich plasma | Bioorg Med Chem Lett 28: 2320-2323 (2018) Article DOI: 10.1016/j.bmcl.2018.05.015 BindingDB Entry DOI: 10.7270/Q2KW5JJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430591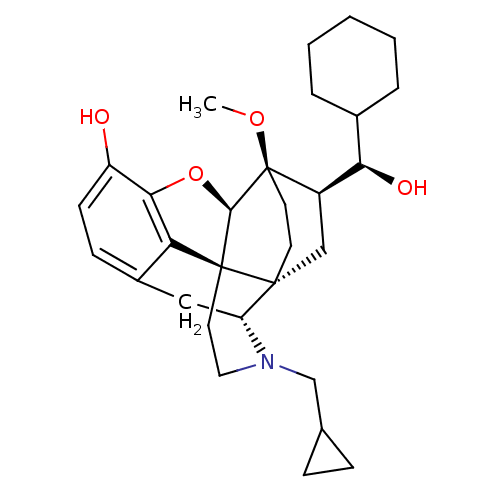 (CHEMBL2338741) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535350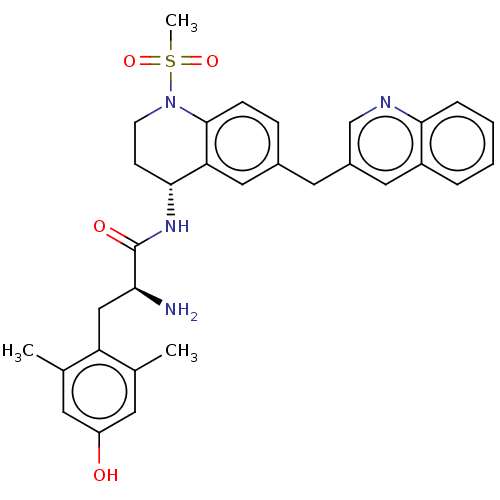 (CHEMBL4471560) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 hr | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50026603 (Buprenorphine | CHEBI:3216) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334995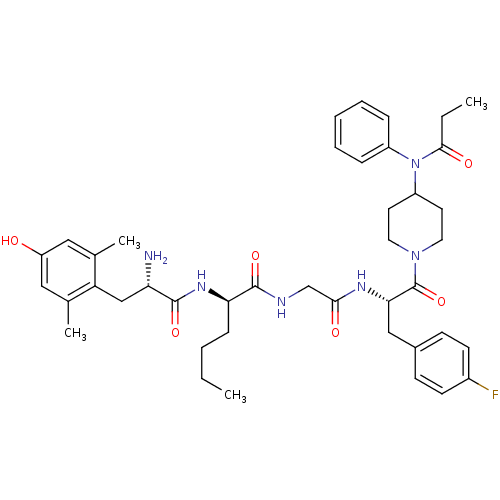 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334995 ((R)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430585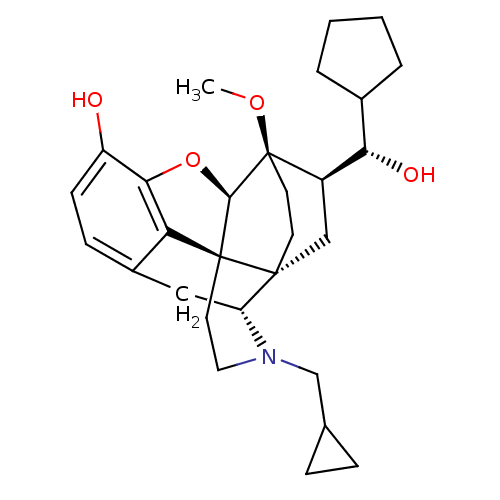 (CHEMBL2338722) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430588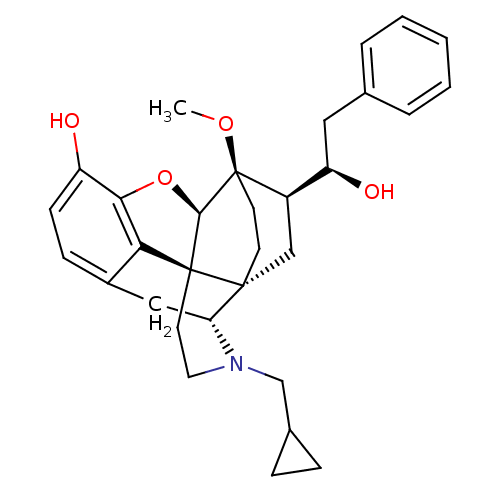 (CHEMBL2338717) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535351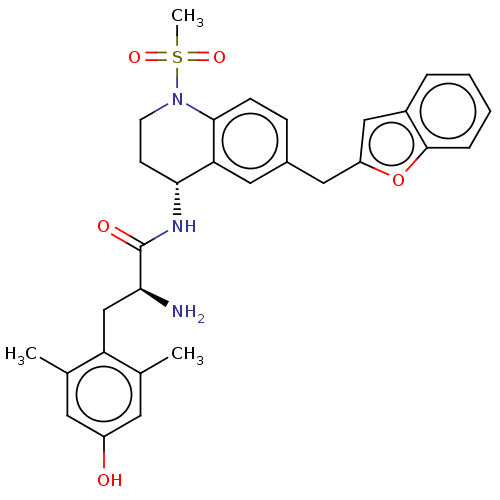 (CHEMBL4459271) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 hr | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535347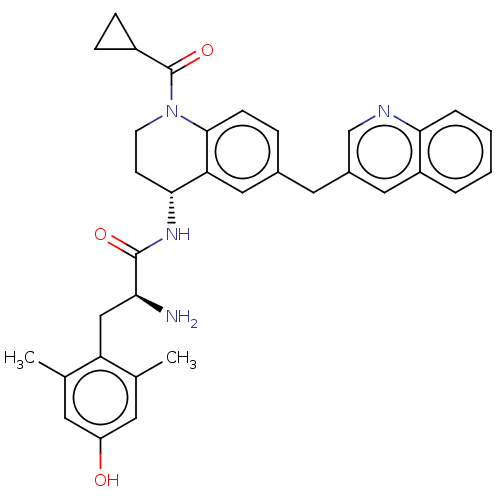 (CHEMBL4476818) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 hr | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334996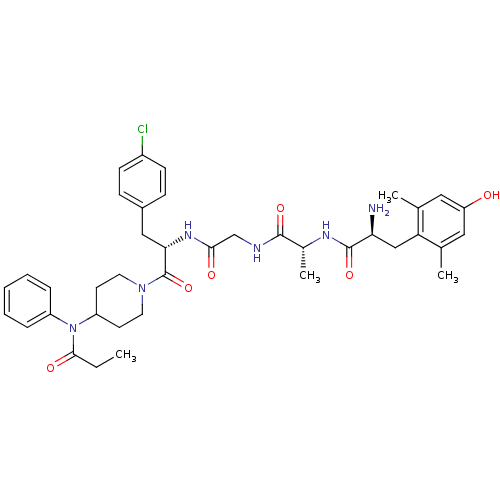 ((S)-2-amino-N-((R)-1-(2-((S)-3-(4-chlorophenyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.347 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50334996 ((S)-2-amino-N-((R)-1-(2-((S)-3-(4-chlorophenyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.347 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 54: 382-6 (2011) Article DOI: 10.1021/jm100982d BindingDB Entry DOI: 10.7270/Q2N29XWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50185330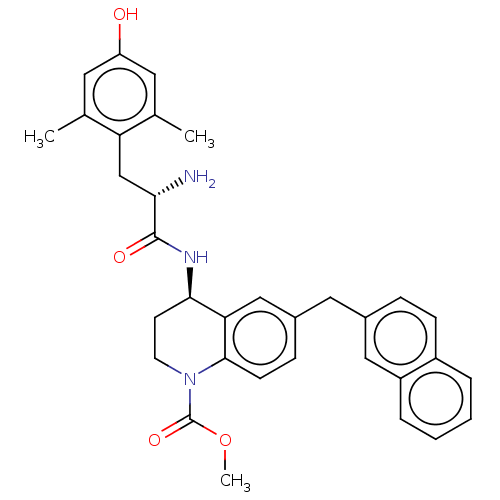 (CHEMBL3824356) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Agonist activity at rat MOR transfected in rat C6 cell membranes after 1 hr by [35S]GTPgammaS assay | J Med Chem 59: 4985-98 (2016) Article DOI: 10.1021/acs.jmedchem.6b00308 BindingDB Entry DOI: 10.7270/Q28917SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535334 (CHEMBL4467875) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 hr | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50535337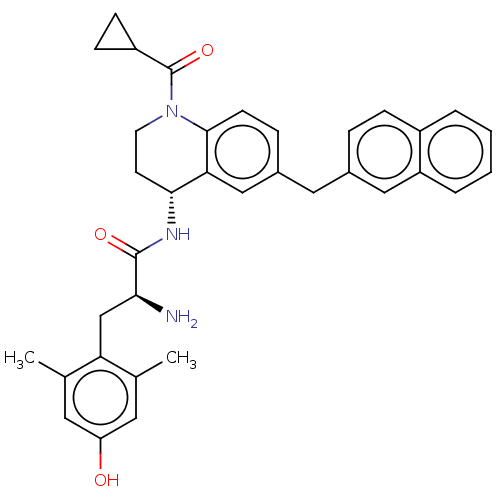 (CHEMBL4446945) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding measured after 1 hr | J Med Chem 62: 4193-4203 (2019) Article DOI: 10.1021/acs.jmedchem.9b00378 BindingDB Entry DOI: 10.7270/Q2542S2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50430598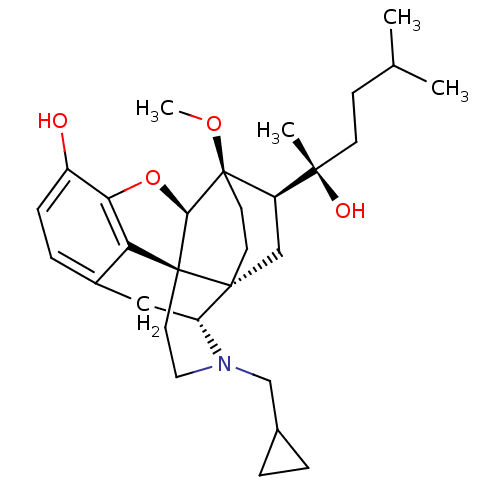 (CHEMBL2338731) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat recombinant MOR expressed in rat C6 cells assessed as stimulation of [35S]GTPgammaS binding after 1 hr | J Med Chem 56: 3207-16 (2013) Article DOI: 10.1021/jm301543e BindingDB Entry DOI: 10.7270/Q28G8N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 474 total ) | Next | Last >> |