

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50442585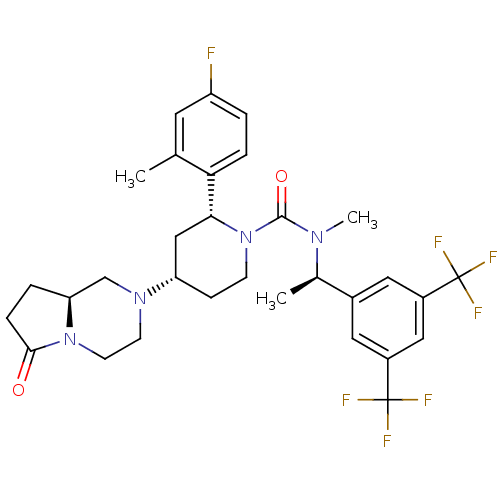 (GW823296X | ORVEPITANT) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]GR205171 from Mongolian gerbil brain NK1 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem 21: 6264-73 (2013) Article DOI: 10.1016/j.bmc.2013.09.001 BindingDB Entry DOI: 10.7270/Q2D79CWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50498575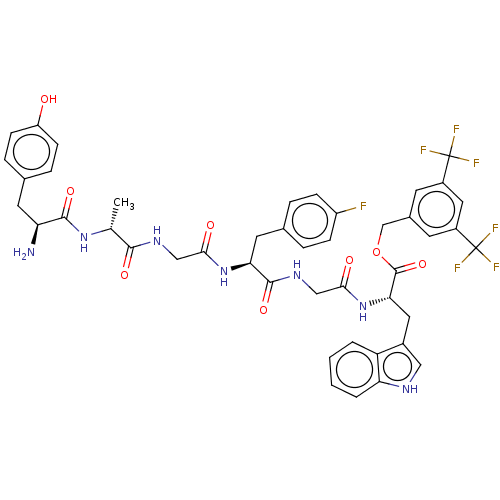 (CHEMBL3608939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]-substance P from rat NK1 receptor transfected in CHO cells | Bioorg Med Chem Lett 25: 3716-20 (2015) Article DOI: 10.1016/j.bmcl.2015.06.030 BindingDB Entry DOI: 10.7270/Q2R78J7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 303: 1171-9 (2002) Article DOI: 10.1124/jpet.102.040162 BindingDB Entry DOI: 10.7270/Q23J3BJH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 303: 1171-9 (2002) Article DOI: 10.1124/jpet.102.040162 BindingDB Entry DOI: 10.7270/Q23J3BJH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50439329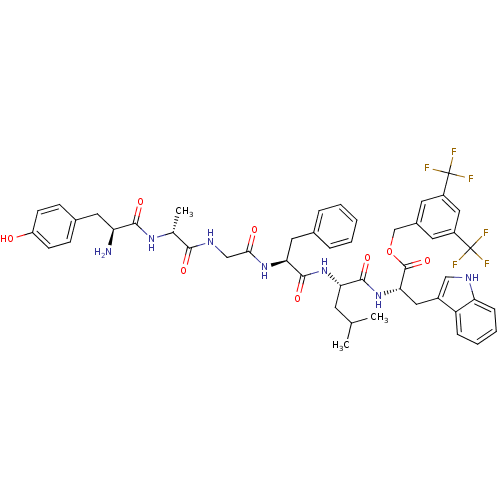 (CHEMBL2419542) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 4975-8 (2013) Article DOI: 10.1016/j.bmcl.2013.06.065 BindingDB Entry DOI: 10.7270/Q2222W64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50498572 (CHEMBL3609616) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]-substance P from rat NK1 receptor transfected in CHO cells | Bioorg Med Chem Lett 25: 3716-20 (2015) Article DOI: 10.1016/j.bmcl.2015.06.030 BindingDB Entry DOI: 10.7270/Q2R78J7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21022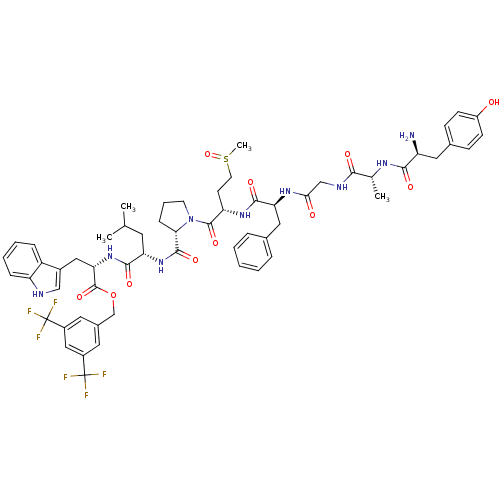 (Bifunctional Peptide Ligand, 6 (TY023) | H-Tyr-D-A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | -13.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 2779-86 (2007) Article DOI: 10.1021/jm061369n BindingDB Entry DOI: 10.7270/Q2SF2TG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50498573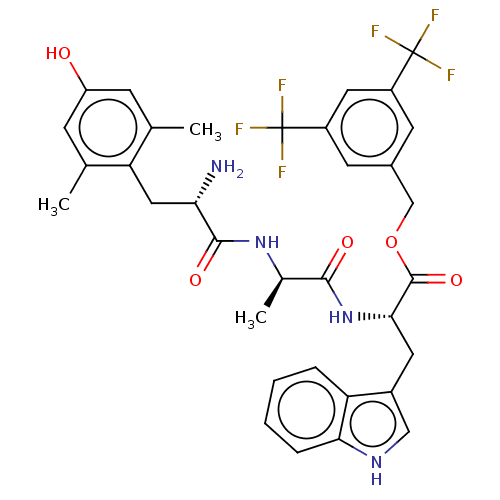 (CHEMBL3609620) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]-substance P from rat NK1 receptor transfected in CHO cells | Bioorg Med Chem Lett 25: 3716-20 (2015) Article DOI: 10.1016/j.bmcl.2015.06.030 BindingDB Entry DOI: 10.7270/Q2R78J7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50439330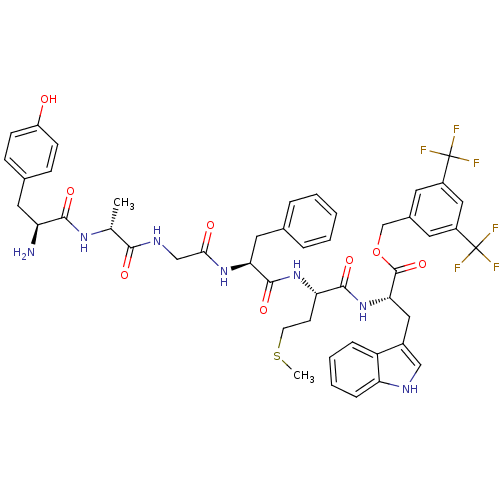 (CHEMBL2419541) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 4975-8 (2013) Article DOI: 10.1016/j.bmcl.2013.06.065 BindingDB Entry DOI: 10.7270/Q2222W64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21021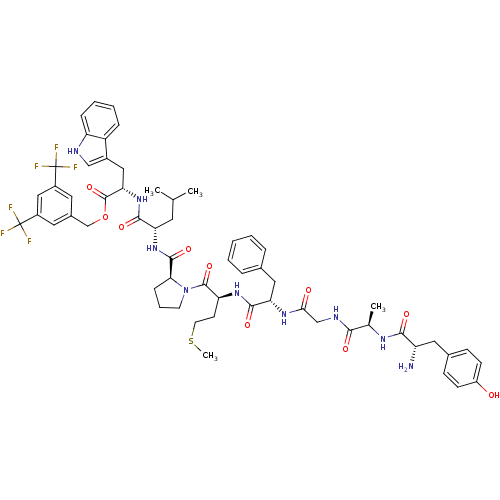 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membrane by liquid scintillation counting | J Med Chem 51: 6334-47 (2008) Article DOI: 10.1021/jm800389v BindingDB Entry DOI: 10.7270/Q26T0MF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | -13.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 2779-86 (2007) Article DOI: 10.1021/jm061369n BindingDB Entry DOI: 10.7270/Q2SF2TG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21021 (Bifunctional Peptide Ligand, 5 (TY005) | CHEMBL389...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 4975-8 (2013) Article DOI: 10.1016/j.bmcl.2013.06.065 BindingDB Entry DOI: 10.7270/Q2222W64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50436546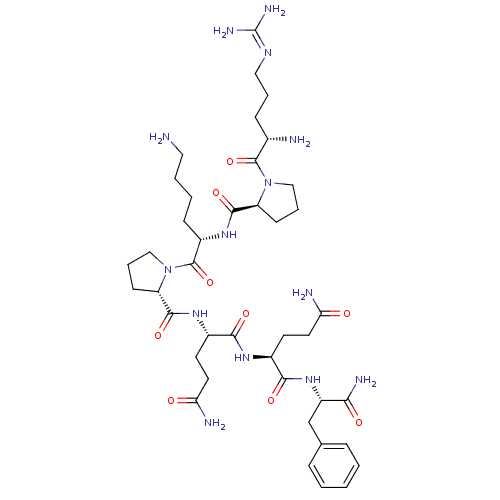 (CHEMBL2397481) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membranes after 60 mins by scintillation counting method | Bioorg Med Chem Lett 28: 2446-2450 (2018) Article DOI: 10.1016/j.bmcl.2018.06.009 BindingDB Entry DOI: 10.7270/Q2QF8WCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50436546 (CHEMBL2397481) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to substance P receptor (1 to 7 amino acids) binding site in rat spinal cord membranes | J Med Chem 56: 4953-65 (2013) Article DOI: 10.1021/jm400209h BindingDB Entry DOI: 10.7270/Q2K075PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50071484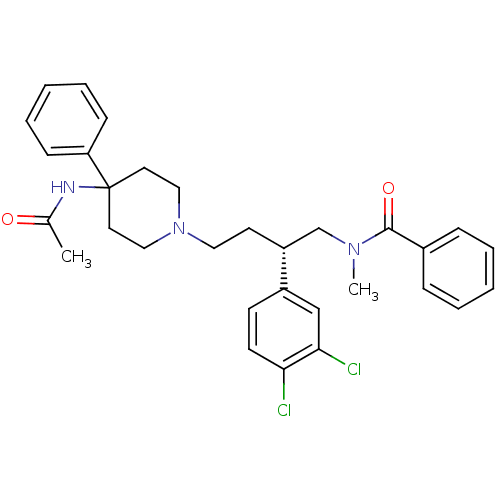 (CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested in vitro to inhibit the binding of [125I]-NKA to its receptor in rat duodenum membrane | Bioorg Med Chem Lett 3: 319-322 (1993) Article DOI: 10.1016/S0960-894X(01)80901-X BindingDB Entry DOI: 10.7270/Q2CR5T9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50498570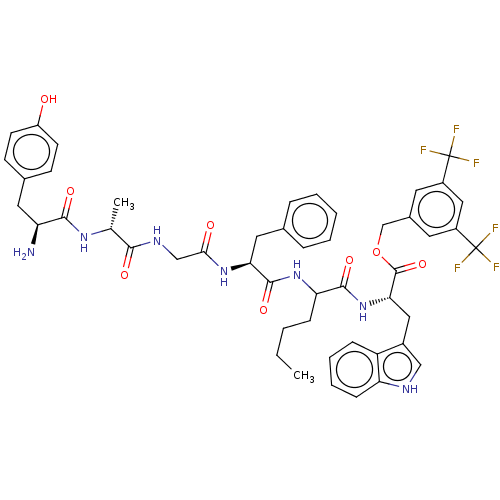 (CHEMBL3608938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]-substance P from rat NK1 receptor transfected in CHO cells | Bioorg Med Chem Lett 25: 3716-20 (2015) Article DOI: 10.1016/j.bmcl.2015.06.030 BindingDB Entry DOI: 10.7270/Q2R78J7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21023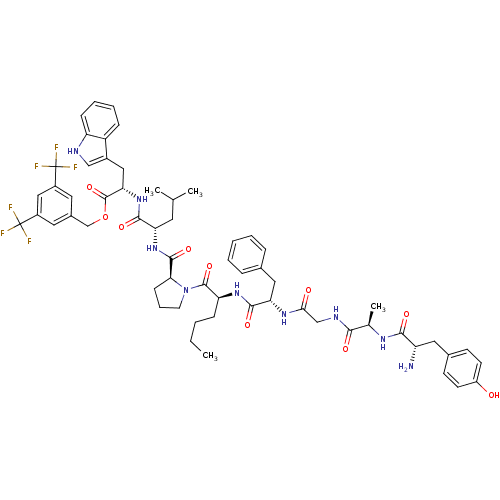 (Bifunctional Peptide Ligand, 7 (TY018) | H-Tyr-D-A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | -12.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 2779-86 (2007) Article DOI: 10.1021/jm061369n BindingDB Entry DOI: 10.7270/Q2SF2TG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21024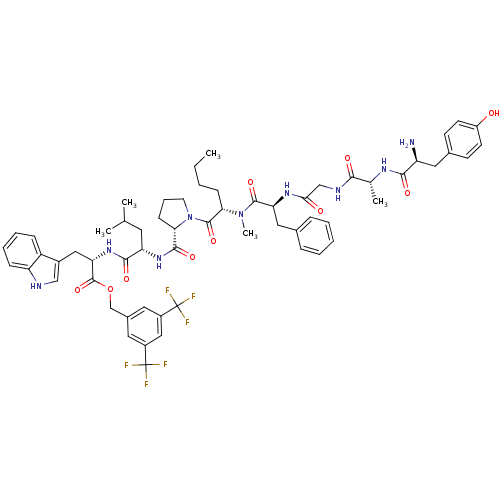 (Bifunctional Peptide Ligand, 8 (TY019) | H-Tyr-D-A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.710 | -12.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 2779-86 (2007) Article DOI: 10.1021/jm061369n BindingDB Entry DOI: 10.7270/Q2SF2TG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21020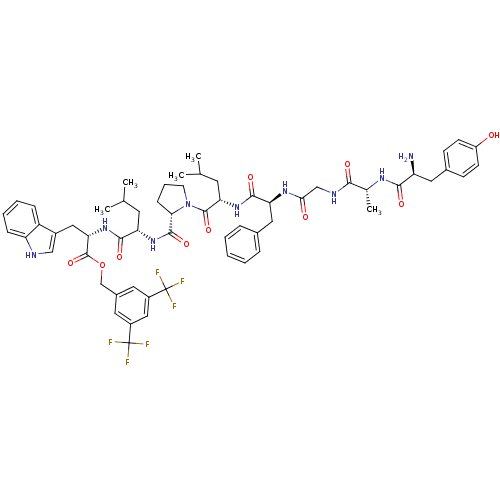 (Bifunctional Peptide Ligand, 4 (TY004) | H-Tyr-D-A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | -12.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 2779-86 (2007) Article DOI: 10.1021/jm061369n BindingDB Entry DOI: 10.7270/Q2SF2TG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21017 (Bifunctional Peptide Ligand, 1 (TY003) | H-Tyr-D-A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.880 | -12.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 2779-86 (2007) Article DOI: 10.1021/jm061369n BindingDB Entry DOI: 10.7270/Q2SF2TG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21019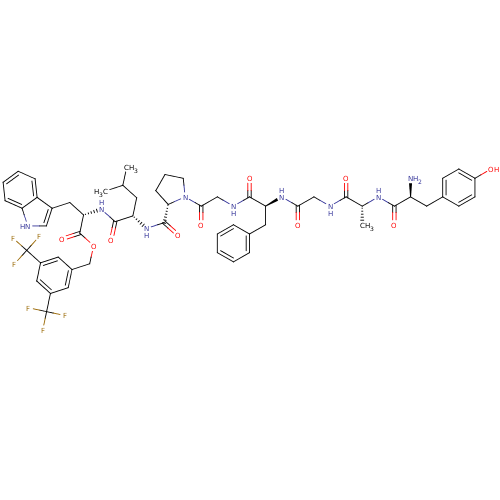 (Bifunctional Peptide Ligand, 3 (TY006) | H-Tyr-D-A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -12.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 2779-86 (2007) Article DOI: 10.1021/jm061369n BindingDB Entry DOI: 10.7270/Q2SF2TG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Curated by PDSP Ki Database | J Pharmacol Exp Ther 277: 840-51 (1996) BindingDB Entry DOI: 10.7270/Q2KD1WF0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM86055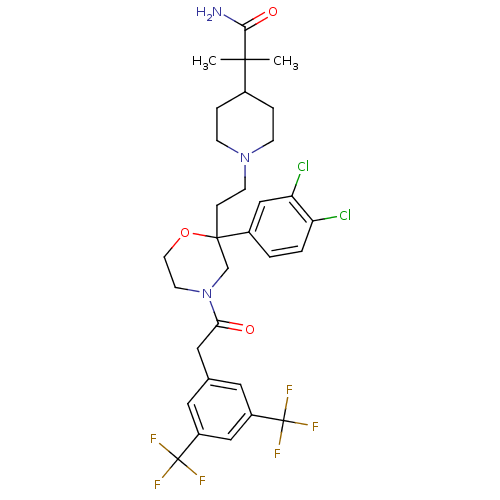 (SSR240600) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 303: 1171-9 (2002) Article DOI: 10.1124/jpet.102.040162 BindingDB Entry DOI: 10.7270/Q23J3BJH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit the binding of [125I]physalaemin to the SP receptors in rat telencephalon slices | J Med Chem 30: 1529-32 (1987) BindingDB Entry DOI: 10.7270/Q24M954B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50439326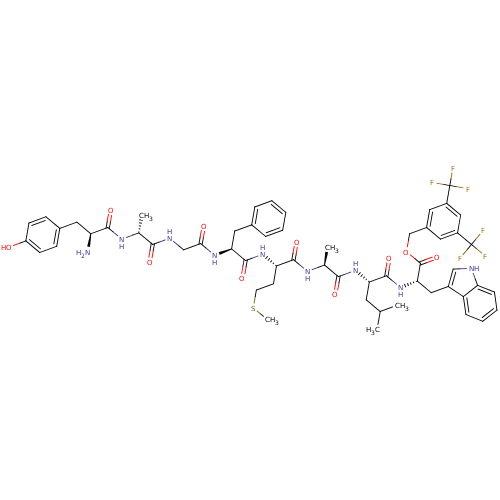 (CHEMBL2419537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 4975-8 (2013) Article DOI: 10.1016/j.bmcl.2013.06.065 BindingDB Entry DOI: 10.7270/Q2222W64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM86055 (SSR240600) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthélabo Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 303: 1171-9 (2002) Article DOI: 10.1124/jpet.102.040162 BindingDB Entry DOI: 10.7270/Q23J3BJH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50295070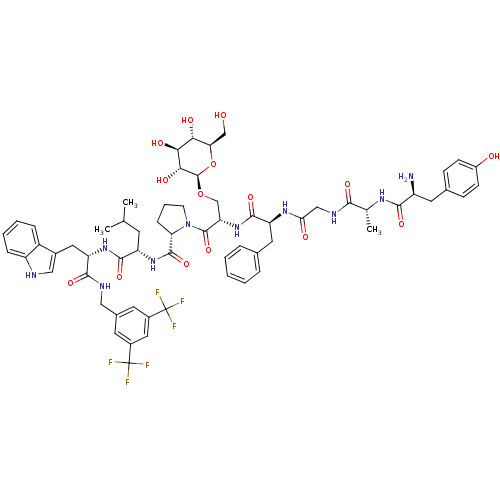 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membrane | J Med Chem 52: 5164-75 (2010) Article DOI: 10.1021/jm900473p BindingDB Entry DOI: 10.7270/Q2MC90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50188489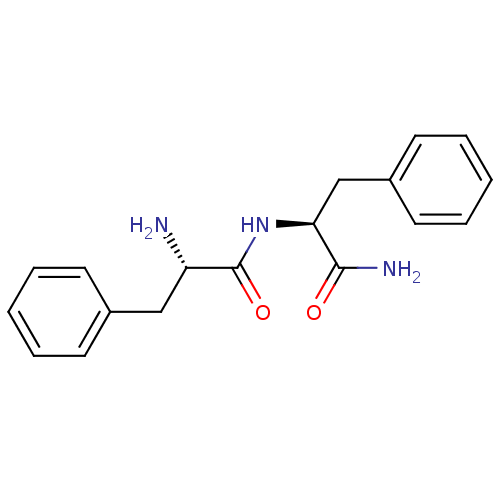 ((S)-2-Amino-N-((S)-1-carbamoyl-2-phenyl-ethyl)-3-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membranes after 60 mins by scintillation counting method | Bioorg Med Chem Lett 28: 2446-2450 (2018) Article DOI: 10.1016/j.bmcl.2018.06.009 BindingDB Entry DOI: 10.7270/Q2QF8WCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50188489 ((S)-2-Amino-N-((S)-1-carbamoyl-2-phenyl-ethyl)-3-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane | J Med Chem 53: 2383-9 (2010) Article DOI: 10.1021/jm901352b BindingDB Entry DOI: 10.7270/Q2542NQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50439328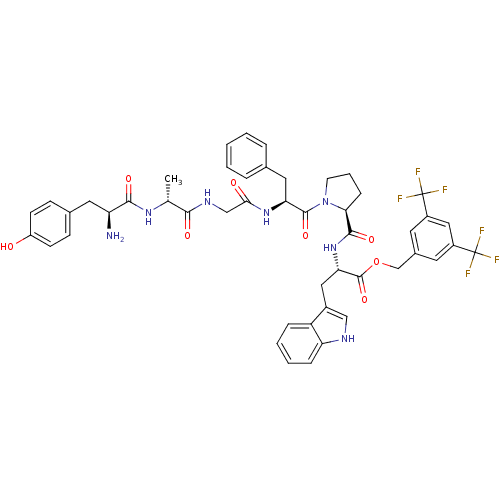 (CHEMBL2419543) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 4975-8 (2013) Article DOI: 10.1016/j.bmcl.2013.06.065 BindingDB Entry DOI: 10.7270/Q2222W64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50308381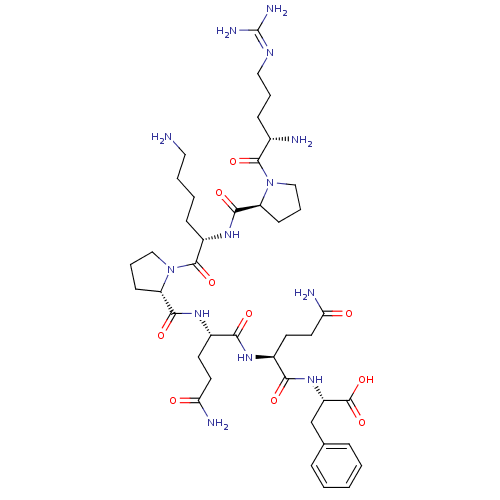 (CHEMBL589979 | H-Arg-Pro-Lys-Pro-Gln-Gln-Phe-OH) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membranes after 60 mins by scintillation counting method | Bioorg Med Chem Lett 28: 2446-2450 (2018) Article DOI: 10.1016/j.bmcl.2018.06.009 BindingDB Entry DOI: 10.7270/Q2QF8WCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50308381 (CHEMBL589979 | H-Arg-Pro-Lys-Pro-Gln-Gln-Phe-OH) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to substance P receptor (1 to 7 amino acids) binding site in rat spinal cord membranes | J Med Chem 56: 4953-65 (2013) Article DOI: 10.1021/jm400209h BindingDB Entry DOI: 10.7270/Q2K075PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50308381 (CHEMBL589979 | H-Arg-Pro-Lys-Pro-Gln-Gln-Phe-OH) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membrane | J Med Chem 53: 2383-9 (2010) Article DOI: 10.1021/jm901352b BindingDB Entry DOI: 10.7270/Q2542NQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21007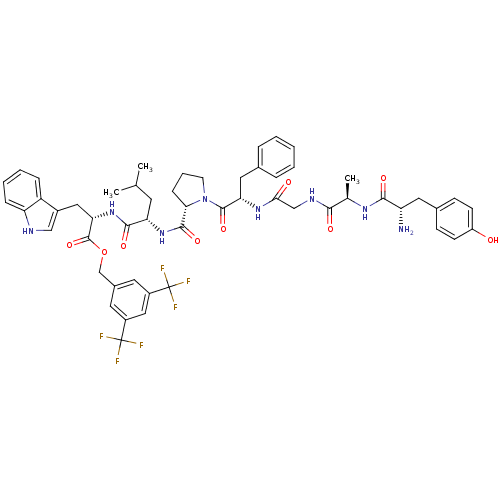 (C-terminal modified bifunctional peptide, 1 | H-Ty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | -12.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50498576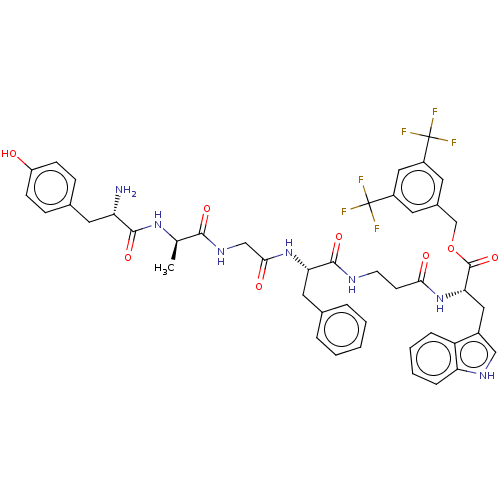 (CHEMBL3608937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]-substance P from rat NK1 receptor transfected in CHO cells | Bioorg Med Chem Lett 25: 3716-20 (2015) Article DOI: 10.1016/j.bmcl.2015.06.030 BindingDB Entry DOI: 10.7270/Q2R78J7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50276158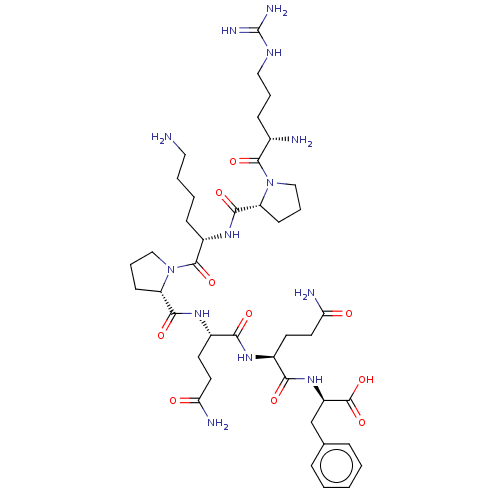 (CHEMBL4127872) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membranes after 60 mins by scintillation counting method | Bioorg Med Chem Lett 28: 2446-2450 (2018) Article DOI: 10.1016/j.bmcl.2018.06.009 BindingDB Entry DOI: 10.7270/Q2QF8WCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50436553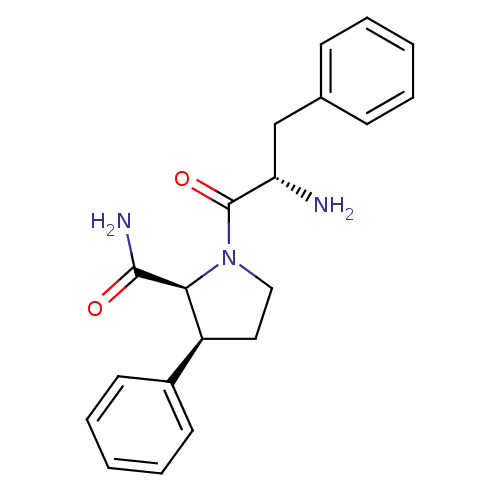 (CHEMBL2396664) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]SP1-7 from substance P receptor (1 to 7 amino acids) binding site in Sprague-Dawley rat spinal cord membranes after 60 mins by li... | J Med Chem 56: 4953-65 (2013) Article DOI: 10.1021/jm400209h BindingDB Entry DOI: 10.7270/Q2K075PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50436553 (CHEMBL2396664) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membranes after 60 mins by scintillation counting method | Bioorg Med Chem Lett 28: 2446-2450 (2018) Article DOI: 10.1016/j.bmcl.2018.06.009 BindingDB Entry DOI: 10.7270/Q2QF8WCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50341318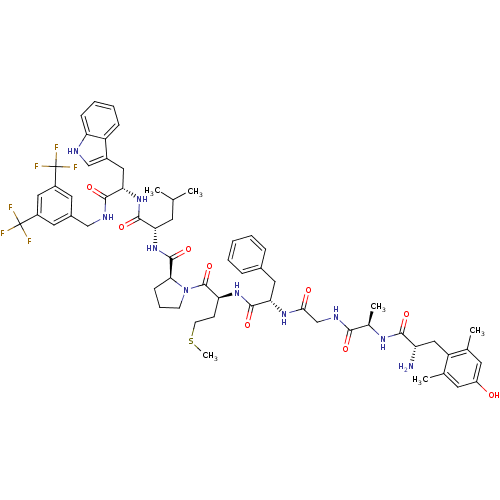 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells | J Med Chem 54: 2029-38 (2011) Article DOI: 10.1021/jm101023r BindingDB Entry DOI: 10.7270/Q2862GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50341318 ((S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-15-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells | J Med Chem 54: 2029-38 (2011) Article DOI: 10.1021/jm101023r BindingDB Entry DOI: 10.7270/Q2862GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50498577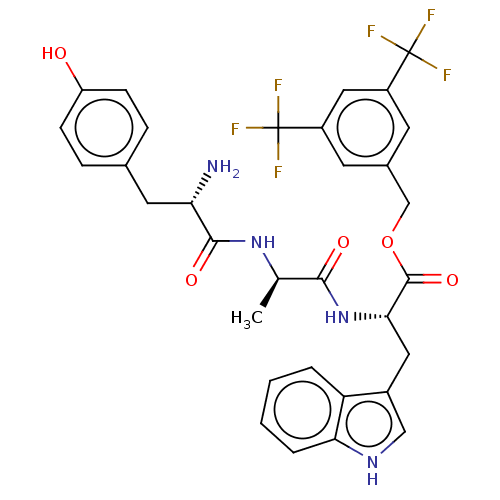 (CHEMBL3609619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]-substance P from rat NK1 receptor transfected in CHO cells | Bioorg Med Chem Lett 25: 3716-20 (2015) Article DOI: 10.1016/j.bmcl.2015.06.030 BindingDB Entry DOI: 10.7270/Q2R78J7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21018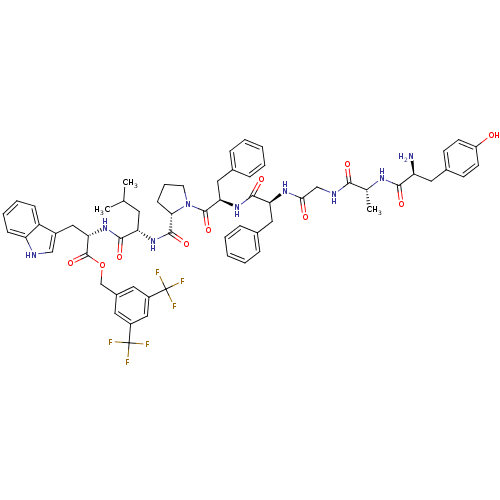 (Bifunctional Peptide Ligand, 2 (TY007) | H-Tyr-D-A...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -11.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 50: 2779-86 (2007) Article DOI: 10.1021/jm061369n BindingDB Entry DOI: 10.7270/Q2SF2TG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50436547 (CHEMBL2397494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]SP1-7 from substance P receptor (1 to 7 amino acids) binding site in Sprague-Dawley rat spinal cord membranes after 60 mins by li... | J Med Chem 56: 4953-65 (2013) Article DOI: 10.1021/jm400209h BindingDB Entry DOI: 10.7270/Q2K075PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50439331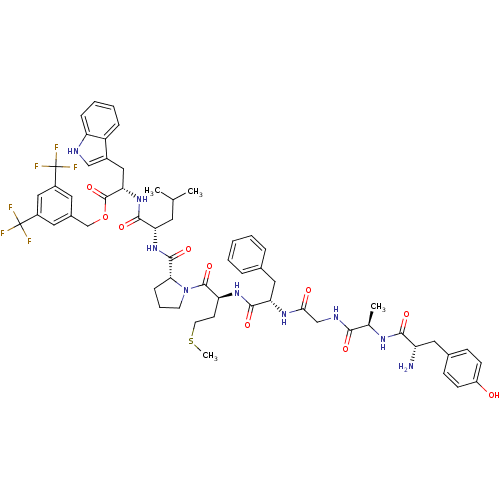 (CHEMBL2419540) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 4975-8 (2013) Article DOI: 10.1016/j.bmcl.2013.06.065 BindingDB Entry DOI: 10.7270/Q2222W64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50439327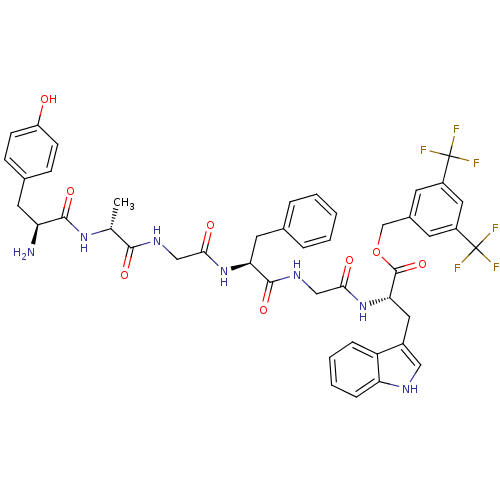 (CHEMBL2419544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 23: 4975-8 (2013) Article DOI: 10.1016/j.bmcl.2013.06.065 BindingDB Entry DOI: 10.7270/Q2222W64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50029885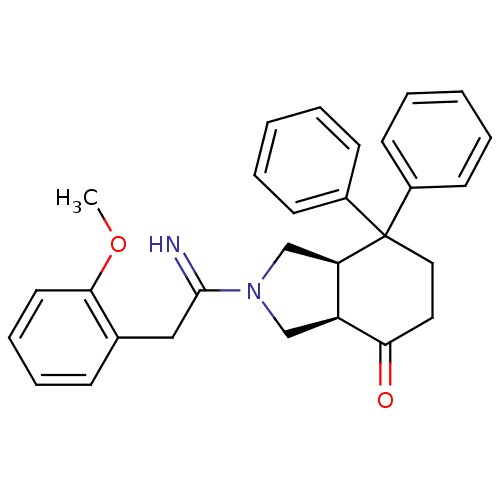 ((3aR,7aR)-2-[1-Imino-2-(2-methoxy-phenyl)-ethyl]-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for binding affinity towards Tachykinin receptor 1 by using [3H]-SP binding assay in rat brain membranes | Bioorg Med Chem Lett 2: 37-40 (1992) Article DOI: 10.1016/S0960-894X(00)80650-2 BindingDB Entry DOI: 10.7270/Q2PR7VW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50498574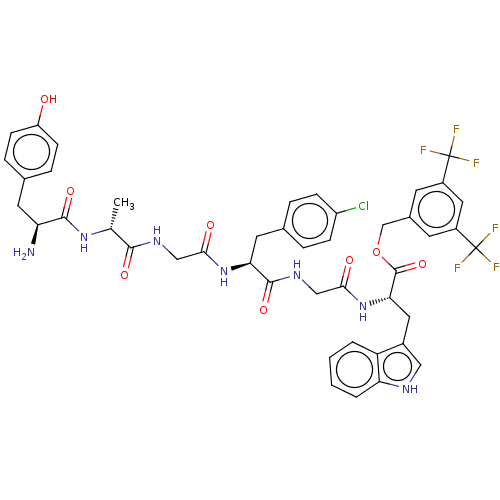 (CHEMBL3609615) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]-substance P from rat NK1 receptor transfected in CHO cells | Bioorg Med Chem Lett 25: 3716-20 (2015) Article DOI: 10.1016/j.bmcl.2015.06.030 BindingDB Entry DOI: 10.7270/Q2R78J7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50041558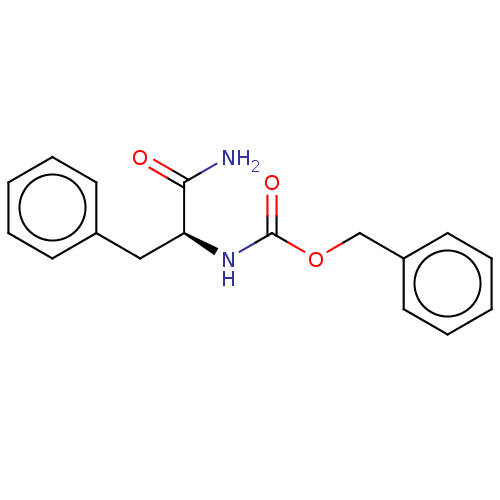 (CHEMBL2042018) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]SP1-7 from NK1 receptor in Sprague-Dawley rat spinal cord membranes after 60 mins by scintillation counting method | Bioorg Med Chem Lett 28: 2446-2450 (2018) Article DOI: 10.1016/j.bmcl.2018.06.009 BindingDB Entry DOI: 10.7270/Q2QF8WCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM21013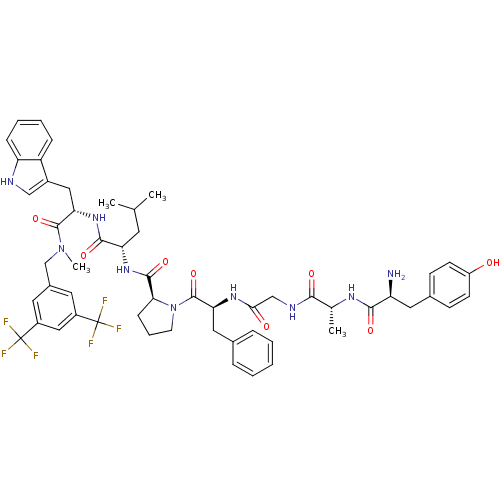 ((2S)-2-{[(2S)-1-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.10 | -11.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 51: 1369-76 (2008) Article DOI: 10.1021/jm070332f BindingDB Entry DOI: 10.7270/Q2X63K7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50436549 (CHEMBL2397492) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]SP1-7 from substance P receptor (1 to 7 amino acids) binding site in Sprague-Dawley rat spinal cord membranes after 60 mins by li... | J Med Chem 56: 4953-65 (2013) Article DOI: 10.1021/jm400209h BindingDB Entry DOI: 10.7270/Q2K075PK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 159 total ) | Next | Last >> |