 Found 604 hits of ki data for polymerid = 2112,9137
Found 604 hits of ki data for polymerid = 2112,9137 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127490
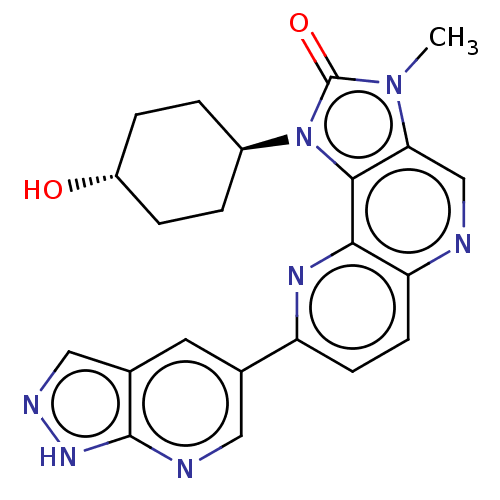
(US8791131, 268)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1cnc2[nH]ncc2c1 |r,wU:13.14,wD:16.18,(7.27,-2.02,;5.78,-1.62,;4.64,-2.65,;4.64,-4.19,;3.31,-4.96,;1.97,-4.19,;.64,-4.96,;-.69,-4.19,;-.69,-2.65,;.64,-1.88,;1.97,-2.65,;3.31,-1.88,;3.63,-.37,;2.86,.96,;1.32,.96,;.55,2.29,;1.32,3.63,;.55,4.96,;2.86,3.63,;3.63,2.29,;5.16,-.21,;5.93,1.12,;-2.23,-2.65,;-2.23,-4.19,;-3.57,-4.96,;-4.9,-4.19,;-6.37,-4.67,;-7.27,-3.42,;-6.37,-2.17,;-4.9,-2.65,;-3.57,-1.88,)| Show InChI InChI=1S/C22H21N7O2/c1-28-18-11-23-17-7-6-16(12-8-13-10-25-27-21(13)24-9-12)26-19(17)20(18)29(22(28)31)14-2-4-15(30)5-3-14/h6-11,14-15,30H,2-5H2,1H3,(H,24,25,27)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.117 | -13.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127403
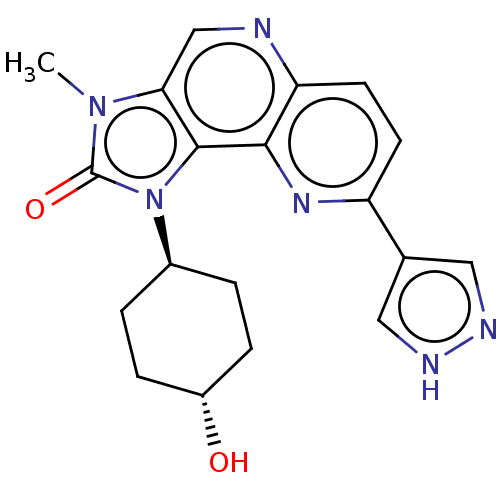
(US8791131, 168)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1cn[nH]c1 |r,wU:13.14,wD:16.18,(5.84,-1.53,;4.35,-1.13,;3.2,-2.16,;3.2,-3.7,;1.87,-4.47,;.54,-3.7,;-.8,-4.47,;-2.13,-3.7,;-2.13,-2.16,;-.8,-1.39,;.54,-2.16,;1.87,-1.39,;2.19,.12,;1.1,1.2,;-.39,.81,;-1.48,1.89,;-1.08,3.38,;-2.17,4.47,;.41,3.78,;1.5,2.69,;3.72,.28,;4.49,1.61,;-3.47,-1.39,;-4.93,-1.87,;-5.84,-.62,;-4.93,.62,;-3.47,.15,)| Show InChI InChI=1S/C19H20N6O2/c1-24-16-10-20-15-7-6-14(11-8-21-22-9-11)23-17(15)18(16)25(19(24)27)12-2-4-13(26)5-3-12/h6-10,12-13,26H,2-5H2,1H3,(H,21,22)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.204 | -13.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127481
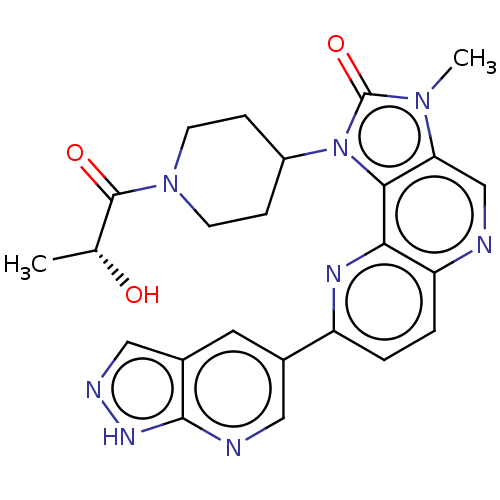
(US8791131, 258)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O |r| Show InChI InChI=1S/C24H24N8O3/c1-13(33)23(34)31-7-5-16(6-8-31)32-21-19(30(2)24(32)35)12-25-18-4-3-17(28-20(18)21)14-9-15-11-27-29-22(15)26-10-14/h3-4,9-13,16,33H,5-8H2,1-2H3,(H,26,27,29)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.241 | -13.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428109
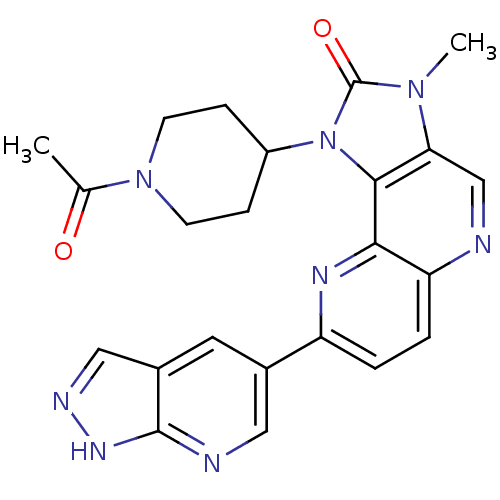
(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428109

(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.243 | -13.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127468
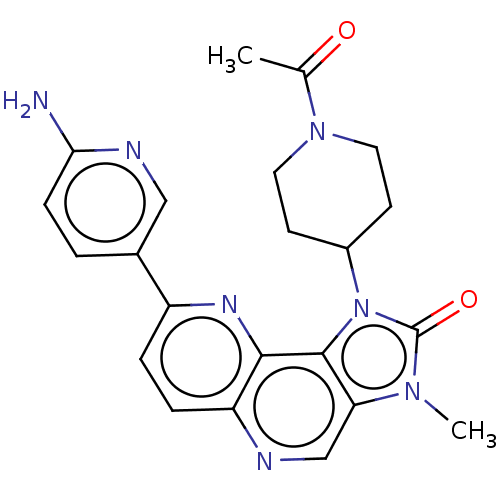
(US8791131, 242)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(N)nc2)n(C)c1=O Show InChI InChI=1S/C22H23N7O2/c1-13(30)28-9-7-15(8-10-28)29-21-18(27(2)22(29)31)12-24-17-5-4-16(26-20(17)21)14-3-6-19(23)25-11-14/h3-6,11-12,15H,7-10H2,1-2H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.299 | -13.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606735
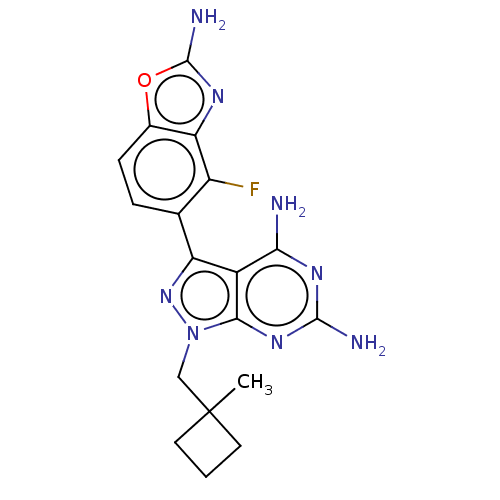
(CHEMBL5219718 | US11731973, Example 3)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3F)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606735

(CHEMBL5219718 | US11731973, Example 3)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3F)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127482
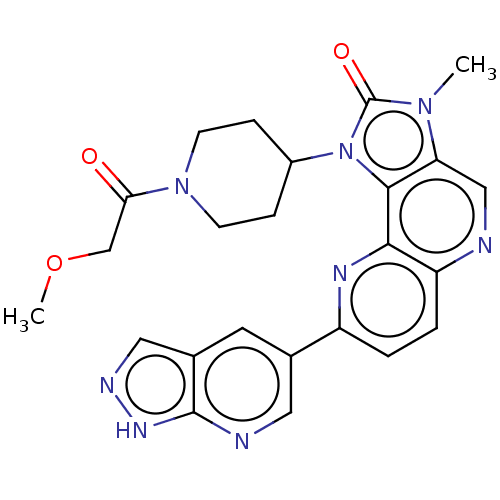
(US8791131, 260)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C24H24N8O3/c1-30-19-12-25-18-4-3-17(14-9-15-11-27-29-23(15)26-10-14)28-21(18)22(19)32(24(30)34)16-5-7-31(8-6-16)20(33)13-35-2/h3-4,9-12,16H,5-8,13H2,1-2H3,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.323 | -12.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127455
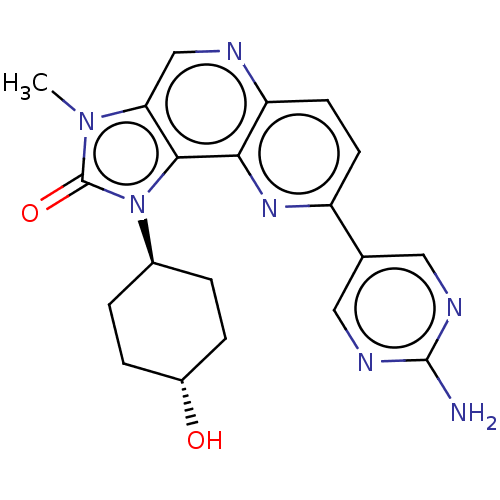
(US8791131, 229)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1cnc(N)nc1 |r,wU:13.14,wD:16.18,(6.65,-1.53,;5.16,-1.13,;4.02,-2.16,;4.02,-3.7,;2.69,-4.47,;1.35,-3.7,;.02,-4.47,;-1.32,-3.7,;-1.32,-2.16,;.02,-1.39,;1.35,-2.16,;2.69,-1.39,;3.01,.12,;1.92,1.2,;.43,.81,;-.66,1.89,;-.26,3.38,;-1.35,4.47,;1.23,3.78,;2.31,2.69,;4.54,.28,;5.31,1.61,;-2.65,-1.39,;-2.65,.15,;-3.98,.92,;-5.32,.15,;-6.65,.92,;-5.32,-1.39,;-3.98,-2.16,)| Show InChI InChI=1S/C20H21N7O2/c1-26-16-10-22-15-7-6-14(11-8-23-19(21)24-9-11)25-17(15)18(16)27(20(26)29)12-2-4-13(28)5-3-12/h6-10,12-13,28H,2-5H2,1H3,(H2,21,23,24)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.330 | -12.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50343775
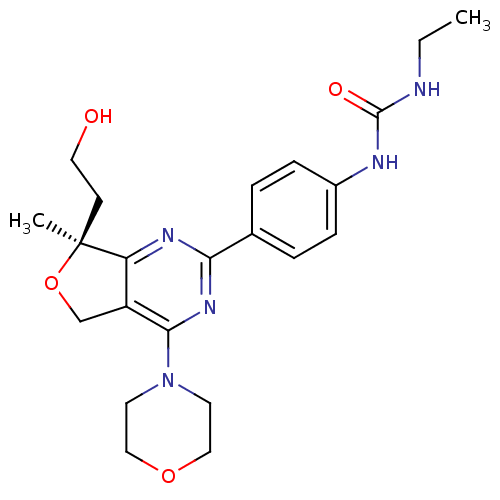
((S)-1-ethyl-3-(4-(7-(2-hydroxyethyl)-7-methyl-4-mo...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2c(CO[C@@]2(C)CCO)c(n1)N1CCOCC1 |r| Show InChI InChI=1S/C22H29N5O4/c1-3-23-21(29)24-16-6-4-15(5-7-16)19-25-18-17(14-31-22(18,2)8-11-28)20(26-19)27-9-12-30-13-10-27/h4-7,28H,3,8-14H2,1-2H3,(H2,23,24,29)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay |
J Med Chem 54: 3426-35 (2011)
Article DOI: 10.1021/jm200215y
BindingDB Entry DOI: 10.7270/Q29C6XRH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127489
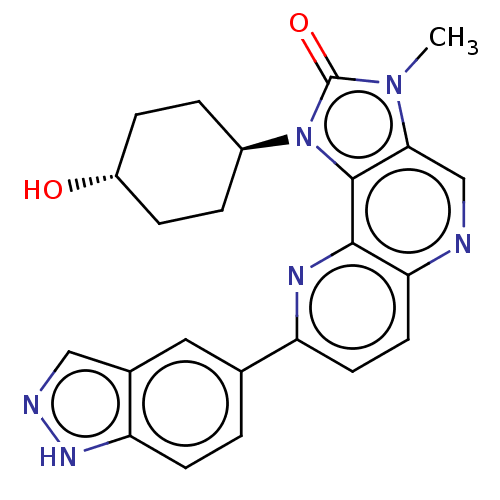
(US8791131, 267)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1ccc2[nH]ncc2c1 |r,wU:13.14,wD:16.18,(7.27,-2.02,;5.78,-1.62,;4.64,-2.65,;4.64,-4.19,;3.31,-4.96,;1.97,-4.19,;.64,-4.96,;-.69,-4.19,;-.69,-2.65,;.64,-1.88,;1.97,-2.65,;3.31,-1.88,;3.63,-.37,;2.86,.96,;1.32,.96,;.55,2.29,;1.32,3.63,;.55,4.96,;2.86,3.63,;3.63,2.29,;5.16,-.21,;5.93,1.12,;-2.23,-2.65,;-2.23,-4.19,;-3.57,-4.96,;-4.9,-4.19,;-6.37,-4.67,;-7.27,-3.42,;-6.37,-2.17,;-4.9,-2.65,;-3.57,-1.88,)| Show InChI InChI=1S/C23H22N6O2/c1-28-20-12-24-19-9-8-17(13-2-7-18-14(10-13)11-25-27-18)26-21(19)22(20)29(23(28)31)15-3-5-16(30)6-4-15/h2,7-12,15-16,30H,3-6H2,1H3,(H,25,27)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.359 | -12.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428111
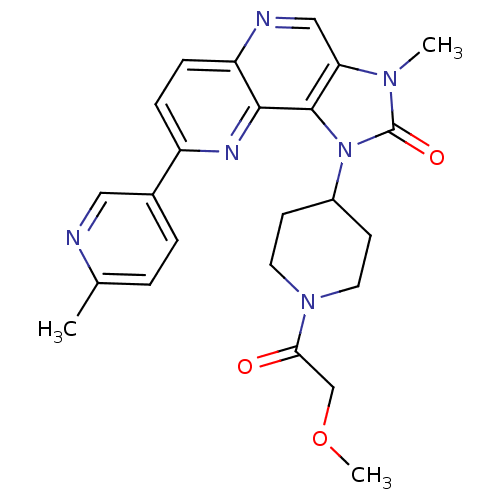
(CHEMBL2331666 | US8791131, 153)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C24H26N6O3/c1-15-4-5-16(12-25-15)18-6-7-19-22(27-18)23-20(13-26-19)28(2)24(32)30(23)17-8-10-29(11-9-17)21(31)14-33-3/h4-7,12-13,17H,8-11,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.377 | -12.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428111

(CHEMBL2331666 | US8791131, 153)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C24H26N6O3/c1-15-4-5-16(12-25-15)18-6-7-19-22(27-18)23-20(13-26-19)28(2)24(32)30(23)17-8-10-29(11-9-17)21(31)14-33-3/h4-7,12-13,17H,8-11,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606737
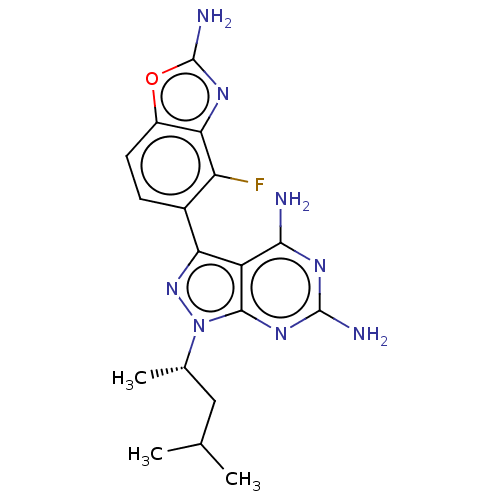
(CHEMBL5218727 | US11731973, Example 5)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428131
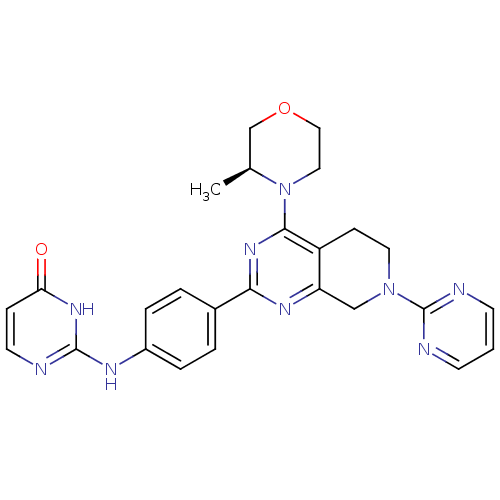
(CHEMBL2331687)Show SMILES C[C@H]1COCCN1c1nc(nc2CN(CCc12)c1ncccn1)-c1ccc(Nc2nccc(=O)[nH]2)cc1 |r| Show InChI InChI=1S/C26H27N9O2/c1-17-16-37-14-13-35(17)24-20-8-12-34(26-28-9-2-10-29-26)15-21(20)31-23(33-24)18-3-5-19(6-4-18)30-25-27-11-7-22(36)32-25/h2-7,9-11,17H,8,12-16H2,1H3,(H2,27,30,32,36)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assay |
ACS Med Chem Lett 4: 103-7 (2013)
Article DOI: 10.1021/ml3003132
BindingDB Entry DOI: 10.7270/Q28C9XK3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606737

(CHEMBL5218727 | US11731973, Example 5)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127462
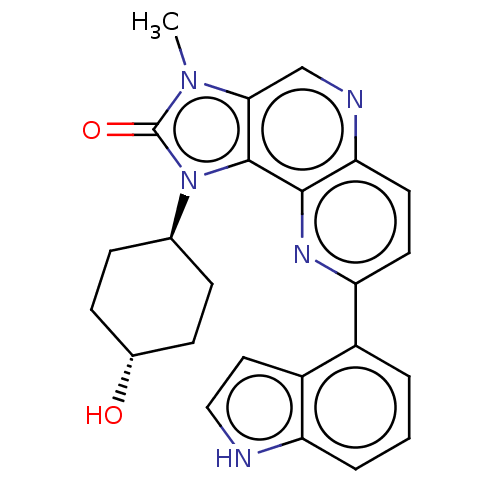
(US8791131, 236)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1cccc2[nH]ccc12 |r,wU:13.14,wD:16.18,(6.56,-1.53,;5.07,-1.13,;3.92,-2.16,;3.92,-3.7,;2.59,-4.47,;1.26,-3.7,;-.08,-4.47,;-1.41,-3.7,;-1.41,-2.16,;-.08,-1.39,;1.26,-2.16,;2.59,-1.39,;2.91,.12,;1.82,1.2,;.33,.81,;-.75,1.89,;-.36,3.38,;-1.45,4.47,;1.13,3.78,;2.22,2.69,;4.44,.28,;5.21,1.61,;-2.74,-1.39,;-2.74,.15,;-4.08,.92,;-5.41,.15,;-5.41,-1.39,;-6.56,-2.42,;-5.93,-3.83,;-4.4,-3.67,;-4.08,-2.16,)| Show InChI InChI=1S/C24H23N5O2/c1-28-21-13-26-20-10-9-19(16-3-2-4-18-17(16)11-12-25-18)27-22(20)23(21)29(24(28)31)14-5-7-15(30)8-6-14/h2-4,9-15,25,30H,5-8H2,1H3/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.404 | -12.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127423
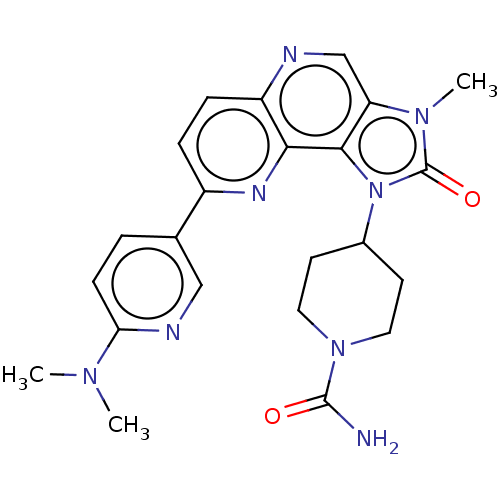
(US8791131, 197)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(N)=O)c3c2n1 Show InChI InChI=1S/C23H26N8O2/c1-28(2)19-7-4-14(12-26-19)16-5-6-17-20(27-16)21-18(13-25-17)29(3)23(33)31(21)15-8-10-30(11-9-15)22(24)32/h4-7,12-13,15H,8-11H2,1-3H3,(H2,24,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.444 | -12.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606738
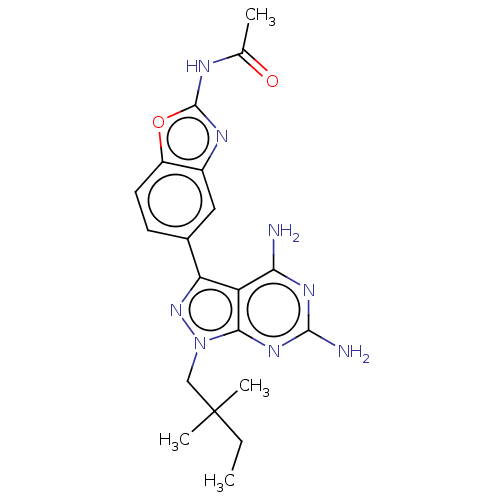
(CHEMBL5218590 | US11731973, Example 6)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(NC(C)=O)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606738

(CHEMBL5218590 | US11731973, Example 6)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(NC(C)=O)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606740
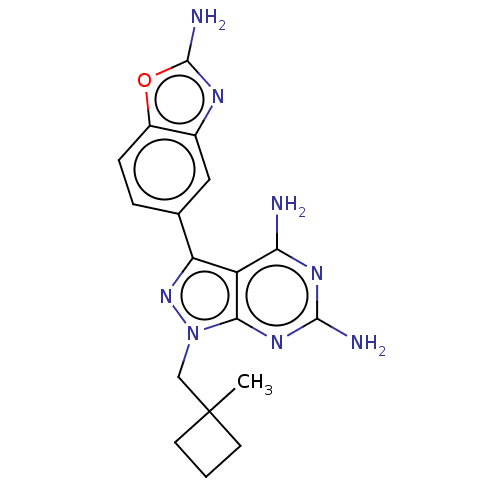
(CHEMBL5220536 | US11731973, Example 30)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606740

(CHEMBL5220536 | US11731973, Example 30)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606742
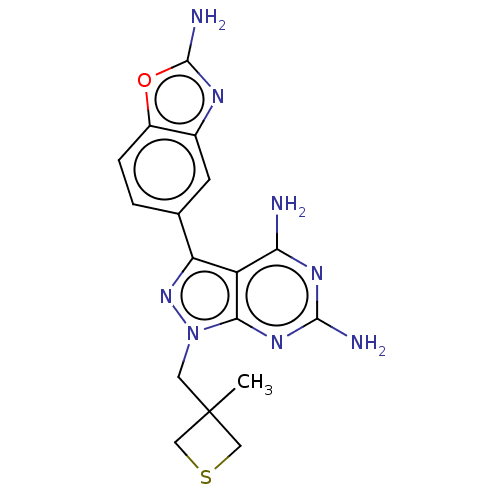
(CHEMBL5220948 | US11731973, Example 9)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CSC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM613740
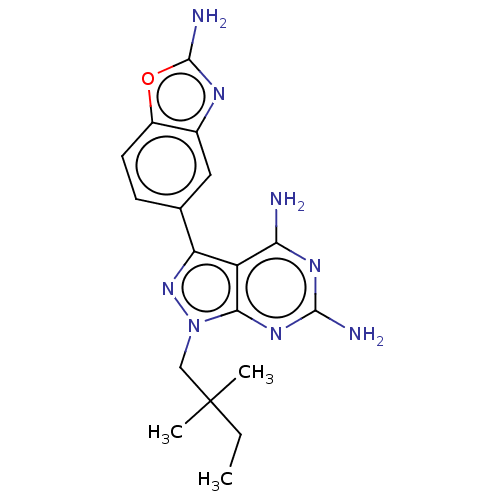
(3-(2-aminobenzoxazol-5-yl)-1-(2,2- dimethylbutyl)-...)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606742

(CHEMBL5220948 | US11731973, Example 9)Show SMILES CC1(Cn2nc(-c3ccc4oc(N)nc4c3)c3c(N)nc(N)nc23)CSC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
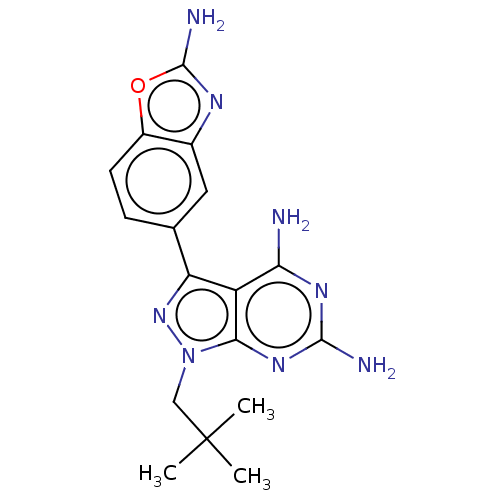
(Homo sapiens (Human)) | BDBM50606739
(CHEMBL5220152 | US11731973, Example 7)Show SMILES CC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606739

(CHEMBL5220152 | US11731973, Example 7)Show SMILES CC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127483
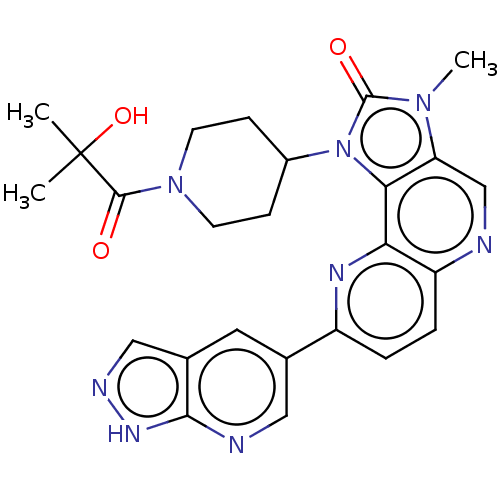
(US8791131, 261)Show SMILES Cn1c2cnc3ccc(nc3c2n(C2CCN(CC2)C(=O)C(C)(C)O)c1=O)-c1cnc2[nH]ncc2c1 Show InChI InChI=1S/C25H26N8O3/c1-25(2,36)23(34)32-8-6-16(7-9-32)33-21-19(31(3)24(33)35)13-26-18-5-4-17(29-20(18)21)14-10-15-12-28-30-22(15)27-11-14/h4-5,10-13,16,36H,6-9H2,1-3H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.658 | -12.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50343776
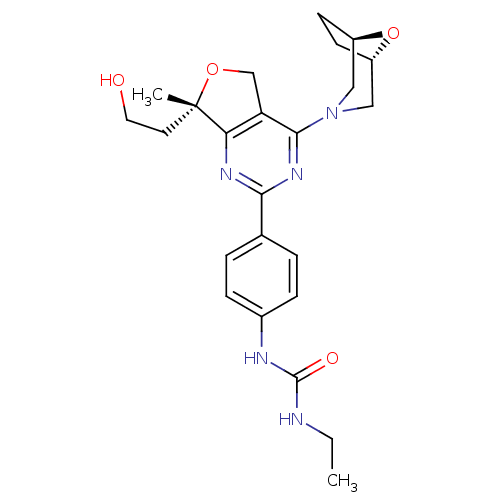
(1-(4-((S)-4-((1R,5S)-8-oxa-3-azabicyclo[3.2.1]octa...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2c(CO[C@@]2(C)CCO)c(n1)N1C[C@@H]2CC[C@H](C1)O2 |r| Show InChI InChI=1S/C24H31N5O4/c1-3-25-23(31)26-16-6-4-15(5-7-16)21-27-20-19(14-32-24(20,2)10-11-30)22(28-21)29-12-17-8-9-18(13-29)33-17/h4-7,17-18,30H,3,8-14H2,1-2H3,(H2,25,26,31)/t17-,18+,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay |
J Med Chem 54: 3426-35 (2011)
Article DOI: 10.1021/jm200215y
BindingDB Entry DOI: 10.7270/Q29C6XRH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50343774
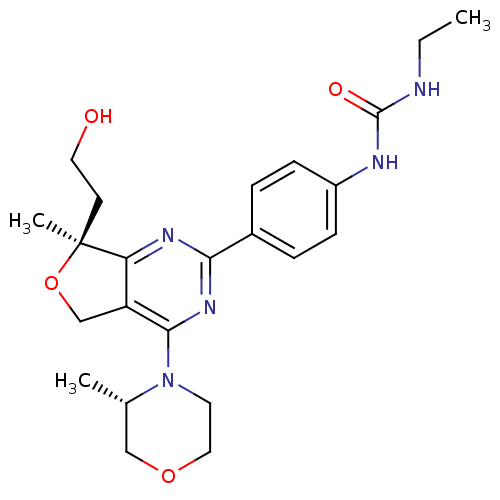
(1-Ethyl-3-(4-((S)-7-(2-hydroxyethyl)-7-methyl-4-((...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2c(CO[C@@]2(C)CCO)c(n1)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C23H31N5O4/c1-4-24-22(30)25-17-7-5-16(6-8-17)20-26-19-18(14-32-23(19,3)9-11-29)21(27-20)28-10-12-31-13-15(28)2/h5-8,15,29H,4,9-14H2,1-3H3,(H2,24,25,30)/t15-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay |
J Med Chem 54: 3426-35 (2011)
Article DOI: 10.1021/jm200215y
BindingDB Entry DOI: 10.7270/Q29C6XRH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606736
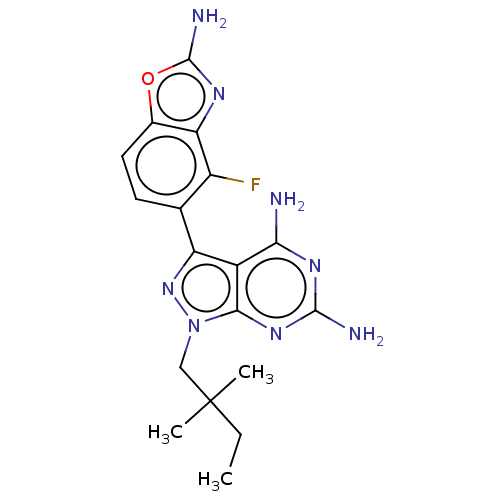
(CHEMBL5218916 | US11731973, Example 10)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM613743

(1-(2,2-dimethylpropyl)-3-(2- ethylaminobenzoxazol-...)Show SMILES CCNc1nc2cc(ccc2o1)-c1nn(CC(C)(C)C)c2nc(N)nc(N)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606736

(CHEMBL5218916 | US11731973, Example 10)Show SMILES CCC(C)(C)Cn1nc(-c2ccc3oc(N)nc3c2F)c2c(N)nc(N)nc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606741
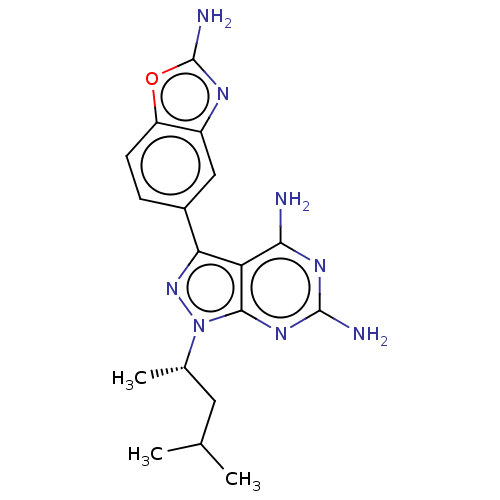
(CHEMBL5219710 | US11731973, Example 1)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50343769
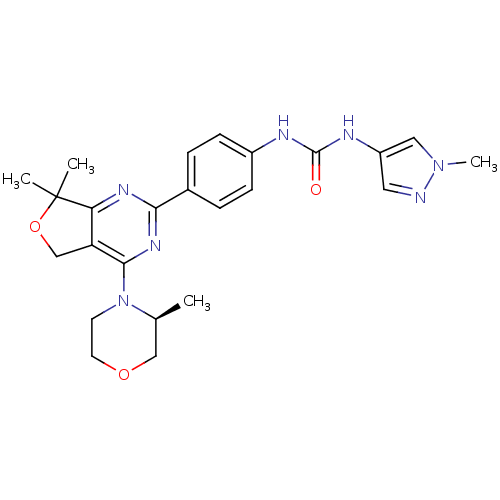
((S)-1-(4-(7,7-Dimethyl-4-(3-methylmorpholino)-5,7-...)Show SMILES C[C@H]1COCCN1c1nc(nc2c1COC2(C)C)-c1ccc(NC(=O)Nc2cnn(C)c2)cc1 |r| Show InChI InChI=1S/C24H29N7O3/c1-15-13-33-10-9-31(15)22-19-14-34-24(2,3)20(19)28-21(29-22)16-5-7-17(8-6-16)26-23(32)27-18-11-25-30(4)12-18/h5-8,11-12,15H,9-10,13-14H2,1-4H3,(H2,26,27,32)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay |
J Med Chem 54: 3426-35 (2011)
Article DOI: 10.1021/jm200215y
BindingDB Entry DOI: 10.7270/Q29C6XRH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327904

(2-amino-4-methyl-6-(1H-pyrazol-3-yl)-8-(tetrahydro...)Show SMILES Cc1nc(N)nc2n(C3CCOCC3)c(=O)c(nc12)-c1cc[nH]n1 Show InChI InChI=1S/C15H17N7O2/c1-8-11-13(20-15(16)18-8)22(9-3-6-24-7-4-9)14(23)12(19-11)10-2-5-17-21-10/h2,5,9H,3-4,6-7H2,1H3,(H,17,21)(H2,16,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50606741

(CHEMBL5219710 | US11731973, Example 1)Show SMILES CC(C)C[C@H](C)n1nc(-c2ccc3oc(N)nc3c2)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127391
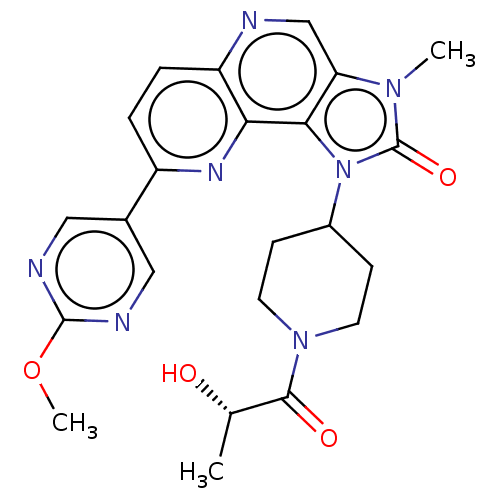
(US8791131, 154)Show SMILES COc1ncc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)[C@H](C)O)c3c2n1 |r| Show InChI InChI=1S/C23H25N7O4/c1-13(31)21(32)29-8-6-15(7-9-29)30-20-18(28(2)23(30)33)12-24-17-5-4-16(27-19(17)20)14-10-25-22(34-3)26-11-14/h4-5,10-13,15,31H,6-9H2,1-3H3/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.822 | -12.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428113
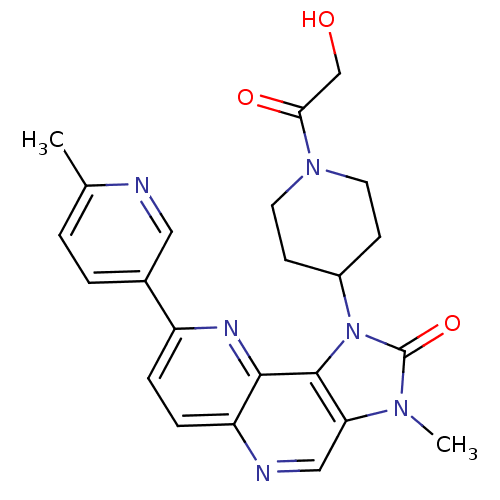
(CHEMBL2331663 | US8791131, 172)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14-3-4-15(11-24-14)17-5-6-18-21(26-17)22-19(12-25-18)27(2)23(32)29(22)16-7-9-28(10-8-16)20(31)13-30/h3-6,11-12,16,30H,7-10,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.842 | -12.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428113

(CHEMBL2331663 | US8791131, 172)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14-3-4-15(11-24-14)17-5-6-18-21(26-17)22-19(12-25-18)27(2)23(32)29(22)16-7-9-28(10-8-16)20(31)13-30/h3-6,11-12,16,30H,7-10,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327907
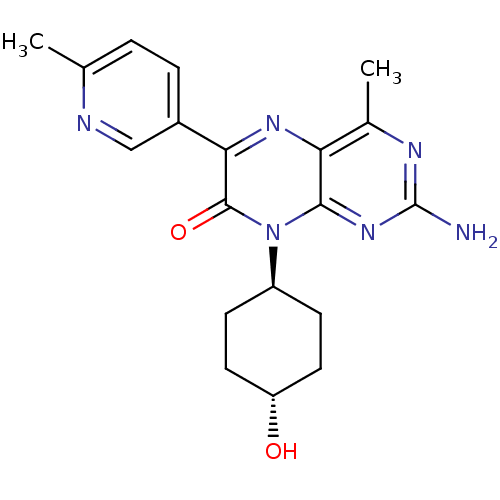
(CHEMBL1257295 | trans-2-amino-8-(4-hydroxycyclohex...)Show SMILES Cc1ccc(cn1)-c1nc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:21.23,wD:18.19,(6.09,-29.48,;4.76,-30.26,;4.77,-31.8,;3.44,-32.58,;2.1,-31.81,;2.09,-30.28,;3.42,-29.5,;.78,-32.59,;-.55,-31.83,;-1.88,-32.6,;-3.22,-31.84,;-3.22,-30.3,;-4.54,-32.61,;-4.55,-34.15,;-5.88,-34.92,;-3.21,-34.92,;-1.88,-34.14,;-.54,-34.91,;-.54,-36.45,;-1.87,-37.22,;-1.87,-38.76,;-.54,-39.53,;-.54,-41.07,;.79,-38.76,;.8,-37.22,;.79,-34.14,;2.13,-34.9,)| Show InChI InChI=1S/C19H22N6O2/c1-10-3-4-12(9-21-10)16-18(27)25(13-5-7-14(26)8-6-13)17-15(23-16)11(2)22-19(20)24-17/h3-4,9,13-14,26H,5-8H2,1-2H3,(H2,20,22,24)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50430787
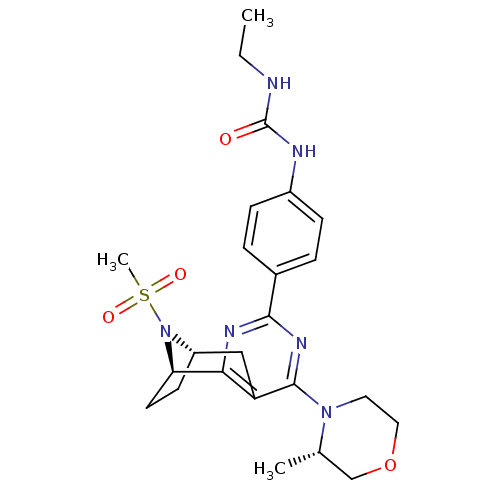
(CHEMBL2334764)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2[C@H]3CC[C@@H](Cc2c(n1)N1CCOC[C@@H]1C)N3S(C)(=O)=O |r,THB:31:30:19.14.20:17.16| Show InChI InChI=1S/C24H32N6O4S/c1-4-25-24(31)26-17-7-5-16(6-8-17)22-27-21-19(23(28-22)29-11-12-34-14-15(29)2)13-18-9-10-20(21)30(18)35(3,32)33/h5-8,15,18,20H,4,9-14H2,1-3H3,(H2,25,26,31)/t15-,18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells assessed as inhibition of phosphorylation of (GFP)-4-EBP1 protein after 30 mins by flu... |
J Med Chem 56: 3090-101 (2013)
Article DOI: 10.1021/jm400194n
BindingDB Entry DOI: 10.7270/Q2QJ7JNF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
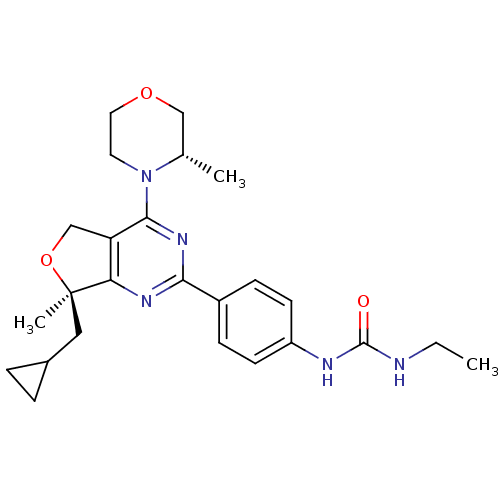
(Homo sapiens (Human)) | BDBM50343772
(1-(4-((R)-7-(cyclopropylmethyl)-7-methyl-4-((S)-3-...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2c(CO[C@]2(C)CC2CC2)c(n1)N1CCOC[C@@H]1C |r| Show InChI InChI=1S/C25H33N5O3/c1-4-26-24(31)27-19-9-7-18(8-10-19)22-28-21-20(15-33-25(21,3)13-17-5-6-17)23(29-22)30-11-12-32-14-16(30)2/h7-10,16-17H,4-6,11-15H2,1-3H3,(H2,26,27,31)/t16-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay |
J Med Chem 54: 3426-35 (2011)
Article DOI: 10.1021/jm200215y
BindingDB Entry DOI: 10.7270/Q29C6XRH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
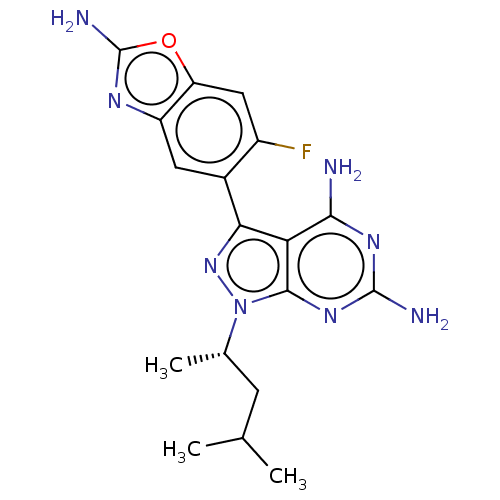
(Homo sapiens (Human)) | BDBM613734
(3-(2-amino-6-fluorobenzoxazol-5-yl)- 1-((S)-1,3-di...)Show SMILES CC(C)C[C@H](C)n1nc(-c2cc3nc(N)oc3cc2F)c2c(N)nc(N)nc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q26W9G6B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
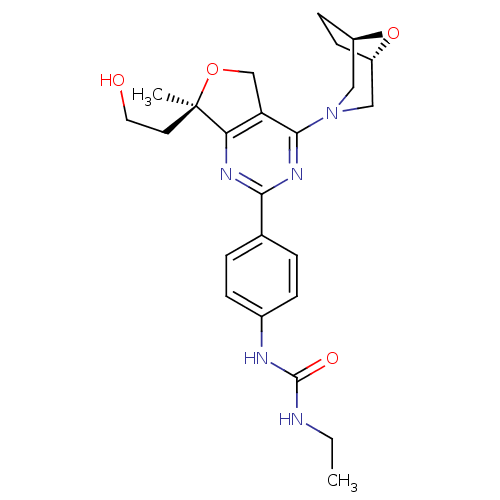
(Homo sapiens (Human)) | BDBM50343779
(1-(4-((R)-4-((1R,5S)-8-oxa-3-azabicyclo[3.2.1]octa...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2c(CO[C@]2(C)CCO)c(n1)N1C[C@@H]2CC[C@H](C1)O2 |r| Show InChI InChI=1S/C24H31N5O4/c1-3-25-23(31)26-16-6-4-15(5-7-16)21-27-20-19(14-32-24(20,2)10-11-30)22(28-21)29-12-17-8-9-18(13-29)33-17/h4-7,17-18,30H,3,8-14H2,1-2H3,(H2,25,26,31)/t17-,18+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR expressed in insect cells using 4E-BP1 substrate after 30 mins by fluorescence resonance energy transfer assay |
J Med Chem 54: 3426-35 (2011)
Article DOI: 10.1021/jm200215y
BindingDB Entry DOI: 10.7270/Q29C6XRH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
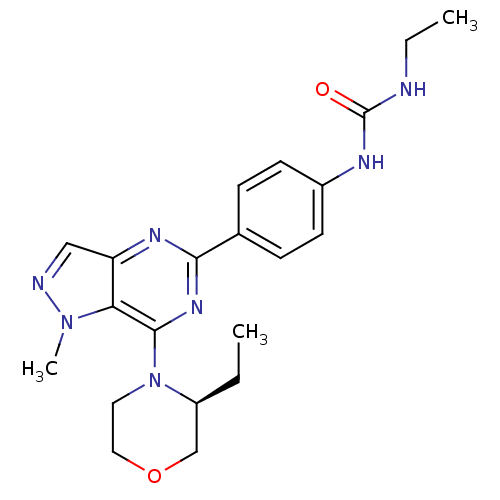
(Homo sapiens (Human)) | BDBM50439517
(CHEMBL2418349)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc(N2CCOC[C@@H]2CC)c2n(C)ncc2n1 |r| Show InChI InChI=1S/C21H27N7O2/c1-4-16-13-30-11-10-28(16)20-18-17(12-23-27(18)3)25-19(26-20)14-6-8-15(9-7-14)24-21(29)22-5-2/h6-9,12,16H,4-5,10-11,13H2,1-3H3,(H2,22,24,29)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mTOR (1360-2549) expressed in insect cells assessed as phosphorylation of recombinant (GFP)-4-EBP1 after 30 mins by L... |
Bioorg Med Chem Lett 23: 5097-104 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.027
BindingDB Entry DOI: 10.7270/Q2QN687D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
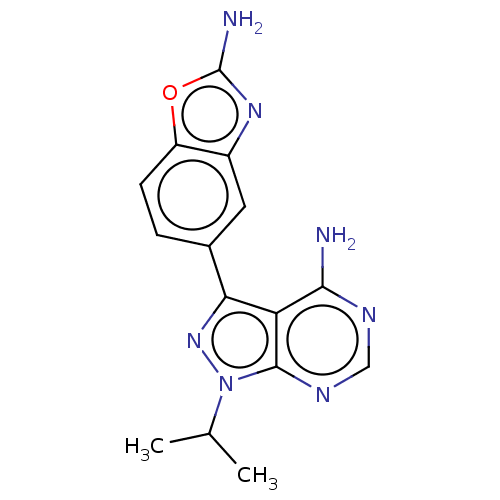
(Homo sapiens (Human)) | BDBM315477
(US10172858, Table 1.1 | US10172858, Table 1.22)Show InChI InChI=1S/C15H15N7O/c1-7(2)22-14-11(13(16)18-6-19-14)12(21-22)8-3-4-10-9(5-8)20-15(17)23-10/h3-7H,1-2H3,(H2,17,20)(H2,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus expression system using GFP-4E-BP1 ... |
ACS Med Chem Lett 10: 1561-1567 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00401
BindingDB Entry DOI: 10.7270/Q2P272KM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
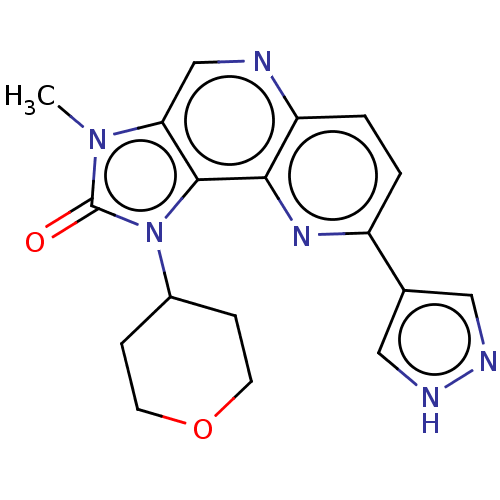
(Homo sapiens (Human)) | BDBM127425
(US8791131, 199)Show SMILES Cn1c2cnc3ccc(nc3c2n(C2CCOCC2)c1=O)-c1cn[nH]c1 Show InChI InChI=1S/C18H18N6O2/c1-23-15-10-19-14-3-2-13(11-8-20-21-9-11)22-16(14)17(15)24(18(23)25)12-4-6-26-7-5-12/h2-3,8-10,12H,4-7H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.03 | -12.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM127412

(US8791131, 181)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)NN)c3c2n1 Show InChI InChI=1S/C22H24N8O2/c1-13-3-4-14(11-24-13)16-5-6-17-19(26-16)20-18(12-25-17)28(2)22(32)30(20)15-7-9-29(10-8-15)21(31)27-23/h3-6,11-12,15H,7-10,23H2,1-2H3,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.06 | -12.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against mTOR using an in vitro kinase assay. mTOR activity is measured in vitro by dete... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data