 Found 9123 hits of ic50 for UniProtKB: P48736
Found 9123 hits of ic50 for UniProtKB: P48736 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326285
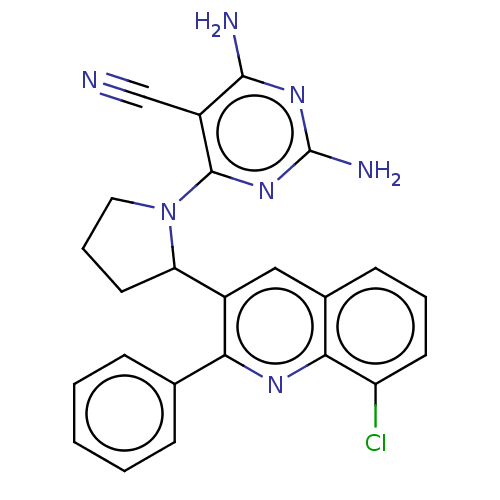
(US9637488, 68)Show SMILES Nc1nc(N)c(C#N)c(n1)N1CCCC1c1cc2cccc(Cl)c2nc1-c1ccccc1 Show InChI InChI=1S/C24H20ClN7/c25-18-9-4-8-15-12-16(20(29-21(15)18)14-6-2-1-3-7-14)19-10-5-11-32(19)23-17(13-26)22(27)30-24(28)31-23/h1-4,6-9,12,19H,5,10-11H2,(H4,27,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326267
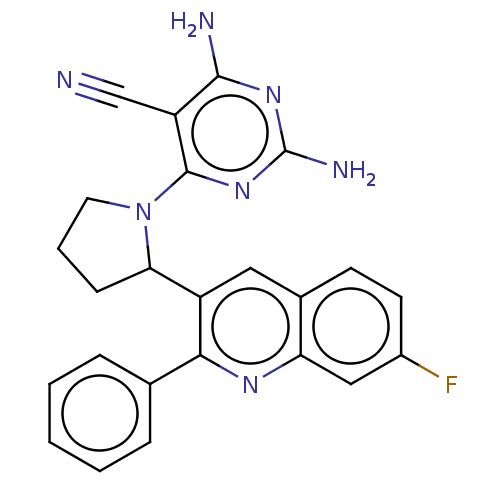
(US9637488, 27)Show SMILES Nc1nc(N)c(C#N)c(n1)N1CCCC1c1cc2ccc(F)cc2nc1-c1ccccc1 Show InChI InChI=1S/C24H20FN7/c25-16-9-8-15-11-17(21(29-19(15)12-16)14-5-2-1-3-6-14)20-7-4-10-32(20)23-18(13-26)22(27)30-24(28)31-23/h1-3,5-6,8-9,11-12,20H,4,7,10H2,(H4,27,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326282

(US9637488, 65)Show SMILES Cc1cccc2cc([C@H]3CCCN3c3ncnc(N)c3C#N)c(nc12)N1CCOCC1 Show InChI InChI=1S/C23H25N7O/c1-15-4-2-5-16-12-17(23(28-20(15)16)29-8-10-31-11-9-29)19-6-3-7-30(19)22-18(13-24)21(25)26-14-27-22/h2,4-5,12,14,19H,3,6-11H2,1H3,(H2,25,26,27)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326284
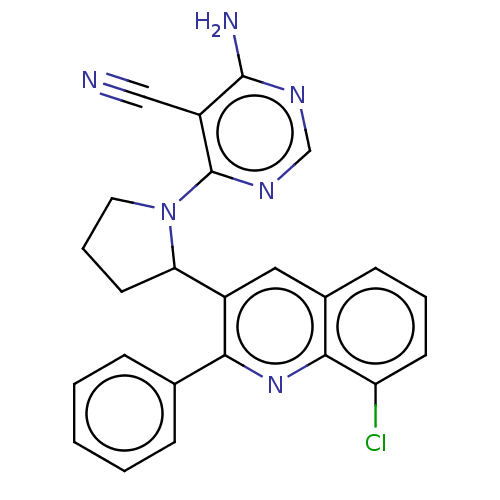
(US9637488, 67)Show SMILES Nc1ncnc(N2CCCC2c2cc3cccc(Cl)c3nc2-c2ccccc2)c1C#N Show InChI InChI=1S/C24H19ClN6/c25-19-9-4-8-16-12-17(21(30-22(16)19)15-6-2-1-3-7-15)20-10-5-11-31(20)24-18(13-26)23(27)28-14-29-24/h1-4,6-9,12,14,20H,5,10-11H2,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326278

(US9637488, 54)Show SMILES Nc1nc(N)c(C#N)c(n1)N1CCC[C@H]1c1cc2cccc(Cl)c2nc1-c1ccccc1 Show InChI InChI=1S/C24H20ClN7/c25-18-9-4-8-15-12-16(20(29-21(15)18)14-6-2-1-3-7-14)19-10-5-11-32(19)23-17(13-26)22(27)30-24(28)31-23/h1-4,6-9,12,19H,5,10-11H2,(H4,27,28,30,31)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326286
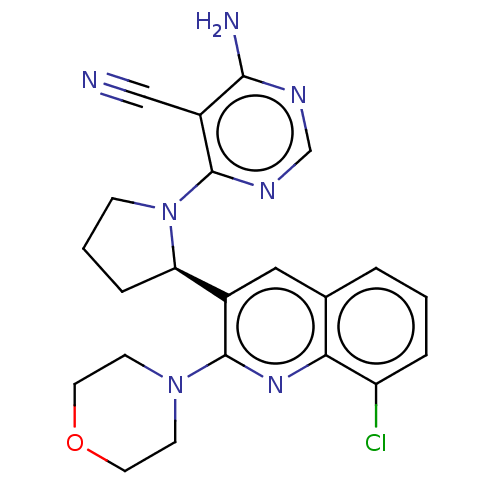
(US9637488, 69)Show SMILES Nc1ncnc(N2CCC[C@@H]2c2cc3cccc(Cl)c3nc2N2CCOCC2)c1C#N Show InChI InChI=1S/C22H22ClN7O/c23-17-4-1-3-14-11-15(22(28-19(14)17)29-7-9-31-10-8-29)18-5-2-6-30(18)21-16(12-24)20(25)26-13-27-21/h1,3-4,11,13,18H,2,5-10H2,(H2,25,26,27)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326259
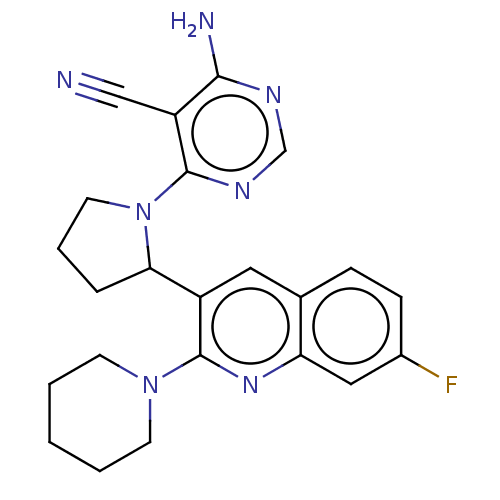
(US9637488, 18)Show SMILES Nc1ncnc(N2CCCC2c2cc3ccc(F)cc3nc2N2CCCCC2)c1C#N Show InChI InChI=1S/C23H24FN7/c24-16-7-6-15-11-17(23(29-19(15)12-16)30-8-2-1-3-9-30)20-5-4-10-31(20)22-18(13-25)21(26)27-14-28-22/h6-7,11-12,14,20H,1-5,8-10H2,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192880
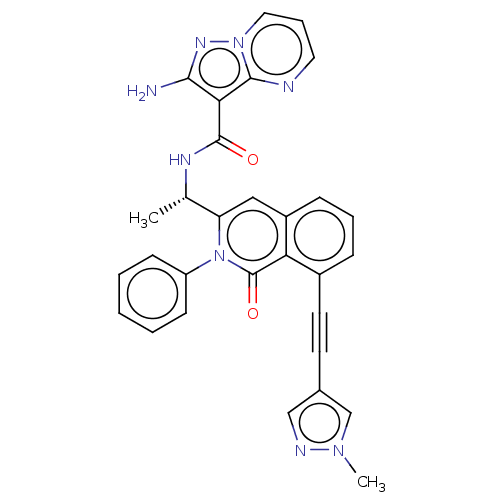
(CHEMBL3984425 | US10329299, Compound 21 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(34-29(39)26-27(31)35-37-15-7-14-32-28(26)37)24-16-22-9-6-8-21(13-12-20-17-33-36(2)18-20)25(22)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,34,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
Eur J Med Chem 163: 413-427 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.072
BindingDB Entry DOI: 10.7270/Q2736V7K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50450680
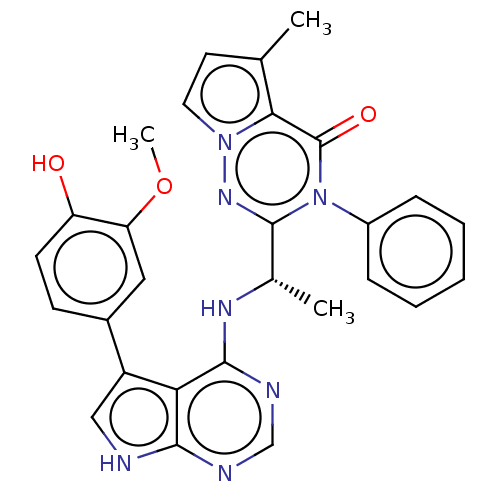
(CHEMBL4168702)Show SMILES COc1cc(ccc1O)-c1c[nH]c2ncnc(N[C@@H](C)c3nn4ccc(C)c4c(=O)n3-c3ccccc3)c12 |r| Show InChI InChI=1S/C28H25N7O3/c1-16-11-12-34-24(16)28(37)35(19-7-5-4-6-8-19)27(33-34)17(2)32-26-23-20(14-29-25(23)30-15-31-26)18-9-10-21(36)22(13-18)38-3/h4-15,17,36H,1-3H3,(H2,29,30,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length p110gamma using PIP2 as substrate preincubated for 30 mins followed by substrate a... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474011

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-({[1-(fluorome...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NCC1(CCF)CC1)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C25H31FN4O4S2/c1-14-22(35-24(28-14)29-16(3)31)18-10-19-12-30(15(2)17-4-5-17)23(32)21(19)20(11-18)36(33,34)27-13-25(6-7-25)8-9-26/h10-11,15,17,27H,4-9,12-13H2,1-3H3,(H,28,29,31)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474007

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-(ethylsulfamoy...)Show SMILES CCNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C21H26N4O4S2/c1-5-22-31(28,29)17-9-15(19-11(2)23-21(30-19)24-13(4)26)8-16-10-25(20(27)18(16)17)12(3)14-6-7-14/h8-9,12,14,22H,5-7,10H2,1-4H3,(H,23,24,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length His-tagged PI3Kgamma expressed in baculovirus expression system using PIP2 as substrate measured after 1 ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113249
BindingDB Entry DOI: 10.7270/Q2PC3641 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50457159

(CHEMBL4217725)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H16ClN5O2S/c24-22-21(29-32(30,31)18-4-2-1-3-5-18)11-17(12-26-22)16-10-19-20(14-28-23(19)27-13-16)15-6-8-25-9-7-15/h1-14,29H,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human PI3K p110gamma expressed in baculovirus expression system using PIP2/PS as substrate after 1 h... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326262

(US9637488, 22 | US9637488, 24)Show SMILES Fc1ccc2cc(C3CCCN3c3ncnc4[nH]cnc34)c(nc2c1)-c1ccccc1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474028

(N-(5-{7-[(3-Cyanophenyl)sulfamoyl]-2-[(1S)-1-cyclo...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)Nc1cccc(c1)C#N)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C26H25N5O4S2/c1-14-24(36-26(28-14)29-16(3)32)19-10-20-13-31(15(2)18-7-8-18)25(33)23(20)22(11-19)37(34,35)30-21-6-4-5-17(9-21)12-27/h4-6,9-11,15,18,30H,7-8,13H2,1-3H3,(H,28,29,32)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50546014

(CHEMBL4780201)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncnc(Nc3cnn(CCO)c3)c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using lipid as substrate incubated for 15 mins followed by substrate addition and measured after 60 mins ADP... |
Citation and Details
Article DOI: 10.1016/j.bmc.2019.04.024
BindingDB Entry DOI: 10.7270/Q2T1577B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM236931
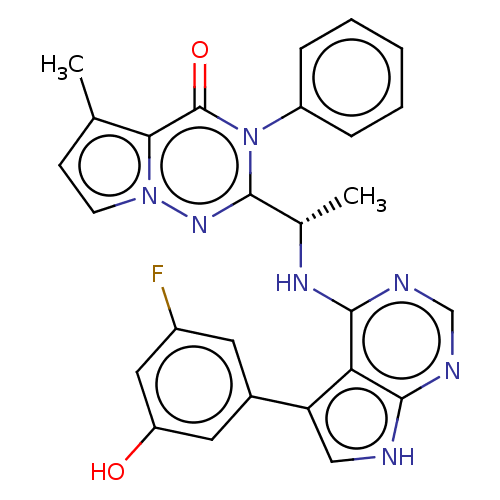
(US9388189, 27)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cc(O)cc(F)c3)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C27H22FN7O2/c1-15-8-9-34-23(15)27(37)35(19-6-4-3-5-7-19)26(33-34)16(2)32-25-22-21(13-29-24(22)30-14-31-25)17-10-18(28)12-20(36)11-17/h3-14,16,36H,1-2H3,(H2,29,30,31,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length p110gamma using PIP2 as substrate preincubated for 30 mins followed by substrate a... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-gamma (unknown origin) after 40 mins by ADP-Glo luminescent kinase assay |
Bioorg Med Chem 24: 957-66 (2016)
Article DOI: 10.1016/j.bmc.2016.01.008
BindingDB Entry DOI: 10.7270/Q2DF6T2X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50433545

(CHEMBL2381271)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cn(C)cn2)s1 Show InChI InChI=1S/C20H20N6OS/c1-12(2)26-19(21-10-23-26)20-24-18-14-5-4-13(15-9-25(3)11-22-15)8-16(14)27-7-6-17(18)28-20/h4-5,8-12H,6-7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kgamma expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 1 hr in presence of ATP by ADP-glo based luminescence assay |
Eur J Med Chem 122: 684-701 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.030
BindingDB Entry DOI: 10.7270/Q2VD71FD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274660
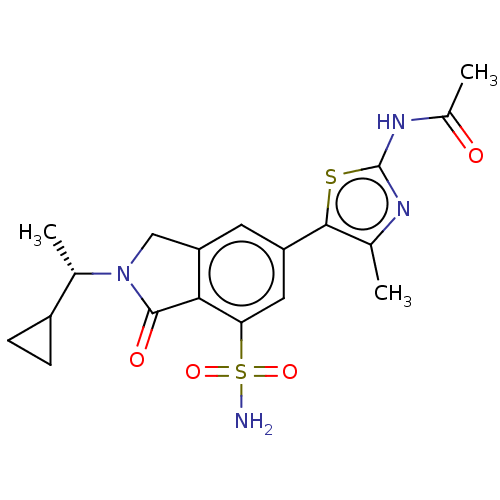
(CHEMBL4128537 | US10858355, Example 12)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(N)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H22N4O4S2/c1-9-17(28-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(7-13)29(20,26)27/h6-7,10,12H,4-5,8H2,1-3H3,(H2,20,26,27)(H,21,22,24)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274640
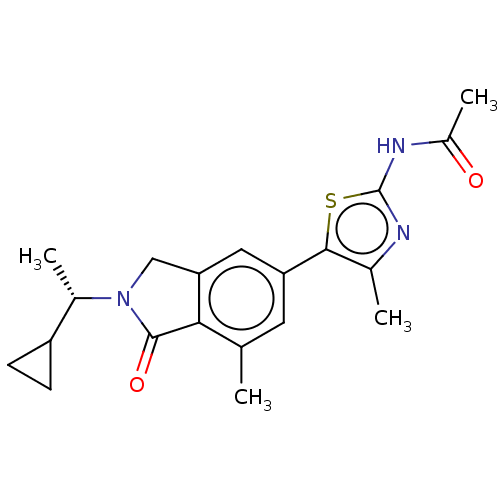
(CHEMBL4126445)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O2S/c1-10-7-15(18-11(2)21-20(26-18)22-13(4)24)8-16-9-23(19(25)17(10)16)12(3)14-5-6-14/h7-8,12,14H,5-6,9H2,1-4H3,(H,21,22,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
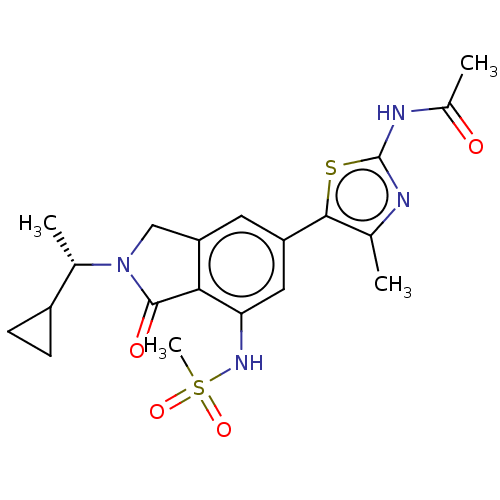
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648
(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50579671
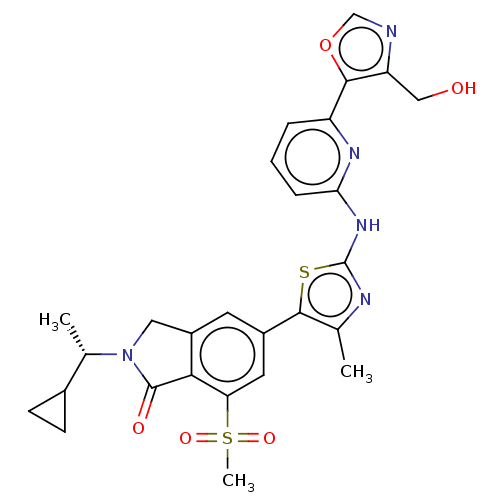
(CHEMBL5090959)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(Nc2cccc(n2)-c2ocnc2CO)nc1C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kgamma assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274638
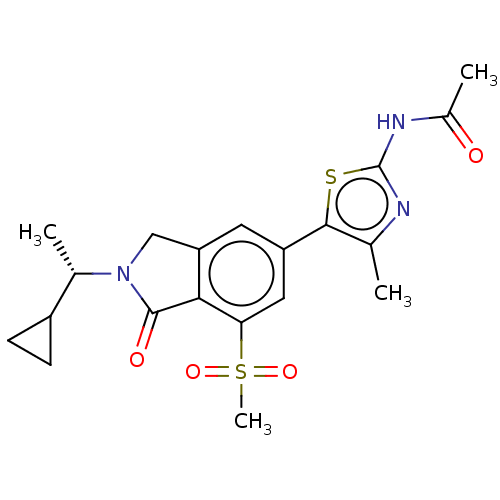
(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474012

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-1-oxo-7-[(2,2,2-...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NCC(F)(F)F)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C21H23F3N4O4S2/c1-10-18(33-20(26-10)27-12(3)29)14-6-15-8-28(11(2)13-4-5-13)19(30)17(15)16(7-14)34(31,32)25-9-21(22,23)24/h6-7,11,13,25H,4-5,8-9H2,1-3H3,(H,26,27,29)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474008

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-(oxetan-3-ylsu...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NC1COC1)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H26N4O5S2/c1-11-20(32-22(23-11)24-13(3)27)15-6-16-8-26(12(2)14-4-5-14)21(28)19(16)18(7-15)33(29,30)25-17-9-31-10-17/h6-7,12,14,17,25H,4-5,8-10H2,1-3H3,(H,23,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474015

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-[(cyclopropyls...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(=O)(=O)C3CC3)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H26N4O4S2/c1-11-20(31-22(23-11)24-13(3)27)15-8-16-10-26(12(2)14-4-5-14)21(28)19(16)18(9-15)25-32(29,30)17-6-7-17/h8-9,12,14,17,25H,4-7,10H2,1-3H3,(H,23,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50204093
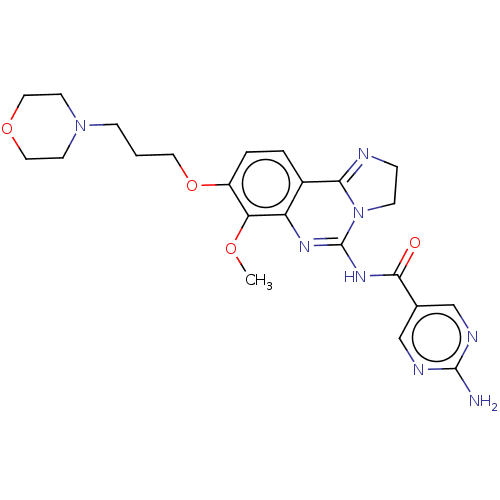
(BAY 80-6946 | BAY-80-6946 | Copanlisib)Show SMILES COc1c(OCCCN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3cnc(N)nc3)=Nc12 |c:35,t:18| Show InChI InChI=1S/C23H28N8O4/c1-33-19-17(35-10-2-6-30-8-11-34-12-9-30)4-3-16-18(19)28-23(31-7-5-25-20(16)31)29-21(32)15-13-26-22(24)27-14-15/h3-4,13-14H,2,5-12H2,1H3,(H2,24,26,27)(H,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Ain Shams University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111718
BindingDB Entry DOI: 10.7270/Q23J3HCB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326261

(US9637488, 21)Show SMILES Fc1ccc2cc(C3CCCN3c3ncnc4[nH]cnc34)c(nc2c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50457173

(CHEMBL4213317)Show SMILES Fc1ccccc1S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H15ClFN5O2S/c24-22-20(30-33(31,32)21-4-2-1-3-19(21)25)10-16(11-27-22)15-9-17-18(13-29-23(17)28-12-15)14-5-7-26-8-6-14/h1-13,30H,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human PI3K p110gamma expressed in baculovirus expression system using PIP2/PS as substrate after 1 h... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50457170
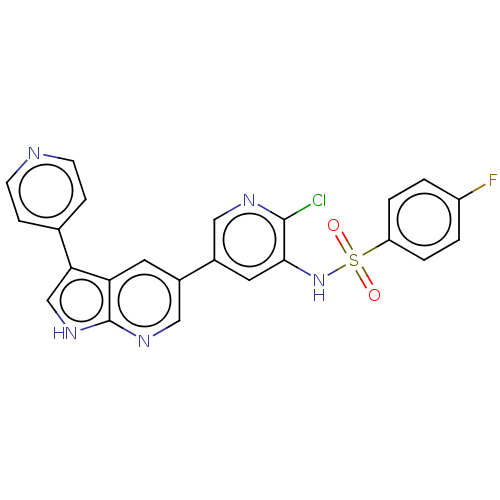
(CHEMBL4208385)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H15ClFN5O2S/c24-22-21(30-33(31,32)18-3-1-17(25)2-4-18)10-16(11-27-22)15-9-19-20(13-29-23(19)28-12-15)14-5-7-26-8-6-14/h1-13,30H,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human PI3K p110gamma expressed in baculovirus expression system using PIP2/PS as substrate after 1 h... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50450695

(CHEMBL4176898)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3ccc(O)cc3)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C27H23N7O2/c1-16-12-13-33-23(16)27(36)34(19-6-4-3-5-7-19)26(32-33)17(2)31-25-22-21(14-28-24(22)29-15-30-25)18-8-10-20(35)11-9-18/h3-15,17,35H,1-2H3,(H2,28,29,30,31)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length p110gamma using PIP2 as substrate preincubated for 30 mins followed by substrate a... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50433559

(CHEMBL2381269)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cn[nH]c2)s1 Show InChI InChI=1S/C19H18N6OS/c1-11(2)25-18(20-10-23-25)19-24-17-14-4-3-12(13-8-21-22-9-13)7-15(14)26-6-5-16(17)27-19/h3-4,7-11H,5-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kgamma expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50450675

(CHEMBL4166977)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cc(O)cc(NS(C)(=O)=O)c3)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C28H26N8O4S/c1-16-9-10-35-24(16)28(38)36(20-7-5-4-6-8-20)27(33-35)17(2)32-26-23-22(14-29-25(23)30-15-31-26)18-11-19(13-21(37)12-18)34-41(3,39)40/h4-15,17,34,37H,1-3H3,(H2,29,30,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length p110gamma using PIP2 as substrate preincubated for 30 mins followed by substrate a... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50606726

(CHEMBL5220911)Show SMILES CC(=O)N1CCN(CC1)c1cnc2ccc(cc2n1)-c1cnc(Cl)c(NS(=O)(=O)c2ccc(F)cc2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114498
BindingDB Entry DOI: 10.7270/Q2ZG6XB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50202547

(CHEMBL3968925)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccc(Cn4cncn4)cc3)c2c1 Show InChI InChI=1S/C30H22F2N6O3S/c1-41-30-28(37-42(39,40)29-9-7-23(31)14-26(29)32)13-22(15-35-30)21-6-8-27-25(12-21)24(10-11-34-27)20-4-2-19(3-5-20)16-38-18-33-17-36-38/h2-15,17-18,37H,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 1 hr in presence of ATP by ADP-glo based luminescence assay |
Eur J Med Chem 122: 684-701 (2016)
Article DOI: 10.1016/j.ejmech.2016.06.030
BindingDB Entry DOI: 10.7270/Q2VD71FD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474017

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-[(ethylsulfony...)Show SMILES CCS(=O)(=O)Nc1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C21H26N4O4S2/c1-5-31(28,29)24-17-9-15(19-11(2)22-21(30-19)23-13(4)26)8-16-10-25(20(27)18(16)17)12(3)14-6-7-14/h8-9,12,14,24H,5-7,10H2,1-4H3,(H,22,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274638

(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274649
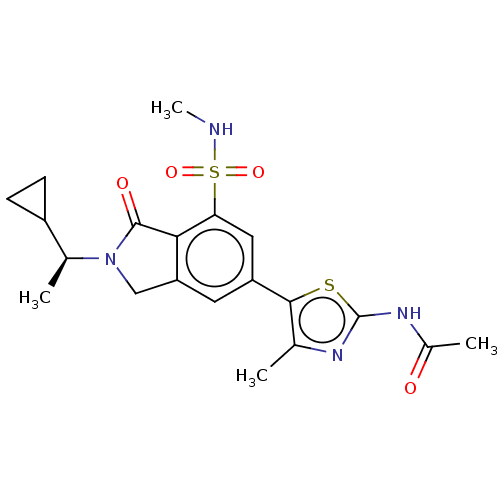
(CHEMBL4129600 | US10858355, Example 30)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(22-10)23-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)30(27,28)21-4/h7-8,11,13,21H,5-6,9H2,1-4H3,(H,22,23,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474020

(US10858355, Example 28)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(=O)(=O)CC3CC3)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C23H28N4O4S2/c1-12-21(32-23(24-12)25-14(3)28)17-8-18-10-27(13(2)16-6-7-16)22(29)20(18)19(9-17)26-33(30,31)11-15-4-5-15/h8-9,13,15-16,26H,4-7,10-11H2,1-3H3,(H,24,25,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM473999

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-(dimethylsulfa...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)N(C)C)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C21H26N4O4S2/c1-11-19(30-21(22-11)23-13(3)26)15-8-16-10-25(12(2)14-6-7-14)20(27)18(16)17(9-15)31(28,29)24(4)5/h8-9,12,14H,6-7,10H2,1-5H3,(H,22,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274649

(CHEMBL4129600 | US10858355, Example 30)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(22-10)23-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)30(27,28)21-4/h7-8,11,13,21H,5-6,9H2,1-4H3,(H,22,23,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kgamma assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274638

(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kgamma assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274649

(CHEMBL4129600 | US10858355, Example 30)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(22-10)23-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)30(27,28)21-4/h7-8,11,13,21H,5-6,9H2,1-4H3,(H,22,23,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274675
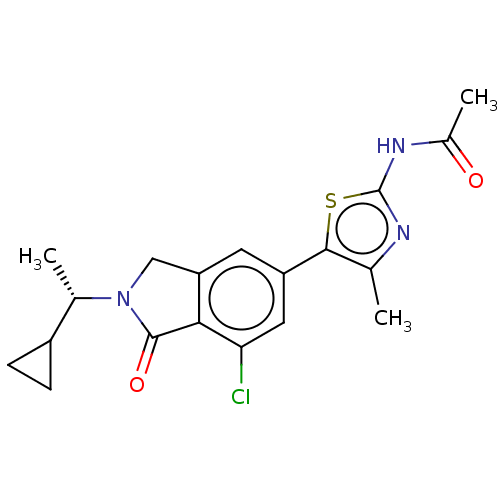
(CHEMBL4126601)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H20ClN3O2S/c1-9-17(26-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(20)7-13/h6-7,10,12H,4-5,8H2,1-3H3,(H,21,22,24)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474028

(N-(5-{7-[(3-Cyanophenyl)sulfamoyl]-2-[(1S)-1-cyclo...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)Nc1cccc(c1)C#N)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C26H25N5O4S2/c1-14-24(36-26(28-14)29-16(3)32)19-10-20-13-31(15(2)18-7-8-18)25(33)23(20)22(11-19)37(34,35)30-21-6-4-5-17(9-21)12-27/h4-6,9-11,15,18,30H,7-8,13H2,1-3H3,(H,28,29,32)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kgamma assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM489240
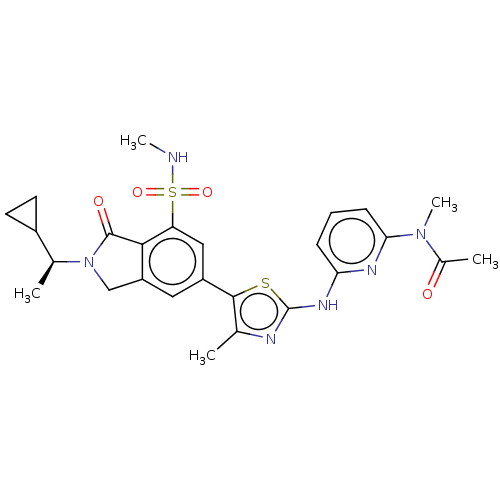
(N-{6-[(5-{2-[(1S)-1-Cyclopropylethyl]-7-(methylsul...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N(C)C(C)=O)nc1C |r| Show InChI InChI=1S/C26H30N6O4S2/c1-14-24(37-26(28-14)30-21-7-6-8-22(29-21)31(5)16(3)33)18-11-19-13-32(15(2)17-9-10-17)25(34)23(19)20(12-18)38(35,36)27-4/h6-8,11-12,15,17,27H,9-10,13H2,1-5H3,(H,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kgamma assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data