 Found 248 hits of ki for UniProtKB: P32238
Found 248 hits of ki for UniProtKB: P32238 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50360276
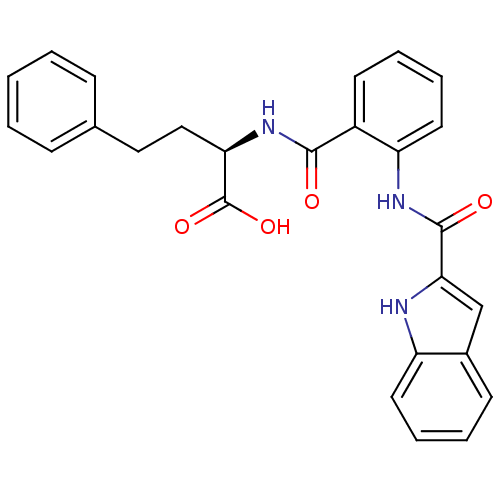
(CHEMBL1933104)Show SMILES OC(=O)[C@@H](CCc1ccccc1)NC(=O)c1ccccc1NC(=O)c1cc2ccccc2[nH]1 |r| Show InChI InChI=1S/C26H23N3O4/c30-24(29-22(26(32)33)15-14-17-8-2-1-3-9-17)19-11-5-7-13-21(19)28-25(31)23-16-18-10-4-6-12-20(18)27-23/h1-13,16,22,27H,14-15H2,(H,28,31)(H,29,30)(H,32,33)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BH-JMV-179 from wild type human CCK1 receptor expressed in COS7 cells after 60 mins by gamma counting |
J Med Chem 54: 5769-85 (2011)
Article DOI: 10.1021/jm200438b
BindingDB Entry DOI: 10.7270/Q2BP036J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321604

((3S,6S,9S,12S,15R,18S,21S,24R,27S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:58.71,74.80,8.20,87.93,69.75,wD:48.59,22.31,4.4,33.48,95.101,(27.14,-11.6,;25.81,-10.83,;25.81,-9.29,;24.47,-8.52,;24.47,-6.97,;23.14,-6.2,;21.8,-6.97,;21.8,-8.5,;20.47,-6.2,;20.47,-4.66,;21.89,-4.07,;23.12,-5,;24.38,-4.1,;23.93,-2.63,;24.71,-1.31,;23.96,.04,;22.43,.05,;21.64,-1.26,;22.38,-2.61,;19.13,-6.97,;17.8,-6.2,;17.8,-4.66,;16.46,-6.97,;16.46,-8.51,;17.8,-9.28,;17.8,-10.82,;19.14,-11.58,;19.13,-13.12,;17.8,-13.89,;20.46,-13.9,;15.12,-6.21,;13.78,-6.97,;13.78,-8.51,;12.45,-6.2,;12.42,-4.66,;13.74,-3.87,;15.09,-4.61,;16.4,-3.81,;16.37,-2.28,;17.69,-1.49,;17.67,.05,;16.32,.8,;15,.01,;15.03,-1.52,;13.71,-2.32,;11.12,-6.97,;9.78,-6.21,;9.78,-4.66,;8.44,-6.98,;8.44,-8.51,;9.78,-9.29,;11.18,-8.65,;12.21,-9.8,;11.45,-11.14,;9.94,-10.82,;7.1,-6.21,;5.76,-6.97,;5.76,-8.5,;4.43,-6.2,;4.43,-4.66,;5.76,-3.89,;7.1,-4.65,;8.42,-3.88,;8.42,-2.34,;7.09,-1.57,;5.75,-2.34,;3.1,-6.99,;1.76,-6.22,;1.76,-4.68,;.42,-6.99,;.42,-8.53,;-.9,-6.21,;-2.25,-6.97,;-2.25,-8.51,;-3.58,-6.2,;-4.91,-6.98,;-3.58,-4.66,;-2.25,-3.89,;-.92,-4.66,;.41,-3.89,;.4,-2.35,;1.77,-1.59,;-.92,-1.58,;-2.25,-2.35,;25.81,-6.21,;25.81,-4.67,;27.15,-6.98,;28.48,-6.21,;28.48,-4.66,;29.82,-3.89,;31.15,-4.66,;29.82,-2.35,;29.82,-6.97,;29.82,-8.51,;31.15,-6.2,;32.49,-6.97,;32.49,-8.5,;33.83,-9.28,;35.16,-8.5,;36.5,-9.28,;36.5,-10.82,;35.16,-11.58,;33.83,-10.81,;33.83,-6.2,;35.15,-6.96,;33.83,-4.65,)| Show InChI InChI=1S/C76H91N17O13/c1-3-4-23-57(69(100)93-64(40-65(95)96)75(106)88-59(66(78)97)35-45-16-7-5-8-17-45)86-73(104)62(38-51-41-83-56-24-14-13-22-54(51)56)91-70(101)58(25-15-32-82-76(79)80)87-71(102)61(37-48-26-29-49-20-11-12-21-50(49)33-48)90-74(105)63(39-52-42-81-43-84-52)92-72(103)60(36-46-18-9-6-10-19-46)89-67(98)44(2)85-68(99)55(77)34-47-27-30-53(94)31-28-47/h5-14,16-22,24,26-31,33,41-44,55,57-64,83,94H,3-4,15,23,25,32,34-40,77H2,1-2H3,(H2,78,97)(H,81,84)(H,85,99)(H,86,104)(H,87,102)(H,88,106)(H,89,98)(H,90,105)(H,91,101)(H,92,103)(H,93,100)(H,95,96)(H4,79,80,82)/t44-,55+,57+,58+,59+,60+,61-,62+,63+,64+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321604

((3S,6S,9S,12S,15R,18S,21S,24R,27S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:58.71,74.80,8.20,87.93,69.75,wD:48.59,22.31,4.4,33.48,95.101,(27.14,-11.6,;25.81,-10.83,;25.81,-9.29,;24.47,-8.52,;24.47,-6.97,;23.14,-6.2,;21.8,-6.97,;21.8,-8.5,;20.47,-6.2,;20.47,-4.66,;21.89,-4.07,;23.12,-5,;24.38,-4.1,;23.93,-2.63,;24.71,-1.31,;23.96,.04,;22.43,.05,;21.64,-1.26,;22.38,-2.61,;19.13,-6.97,;17.8,-6.2,;17.8,-4.66,;16.46,-6.97,;16.46,-8.51,;17.8,-9.28,;17.8,-10.82,;19.14,-11.58,;19.13,-13.12,;17.8,-13.89,;20.46,-13.9,;15.12,-6.21,;13.78,-6.97,;13.78,-8.51,;12.45,-6.2,;12.42,-4.66,;13.74,-3.87,;15.09,-4.61,;16.4,-3.81,;16.37,-2.28,;17.69,-1.49,;17.67,.05,;16.32,.8,;15,.01,;15.03,-1.52,;13.71,-2.32,;11.12,-6.97,;9.78,-6.21,;9.78,-4.66,;8.44,-6.98,;8.44,-8.51,;9.78,-9.29,;11.18,-8.65,;12.21,-9.8,;11.45,-11.14,;9.94,-10.82,;7.1,-6.21,;5.76,-6.97,;5.76,-8.5,;4.43,-6.2,;4.43,-4.66,;5.76,-3.89,;7.1,-4.65,;8.42,-3.88,;8.42,-2.34,;7.09,-1.57,;5.75,-2.34,;3.1,-6.99,;1.76,-6.22,;1.76,-4.68,;.42,-6.99,;.42,-8.53,;-.9,-6.21,;-2.25,-6.97,;-2.25,-8.51,;-3.58,-6.2,;-4.91,-6.98,;-3.58,-4.66,;-2.25,-3.89,;-.92,-4.66,;.41,-3.89,;.4,-2.35,;1.77,-1.59,;-.92,-1.58,;-2.25,-2.35,;25.81,-6.21,;25.81,-4.67,;27.15,-6.98,;28.48,-6.21,;28.48,-4.66,;29.82,-3.89,;31.15,-4.66,;29.82,-2.35,;29.82,-6.97,;29.82,-8.51,;31.15,-6.2,;32.49,-6.97,;32.49,-8.5,;33.83,-9.28,;35.16,-8.5,;36.5,-9.28,;36.5,-10.82,;35.16,-11.58,;33.83,-10.81,;33.83,-6.2,;35.15,-6.96,;33.83,-4.65,)| Show InChI InChI=1S/C76H91N17O13/c1-3-4-23-57(69(100)93-64(40-65(95)96)75(106)88-59(66(78)97)35-45-16-7-5-8-17-45)86-73(104)62(38-51-41-83-56-24-14-13-22-54(51)56)91-70(101)58(25-15-32-82-76(79)80)87-71(102)61(37-48-26-29-49-20-11-12-21-50(49)33-48)90-74(105)63(39-52-42-81-43-84-52)92-72(103)60(36-46-18-9-6-10-19-46)89-67(98)44(2)85-68(99)55(77)34-47-27-30-53(94)31-28-47/h5-14,16-22,24,26-31,33,41-44,55,57-64,83,94H,3-4,15,23,25,32,34-40,77H2,1-2H3,(H2,78,97)(H,81,84)(H,85,99)(H,86,104)(H,87,102)(H,88,106)(H,89,98)(H,90,105)(H,91,101)(H,92,103)(H,93,100)(H,95,96)(H4,79,80,82)/t44-,55+,57+,58+,59+,60+,61-,62+,63+,64+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50360276

(CHEMBL1933104)Show SMILES OC(=O)[C@@H](CCc1ccccc1)NC(=O)c1ccccc1NC(=O)c1cc2ccccc2[nH]1 |r| Show InChI InChI=1S/C26H23N3O4/c30-24(29-22(26(32)33)15-14-17-8-2-1-3-9-17)19-11-5-7-13-21(19)28-25(31)23-16-18-10-4-6-12-20(18)27-23/h1-13,16,22,27H,14-15H2,(H,28,31)(H,29,30)(H,32,33)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste
Curated by ChEMBL
| Assay Description
Displacement of [125I]BH-(Thr,-Nle)-CCK-9 from human CCK1 receptor expressed in COS-7 cells after 60 mins by gamma counting |
J Med Chem 54: 5769-85 (2011)
Article DOI: 10.1021/jm200438b
BindingDB Entry DOI: 10.7270/Q2BP036J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50281633
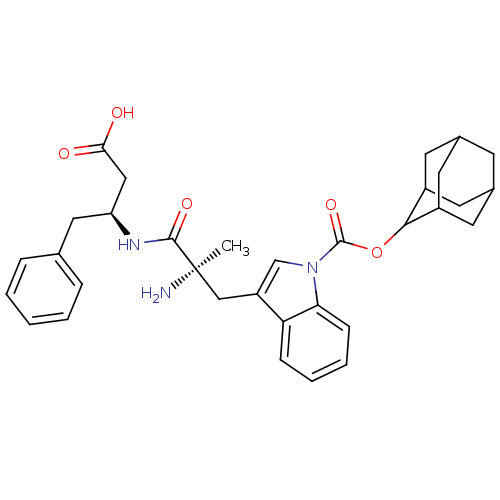
(3-[(R)-2-Amino-2-((S)-1-benzyl-2-carboxy-ethylcarb...)Show SMILES C[C@@](N)(Cc1cn(C(=O)OC2C3CC4CC(C3)CC2C4)c2ccccc12)C(=O)N[C@H](CC(O)=O)Cc1ccccc1 |wU:1.0,wD:29.33,1.1,TLB:12:13:10.11.16:17,9:10:17:13.19.14,THB:12:11:17:13.19.14,14:15:10:13.12.19,14:13:10:15.16.17,(17.69,-2.7,;17.7,-1.17,;16.35,-1.89,;17.77,.37,;16.46,1.21,;15.02,.67,;14.06,1.84,;12.71,1.11,;12.69,-.4,;11.41,1.9,;10.05,1.16,;8.75,1.95,;7.23,1.72,;7,.2,;5.91,-.89,;7.42,-.56,;7.56,.98,;8.77,-1.31,;9.89,-.23,;8.38,-.49,;14.9,3.15,;14.48,4.63,;15.58,5.71,;17.07,5.31,;17.47,3.85,;16.37,2.75,;19,-1.99,;18.93,-3.54,;20.38,-1.28,;21.66,-2.1,;23.03,-1.38,;24.32,-2.2,;25.69,-1.48,;24.26,-3.74,;21.65,-4.65,;22.94,-5.48,;24.3,-4.76,;25.6,-5.57,;25.55,-7.12,;24.18,-7.82,;22.87,-7.02,)| Show InChI InChI=1S/C33H39N3O5/c1-33(34,31(39)35-26(17-29(37)38)16-20-7-3-2-4-8-20)18-25-19-36(28-10-6-5-9-27(25)28)32(40)41-30-23-12-21-11-22(14-23)15-24(30)13-21/h2-10,19,21-24,26,30H,11-18,34H2,1H3,(H,35,39)(H,37,38)/t21?,22?,23?,24?,26-,30?,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against CCK-A receptor |
Bioorg Med Chem Lett 3: 799-802 (1993)
Article DOI: 10.1016/S0960-894X(00)80669-1
BindingDB Entry DOI: 10.7270/Q29S1QZZ |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50413478
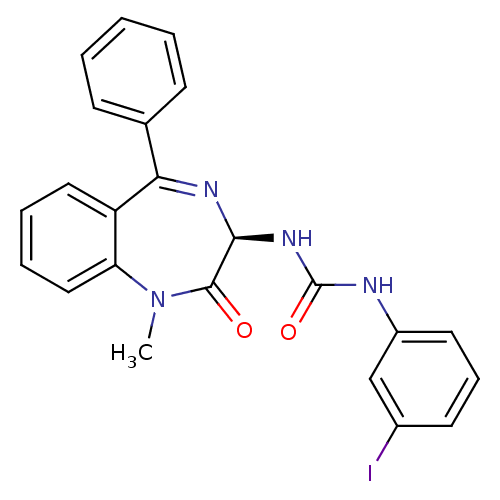
(CHEMBL515288)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(I)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C23H19IN4O2/c1-28-19-13-6-5-12-18(19)20(15-8-3-2-4-9-15)26-21(22(28)29)27-23(30)25-17-11-7-10-16(24)14-17/h2-14,21H,1H3,(H2,25,27,30)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I](S)-1-(3-iodophenyl)-3-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)urea from human CCK1 receptor express... |
J Med Chem 52: 2138-47 (2009)
Article DOI: 10.1021/jm801439x
BindingDB Entry DOI: 10.7270/Q22B908P |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50360273
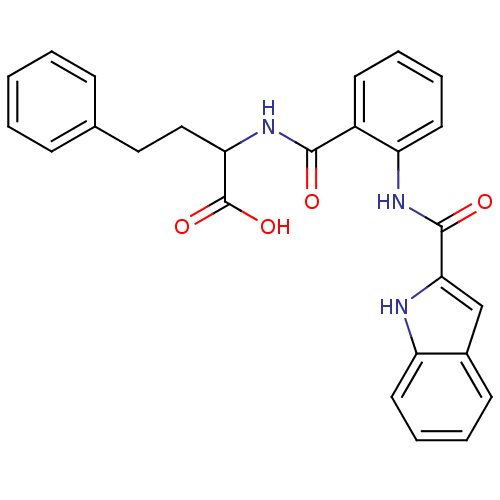
(CHEMBL1933100)Show SMILES OC(=O)C(CCc1ccccc1)NC(=O)c1ccccc1NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C26H23N3O4/c30-24(29-22(26(32)33)15-14-17-8-2-1-3-9-17)19-11-5-7-13-21(19)28-25(31)23-16-18-10-4-6-12-20(18)27-23/h1-13,16,22,27H,14-15H2,(H,28,31)(H,29,30)(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste
Curated by ChEMBL
| Assay Description
Displacement of [125I]BH-(Thr,-Nle)-CCK-9 from human CCK1 receptor expressed in COS-7 cells after 60 mins by gamma counting |
J Med Chem 54: 5769-85 (2011)
Article DOI: 10.1021/jm200438b
BindingDB Entry DOI: 10.7270/Q2BP036J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50360276

(CHEMBL1933104)Show SMILES OC(=O)[C@@H](CCc1ccccc1)NC(=O)c1ccccc1NC(=O)c1cc2ccccc2[nH]1 |r| Show InChI InChI=1S/C26H23N3O4/c30-24(29-22(26(32)33)15-14-17-8-2-1-3-9-17)19-11-5-7-13-21(19)28-25(31)23-16-18-10-4-6-12-20(18)27-23/h1-13,16,22,27H,14-15H2,(H,28,31)(H,29,30)(H,32,33)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SR-27897 from wild type human CCK1 receptor expressed in COS7 cells after 60 mins by gamma counting |
J Med Chem 54: 5769-85 (2011)
Article DOI: 10.1021/jm200438b
BindingDB Entry DOI: 10.7270/Q2BP036J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321607
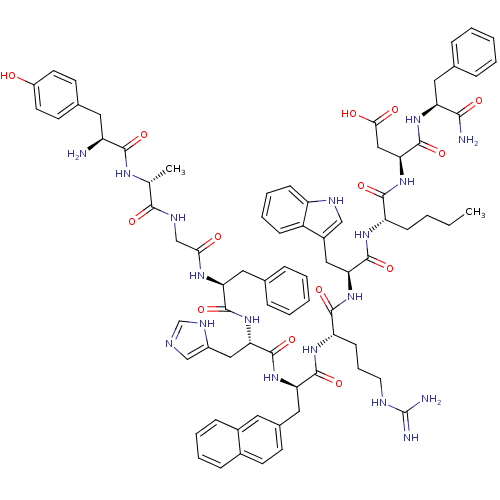
((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C78H94N18O14/c1-3-4-23-58(71(104)96-65(40-67(99)100)77(110)92-60(68(80)101)35-46-16-7-5-8-17-46)90-75(108)63(38-52-41-85-57-24-14-13-22-55(52)57)94-72(105)59(25-15-32-84-78(81)82)91-74(107)62(37-49-26-29-50-20-11-12-21-51(50)33-49)93-76(109)64(39-53-42-83-44-87-53)95-73(106)61(36-47-18-9-6-10-19-47)89-66(98)43-86-69(102)45(2)88-70(103)56(79)34-48-27-30-54(97)31-28-48/h5-14,16-22,24,26-31,33,41-42,44-45,56,58-65,85,97H,3-4,15,23,25,32,34-40,43,79H2,1-2H3,(H2,80,101)(H,83,87)(H,86,102)(H,88,103)(H,89,98)(H,90,108)(H,91,107)(H,92,110)(H,93,109)(H,94,105)(H,95,106)(H,96,104)(H,99,100)(H4,81,82,84)/t45-,56+,58+,59+,60+,61+,62-,63+,64+,65+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321607

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C78H94N18O14/c1-3-4-23-58(71(104)96-65(40-67(99)100)77(110)92-60(68(80)101)35-46-16-7-5-8-17-46)90-75(108)63(38-52-41-85-57-24-14-13-22-55(52)57)94-72(105)59(25-15-32-84-78(81)82)91-74(107)62(37-49-26-29-50-20-11-12-21-51(50)33-49)93-76(109)64(39-53-42-83-44-87-53)95-73(106)61(36-47-18-9-6-10-19-47)89-66(98)43-86-69(102)45(2)88-70(103)56(79)34-48-27-30-54(97)31-28-48/h5-14,16-22,24,26-31,33,41-42,44-45,56,58-65,85,97H,3-4,15,23,25,32,34-40,43,79H2,1-2H3,(H2,80,101)(H,83,87)(H,86,102)(H,88,103)(H,89,98)(H,90,108)(H,91,107)(H,92,110)(H,93,109)(H,94,105)(H,95,106)(H,96,104)(H,99,100)(H4,81,82,84)/t45-,56+,58+,59+,60+,61+,62-,63+,64+,65+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM21139
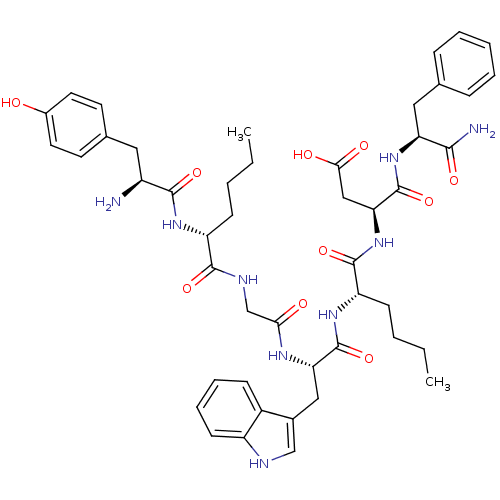
((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...)Show SMILES CCCC[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C47H61N9O10/c1-3-5-15-35(53-43(62)33(48)22-29-18-20-31(57)21-19-29)44(63)51-27-40(58)52-38(24-30-26-50-34-17-11-10-14-32(30)34)46(65)54-36(16-6-4-2)45(64)56-39(25-41(59)60)47(66)55-37(42(49)61)23-28-12-8-7-9-13-28/h7-14,17-21,26,33,35-39,50,57H,3-6,15-16,22-25,27,48H2,1-2H3,(H2,49,61)(H,51,63)(H,52,58)(H,53,62)(H,54,65)(H,55,66)(H,56,64)(H,59,60)/t33-,35+,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 49: 2868-75 (2006)
Article DOI: 10.1021/jm050921q
BindingDB Entry DOI: 10.7270/Q24Q7S99 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50214807
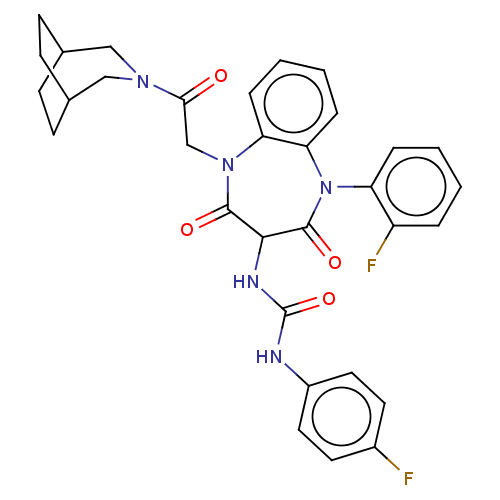
(CHEMBL320624)Show SMILES Fc1ccc(NC(=O)NC2C(=O)N(CC(=O)N3CC4CCC(CC4)C3)c3ccccc3N(c3ccccc3F)C2=O)cc1 |(7.19,-15.84,;7.2,-14.3,;8.53,-13.53,;8.53,-12,;7.2,-11.23,;7.2,-9.69,;5.87,-8.92,;5.87,-7.39,;4.55,-9.69,;3.22,-8.92,;3.15,-7.36,;4.49,-6.61,;1.79,-6.46,;2.01,-4.83,;.72,-3.83,;-.79,-4.45,;.94,-2.22,;2.59,-1.75,;3.29,-.52,;2.59,.99,;.89,1.35,;-.46,.21,;.64,-.87,;1.72,.22,;-.32,-1.45,;.22,-6.97,;-.84,-5.83,;-2.37,-6.17,;-2.83,-7.67,;-1.77,-8.81,;-.25,-8.47,;.57,-9.58,;-.2,-10.9,;.57,-12.23,;-.2,-13.56,;-1.74,-13.56,;-2.49,-12.24,;-1.74,-10.92,;-2.51,-9.58,;2.17,-9.76,;2.57,-11.24,;5.87,-12,;5.87,-13.53,)| Show InChI InChI=1S/C32H31F2N5O4/c33-22-13-15-23(16-14-22)35-32(43)36-29-30(41)38(19-28(40)37-17-20-9-10-21(18-37)12-11-20)26-7-3-4-8-27(26)39(31(29)42)25-6-2-1-5-24(25)34/h1-8,13-16,20-21,29H,9-12,17-19H2,(H2,35,36,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tested for its selectivity against human Cholecystokinin type A receptor isolated from a human gallbladder cDNA library and stably transfected into a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PZ5C0X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste
Curated by ChEMBL
| Assay Description
Displacement of [125I]BH-(Thr,-Nle)-CCK-9 from human CCK1 receptor expressed in COS-7 cells after 60 mins by gamma counting |
J Med Chem 54: 5769-85 (2011)
Article DOI: 10.1021/jm200438b
BindingDB Entry DOI: 10.7270/Q2BP036J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415053
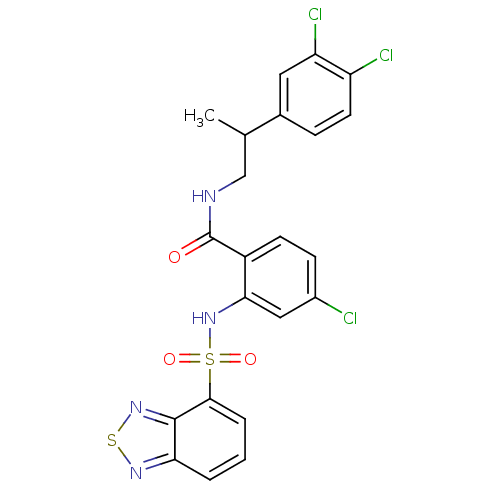
(CHEMBL583457)Show SMILES CC(CNC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H17Cl3N4O3S2/c1-12(13-5-8-16(24)17(25)9-13)11-26-22(30)15-7-6-14(23)10-19(15)29-34(31,32)20-4-2-3-18-21(20)28-33-27-18/h2-10,12,29H,11H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321596

((3S,6R,9S,12S,15S,18S)-3-((1H-imidazol-5-yl)methyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C76H90N16O12/c1-4-5-23-58(68(97)90-64(38-66(94)95)73(102)87-60(67(78)96)32-45-16-7-6-8-17-45)85-71(100)62(34-51-39-83-57-24-14-13-22-54(51)57)89-69(98)59(25-15-28-82-76(79)80)86-70(99)61(33-46-26-27-47-18-9-10-19-48(47)31-46)88-72(101)63(36-52-40-81-42-84-52)91-74(103)65-35-49-20-11-12-21-50(49)41-92(65)75(104)56(77)37-55-43(2)29-53(93)30-44(55)3/h6-14,16-22,24,26-27,29-31,39-40,42,56,58-65,83,93H,4-5,15,23,25,28,32-38,41,77H2,1-3H3,(H2,78,96)(H,81,84)(H,85,100)(H,86,99)(H,87,102)(H,88,101)(H,89,98)(H,90,97)(H,91,103)(H,94,95)(H4,79,80,82)/t56-,58-,59-,60-,61+,62-,63-,64-,65?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321596

((3S,6R,9S,12S,15S,18S)-3-((1H-imidazol-5-yl)methyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C76H90N16O12/c1-4-5-23-58(68(97)90-64(38-66(94)95)73(102)87-60(67(78)96)32-45-16-7-6-8-17-45)85-71(100)62(34-51-39-83-57-24-14-13-22-54(51)57)89-69(98)59(25-15-28-82-76(79)80)86-70(99)61(33-46-26-27-47-18-9-10-19-48(47)31-46)88-72(101)63(36-52-40-81-42-84-52)91-74(103)65-35-49-20-11-12-21-50(49)41-92(65)75(104)56(77)37-55-43(2)29-53(93)30-44(55)3/h6-14,16-22,24,26-27,29-31,39-40,42,56,58-65,83,93H,4-5,15,23,25,28,32-38,41,77H2,1-3H3,(H2,78,96)(H,81,84)(H,85,100)(H,86,99)(H,87,102)(H,88,101)(H,89,98)(H,90,97)(H,91,103)(H,94,95)(H4,79,80,82)/t56-,58-,59-,60-,61+,62-,63-,64-,65?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321606
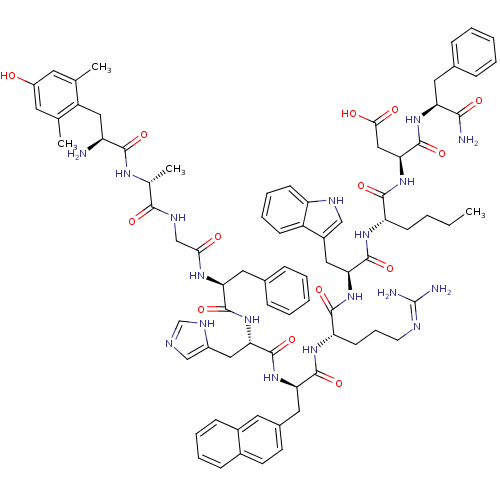
((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:8.20,58.71,93.99,78.84,wD:73.79,48.59,22.31,33.48,4.4,101.107,(24.59,-5.48,;25.93,-4.72,;25.93,-3.18,;24.59,-2.4,;24.59,-.86,;23.26,-.1,;21.93,-.87,;21.93,-2.41,;20.59,-.11,;20.59,1.44,;22,2.07,;23.25,1.19,;24.48,2.12,;23.98,3.58,;24.73,4.92,;23.94,6.24,;22.4,6.22,;21.65,4.87,;22.44,3.55,;19.25,-.87,;17.91,-.12,;17.91,1.43,;16.57,-.89,;16.57,-2.43,;17.91,-3.2,;17.91,-4.73,;19.24,-5.51,;19.24,-7.05,;17.93,-7.8,;20.61,-7.81,;15.25,-.1,;13.92,-.87,;13.92,-2.41,;12.59,-.1,;12.6,1.44,;13.92,2.21,;15.26,1.44,;16.59,2.21,;16.6,3.75,;17.93,4.53,;17.92,6.07,;16.58,6.84,;15.25,6.06,;15.26,4.52,;13.92,3.75,;11.26,-.86,;9.92,-.1,;9.92,1.45,;8.58,-.87,;8.58,-2.41,;9.92,-3.18,;11.32,-2.55,;12.36,-3.69,;11.59,-5.03,;10.08,-4.71,;7.25,-.09,;5.91,-.85,;5.91,-2.39,;4.58,-.08,;4.58,1.46,;5.91,2.23,;7.25,1.47,;8.58,2.24,;8.58,3.78,;7.24,4.55,;5.91,3.78,;3.25,-.85,;1.92,-.07,;1.92,1.47,;.58,-.85,;-.75,-.07,;-2.08,-.85,;-2.08,-2.38,;-3.41,-.07,;-3.41,1.47,;-4.75,-.84,;-6.09,-.08,;-6.09,1.47,;-7.42,-.86,;-7.4,-2.4,;-8.75,-.1,;-10.08,-.88,;-10.06,-2.42,;-8.72,-3.17,;-11.4,-3.2,;-12.73,-2.44,;-14.07,-3.22,;-12.76,-.9,;-11.41,-.13,;-11.43,1.41,;25.93,-.09,;25.93,1.45,;27.26,-.87,;28.59,-.09,;28.59,1.45,;29.93,2.21,;31.25,1.45,;29.93,3.76,;29.93,-.87,;29.93,-2.4,;31.26,-.1,;32.6,-.87,;32.6,-2.4,;33.93,-3.17,;35.28,-2.4,;36.61,-3.17,;36.61,-4.71,;35.27,-5.48,;33.94,-4.71,;33.93,-.1,;35.27,-.86,;33.93,1.45,)| Show InChI InChI=1S/C80H98N18O14/c1-5-6-25-60(73(106)98-67(40-69(101)102)79(112)94-62(70(82)103)34-48-18-9-7-10-19-48)92-77(110)65(37-53-41-87-59-26-16-15-24-56(53)59)96-74(107)61(27-17-30-86-80(83)84)93-76(109)64(36-50-28-29-51-22-13-14-23-52(51)33-50)95-78(111)66(38-54-42-85-44-89-54)97-75(108)63(35-49-20-11-8-12-21-49)91-68(100)43-88-71(104)47(4)90-72(105)58(81)39-57-45(2)31-55(99)32-46(57)3/h7-16,18-24,26,28-29,31-33,41-42,44,47,58,60-67,87,99H,5-6,17,25,27,30,34-40,43,81H2,1-4H3,(H2,82,103)(H,85,89)(H,88,104)(H,90,105)(H,91,100)(H,92,110)(H,93,109)(H,94,112)(H,95,111)(H,96,107)(H,97,108)(H,98,106)(H,101,102)(H4,83,84,86)/t47-,58+,60+,61+,62+,63+,64-,65+,66+,67+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321606

((3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazo...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:8.20,58.71,93.99,78.84,wD:73.79,48.59,22.31,33.48,4.4,101.107,(24.59,-5.48,;25.93,-4.72,;25.93,-3.18,;24.59,-2.4,;24.59,-.86,;23.26,-.1,;21.93,-.87,;21.93,-2.41,;20.59,-.11,;20.59,1.44,;22,2.07,;23.25,1.19,;24.48,2.12,;23.98,3.58,;24.73,4.92,;23.94,6.24,;22.4,6.22,;21.65,4.87,;22.44,3.55,;19.25,-.87,;17.91,-.12,;17.91,1.43,;16.57,-.89,;16.57,-2.43,;17.91,-3.2,;17.91,-4.73,;19.24,-5.51,;19.24,-7.05,;17.93,-7.8,;20.61,-7.81,;15.25,-.1,;13.92,-.87,;13.92,-2.41,;12.59,-.1,;12.6,1.44,;13.92,2.21,;15.26,1.44,;16.59,2.21,;16.6,3.75,;17.93,4.53,;17.92,6.07,;16.58,6.84,;15.25,6.06,;15.26,4.52,;13.92,3.75,;11.26,-.86,;9.92,-.1,;9.92,1.45,;8.58,-.87,;8.58,-2.41,;9.92,-3.18,;11.32,-2.55,;12.36,-3.69,;11.59,-5.03,;10.08,-4.71,;7.25,-.09,;5.91,-.85,;5.91,-2.39,;4.58,-.08,;4.58,1.46,;5.91,2.23,;7.25,1.47,;8.58,2.24,;8.58,3.78,;7.24,4.55,;5.91,3.78,;3.25,-.85,;1.92,-.07,;1.92,1.47,;.58,-.85,;-.75,-.07,;-2.08,-.85,;-2.08,-2.38,;-3.41,-.07,;-3.41,1.47,;-4.75,-.84,;-6.09,-.08,;-6.09,1.47,;-7.42,-.86,;-7.4,-2.4,;-8.75,-.1,;-10.08,-.88,;-10.06,-2.42,;-8.72,-3.17,;-11.4,-3.2,;-12.73,-2.44,;-14.07,-3.22,;-12.76,-.9,;-11.41,-.13,;-11.43,1.41,;25.93,-.09,;25.93,1.45,;27.26,-.87,;28.59,-.09,;28.59,1.45,;29.93,2.21,;31.25,1.45,;29.93,3.76,;29.93,-.87,;29.93,-2.4,;31.26,-.1,;32.6,-.87,;32.6,-2.4,;33.93,-3.17,;35.28,-2.4,;36.61,-3.17,;36.61,-4.71,;35.27,-5.48,;33.94,-4.71,;33.93,-.1,;35.27,-.86,;33.93,1.45,)| Show InChI InChI=1S/C80H98N18O14/c1-5-6-25-60(73(106)98-67(40-69(101)102)79(112)94-62(70(82)103)34-48-18-9-7-10-19-48)92-77(110)65(37-53-41-87-59-26-16-15-24-56(53)59)96-74(107)61(27-17-30-86-80(83)84)93-76(109)64(36-50-28-29-51-22-13-14-23-52(51)33-50)95-78(111)66(38-54-42-85-44-89-54)97-75(108)63(35-49-20-11-8-12-21-49)91-68(100)43-88-71(104)47(4)90-72(105)58(81)39-57-45(2)31-55(99)32-46(57)3/h7-16,18-24,26,28-29,31-33,41-42,44,47,58,60-67,87,99H,5-6,17,25,27,30,34-40,43,81H2,1-4H3,(H2,82,103)(H,85,89)(H,88,104)(H,90,105)(H,91,100)(H,92,110)(H,93,109)(H,94,112)(H,95,111)(H,96,107)(H,97,108)(H,98,106)(H,101,102)(H4,83,84,86)/t47-,58+,60+,61+,62+,63+,64-,65+,66+,67+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415058
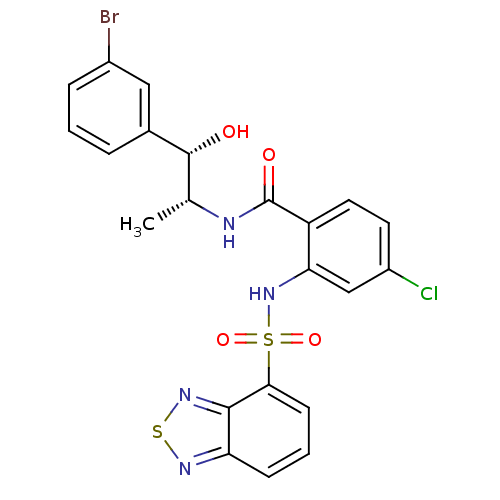
(CHEMBL571206)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12)[C@@H](O)c1cccc(Br)c1 |r| Show InChI InChI=1S/C22H18BrClN4O4S2/c1-12(21(29)13-4-2-5-14(23)10-13)25-22(30)16-9-8-15(24)11-18(16)28-34(31,32)19-7-3-6-17-20(19)27-33-26-17/h2-12,21,28-29H,1H3,(H,25,30)/t12-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728
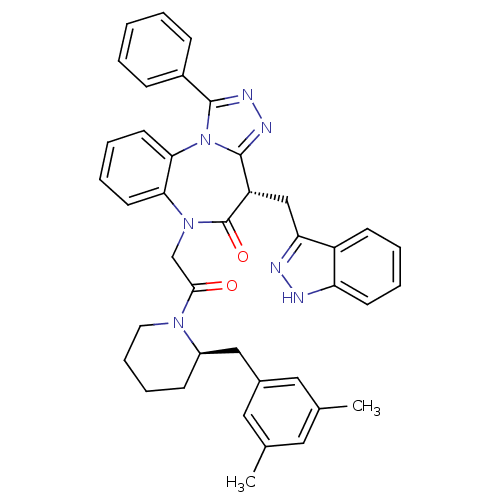
(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50056102
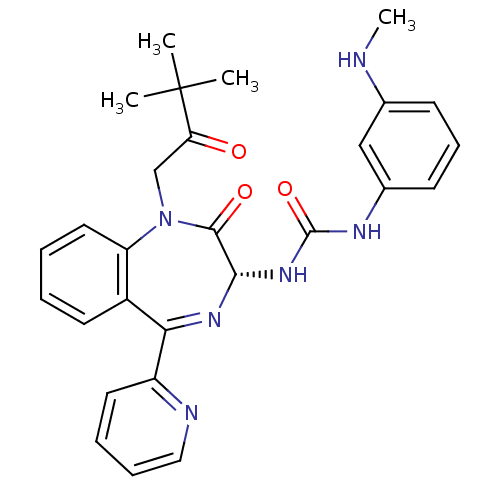
((R)-1-(1-(3,3-dimethyl-2-oxobutyl)-2-oxo-5-(pyridi...)Show SMILES CNc1cccc(NC(=O)N[C@@H]2N=C(c3ccccn3)c3ccccc3N(CC(=O)C(C)(C)C)C2=O)c1 |t:12| Show InChI InChI=1S/C28H30N6O3/c1-28(2,3)23(35)17-34-22-14-6-5-12-20(22)24(21-13-7-8-15-30-21)32-25(26(34)36)33-27(37)31-19-11-9-10-18(16-19)29-4/h5-16,25,29H,17H2,1-4H3,(H2,31,33,37)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [3H]L-364718 from human recombinant CCK1 receptor expressed in CHOK1 cells |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415052
(CHEMBL583343)Show SMILES Clc1ccc(cc1)C(CNC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C27H19Cl3N4O3S2/c28-18-8-4-16(5-9-18)22(17-6-10-19(29)11-7-17)15-31-27(35)21-13-12-20(30)14-24(21)34-39(36,37)25-3-1-2-23-26(25)33-38-32-23/h1-14,22,34H,15H2,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM21141
((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...)Show SMILES CCCC[C@H](N(C)C(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C45H57N9O10/c1-4-5-15-37(44(63)53-35(23-39(57)58)43(62)52-34(40(47)59)21-27-11-7-6-8-12-27)54(3)45(64)36(22-29-24-48-33-14-10-9-13-31(29)33)51-38(56)25-49-41(60)26(2)50-42(61)32(46)20-28-16-18-30(55)19-17-28/h6-14,16-19,24,26,32,34-37,48,55H,4-5,15,20-23,25,46H2,1-3H3,(H2,47,59)(H,49,60)(H,50,61)(H,51,56)(H,52,62)(H,53,63)(H,57,58)/t26-,32+,34+,35+,36-,37+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type A receptor (CCK-A) receptor was determined |
J Med Chem 46: 4215-31 (2003)
Article DOI: 10.1021/jm0303103
BindingDB Entry DOI: 10.7270/Q2JW8FM1 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50321597
((3S,6R,9S,12S,15S,18S)-3-((1H-imidazol-5-yl)methyl...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:33.48,22.31,4.4,48.59,93.100,wD:70.77,58.62,8.20,85.92,(16.65,-3.98,;15.32,-4.75,;15.32,-6.29,;13.98,-7.06,;13.98,-8.6,;12.65,-9.36,;11.32,-8.59,;11.32,-7.05,;9.99,-9.35,;9.99,-10.9,;11.32,-11.67,;12.72,-11.05,;13.76,-12.19,;12.98,-13.52,;13.46,-14.99,;12.42,-16.14,;10.92,-15.81,;10.44,-14.35,;11.48,-13.21,;8.66,-8.59,;7.33,-9.38,;7.33,-10.92,;6,-8.61,;6,-7.07,;7.33,-6.3,;7.33,-4.76,;8.66,-3.97,;8.68,-2.43,;7.33,-1.66,;10,-1.66,;4.65,-9.35,;3.32,-8.59,;3.32,-7.05,;1.99,-9.36,;1.76,-10.89,;2.96,-11.84,;2.73,-13.37,;3.92,-14.33,;5.36,-13.77,;6.57,-14.74,;8.01,-14.17,;8.23,-12.64,;7.03,-11.68,;5.59,-12.24,;4.39,-11.29,;.66,-8.58,;-.66,-9.38,;-.66,-10.92,;-2,-8.61,;-2,-7.07,;-.66,-6.29,;.75,-6.93,;1.79,-5.78,;1.01,-4.45,;-.5,-4.76,;-3.33,-9.38,;-4.67,-8.61,;-4.67,-7.07,;-6,-9.38,;-7.33,-8.62,;-8.66,-9.39,;-9.99,-8.62,;-11.33,-9.39,;-11.33,-10.93,;-9.99,-11.69,;-8.66,-10.92,;-7.33,-11.7,;-6,-10.91,;-4.66,-11.68,;-3.33,-10.91,;-4.66,-13.22,;-5.99,-13.99,;-3.32,-13.99,;-3.32,-15.53,;-4.65,-16.3,;-5.98,-15.53,;-4.65,-17.84,;-3.31,-18.61,;-3.31,-20.15,;-1.98,-17.84,;-1.98,-16.29,;-.65,-15.52,;15.32,-9.37,;15.32,-10.91,;16.65,-8.59,;17.98,-9.36,;17.98,-10.91,;19.32,-11.67,;20.64,-10.9,;19.32,-13.21,;19.32,-8.59,;19.32,-7.05,;20.65,-9.36,;21.98,-8.59,;21.98,-7.05,;23.31,-6.28,;24.65,-7.06,;25.98,-6.29,;25.99,-4.75,;24.65,-3.98,;23.32,-4.75,;23.31,-9.36,;24.65,-8.59,;23.31,-10.91,)| Show InChI InChI=1S/C76H90N16O12/c1-4-5-23-58(68(97)90-64(38-66(94)95)73(102)87-60(67(78)96)32-45-16-7-6-8-17-45)85-71(100)62(34-51-39-83-57-24-14-13-22-54(51)57)89-69(98)59(25-15-28-82-76(79)80)86-70(99)61(33-46-26-27-47-18-9-10-19-48(47)31-46)88-72(101)63(36-52-40-81-42-84-52)91-74(103)65-35-49-20-11-12-21-50(49)41-92(65)75(104)56(77)37-55-43(2)29-53(93)30-44(55)3/h6-14,16-22,24,26-27,29-31,39-40,42,56,58-65,83,93H,4-5,15,23,25,28,32-38,41,77H2,1-3H3,(H2,78,96)(H,81,84)(H,85,100)(H,86,99)(H,87,102)(H,88,101)(H,89,98)(H,90,97)(H,91,103)(H,94,95)(H4,79,80,82)/t56-,58-,59-,60-,61+,62-,63-,64-,65+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8(SO3) from human CCK1 receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4080-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.078
BindingDB Entry DOI: 10.7270/Q2D79CCN |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50214809
(CHEMBL421575)Show SMILES Fc1ccc(NC(=O)NC2C(=O)N(CC(=O)N3CCCC3)c3ccccc3N(c3ccccc3F)C2=O)cc1 Show InChI InChI=1S/C28H25F2N5O4/c29-18-11-13-19(14-12-18)31-28(39)32-25-26(37)34(17-24(36)33-15-5-6-16-33)22-9-3-4-10-23(22)35(27(25)38)21-8-2-1-7-20(21)30/h1-4,7-14,25H,5-6,15-17H2,(H2,31,32,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tested for its selectivity against human Cholecystokinin type A receptor isolated from a human gallbladder cDNA library and stably transfected into a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PZ5C0X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415072
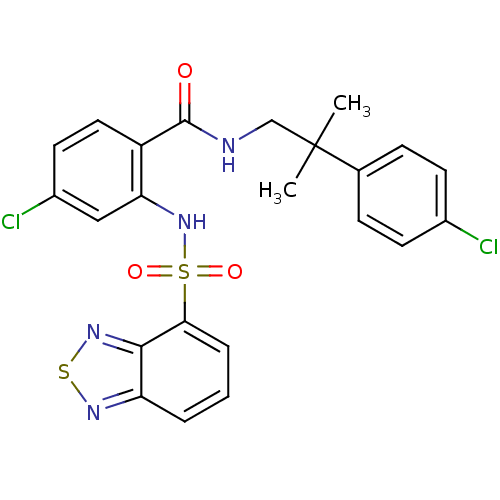
(CHEMBL565511)Show SMILES CC(C)(CNC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H20Cl2N4O3S2/c1-23(2,14-6-8-15(24)9-7-14)13-26-22(30)17-11-10-16(25)12-19(17)29-34(31,32)20-5-3-4-18-21(20)28-33-27-18/h3-12,29H,13H2,1-2H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50185262

((+/-)-2-(2-(1H-indole-2-carboxamido)benzamido)-3-p...)Show SMILES OC(=O)C(Cc1ccccc1)NC(=O)c1ccccc1NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C25H21N3O4/c29-23(28-22(25(31)32)14-16-8-2-1-3-9-16)18-11-5-7-13-20(18)27-24(30)21-15-17-10-4-6-12-19(17)26-21/h1-13,15,22,26H,14H2,(H,27,30)(H,28,29)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste
Curated by ChEMBL
| Assay Description
Displacement of [125I]BH-(Thr,-Nle)-CCK-9 from human CCK1 receptor expressed in COS-7 cells after 60 mins by gamma counting |
J Med Chem 54: 5769-85 (2011)
Article DOI: 10.1021/jm200438b
BindingDB Entry DOI: 10.7270/Q2BP036J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415074
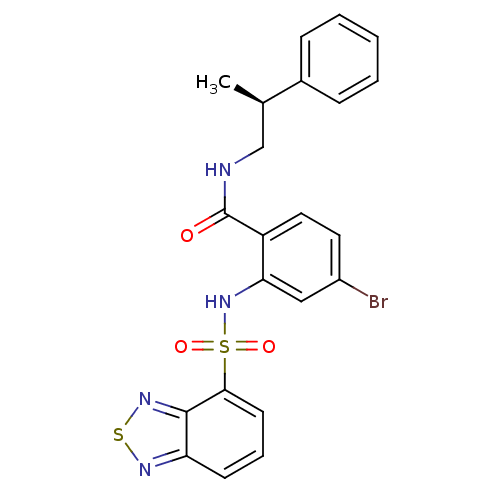
(CHEMBL566604)Show SMILES C[C@H](CNC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccccc1 |r| Show InChI InChI=1S/C22H19BrN4O3S2/c1-14(15-6-3-2-4-7-15)13-24-22(28)17-11-10-16(23)12-19(17)27-32(29,30)20-9-5-8-18-21(20)26-31-25-18/h2-12,14,27H,13H2,1H3,(H,24,28)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50214808
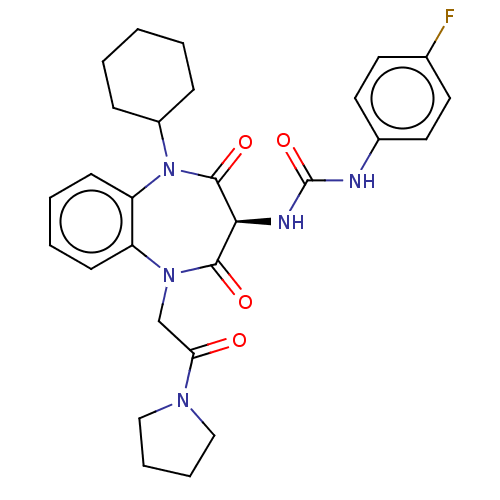
(CHEMBL320141 | GR-199114X)Show SMILES Fc1ccc(NC(=O)N[C@H]2C(=O)N(CC(=O)N3CCCC3)c3ccccc3N(C3CCCCC3)C2=O)cc1 Show InChI InChI=1S/C28H32FN5O4/c29-19-12-14-20(15-13-19)30-28(38)31-25-26(36)33(18-24(35)32-16-6-7-17-32)22-10-4-5-11-23(22)34(27(25)37)21-8-2-1-3-9-21/h4-5,10-15,21,25H,1-3,6-9,16-18H2,(H2,30,31,38)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of specific binding of [125I]-ET-1 (endothelin 1) to the endothelin A receptor of rat aorta membranes |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PZ5C0X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50214810
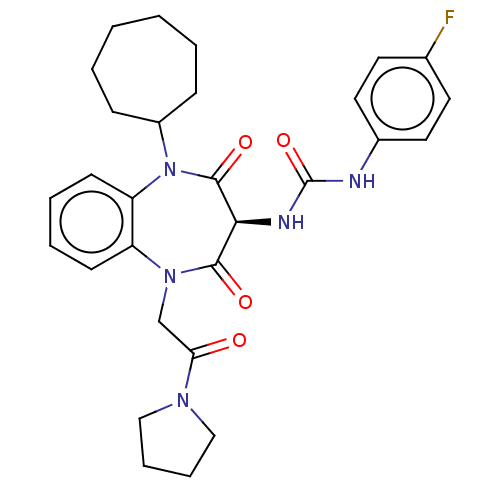
(CHEMBL95200)Show SMILES Fc1ccc(NC(=O)N[C@H]2C(=O)N(CC(=O)N3CCCC3)c3ccccc3N(C3CCCCCC3)C2=O)cc1 Show InChI InChI=1S/C29H34FN5O4/c30-20-13-15-21(16-14-20)31-29(39)32-26-27(37)34(19-25(36)33-17-7-8-18-33)23-11-5-6-12-24(23)35(28(26)38)22-9-3-1-2-4-10-22/h5-6,11-16,22,26H,1-4,7-10,17-19H2,(H2,31,32,39)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Tested for its selectivity against human Cholecystokinin type A receptor isolated from a human gallbladder cDNA library and stably transfected into a... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PZ5C0X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415051
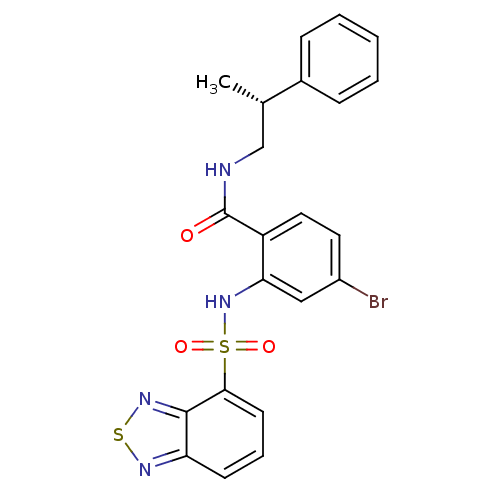
(CHEMBL584063)Show SMILES C[C@@H](CNC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccccc1 |r| Show InChI InChI=1S/C22H19BrN4O3S2/c1-14(15-6-3-2-4-7-15)13-24-22(28)17-11-10-16(23)12-19(17)27-32(29,30)20-9-5-8-18-21(20)26-31-25-18/h2-12,14,27H,13H2,1H3,(H,24,28)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415051

(CHEMBL584063)Show SMILES C[C@@H](CNC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccccc1 |r| Show InChI InChI=1S/C22H19BrN4O3S2/c1-14(15-6-3-2-4-7-15)13-24-22(28)17-11-10-16(23)12-19(17)27-32(29,30)20-9-5-8-18-21(20)26-31-25-18/h2-12,14,27H,13H2,1H3,(H,24,28)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415061
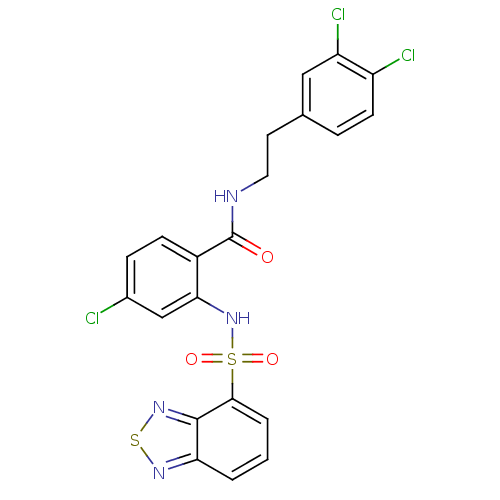
(CHEMBL570521)Show SMILES Clc1ccc(C(=O)NCCc2ccc(Cl)c(Cl)c2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C21H15Cl3N4O3S2/c22-13-5-6-14(21(29)25-9-8-12-4-7-15(23)16(24)10-12)18(11-13)28-33(30,31)19-3-1-2-17-20(19)27-32-26-17/h1-7,10-11,28H,8-9H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415067
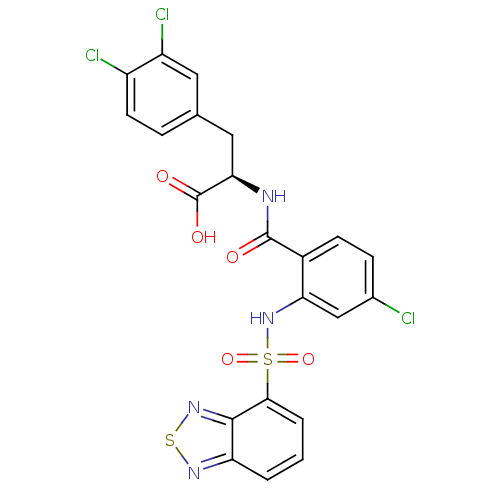
(CHEMBL566359)Show SMILES OC(=O)[C@@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Cl3N4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-14(24)15(25)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM21137
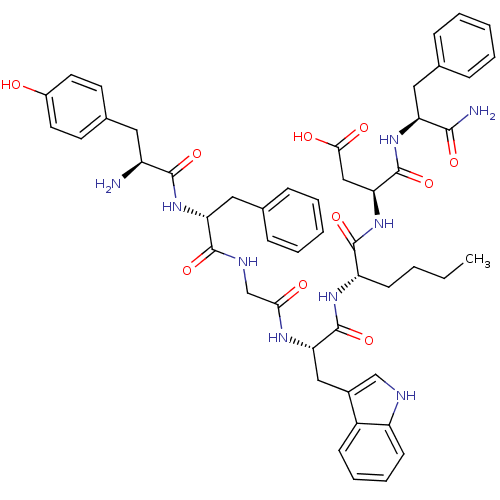
((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H59N9O10/c1-2-3-17-38(48(67)59-42(27-44(62)63)50(69)57-39(45(52)64)24-30-12-6-4-7-13-30)56-49(68)41(26-33-28-53-37-18-11-10-16-35(33)37)55-43(61)29-54-47(66)40(25-31-14-8-5-9-15-31)58-46(65)36(51)23-32-19-21-34(60)22-20-32/h4-16,18-22,28,36,38-42,53,60H,2-3,17,23-27,29,51H2,1H3,(H2,52,64)(H,54,66)(H,55,61)(H,56,68)(H,57,69)(H,58,65)(H,59,67)(H,62,63)/t36-,38-,39-,40+,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 49: 2868-75 (2006)
Article DOI: 10.1021/jm050921q
BindingDB Entry DOI: 10.7270/Q24Q7S99 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415071
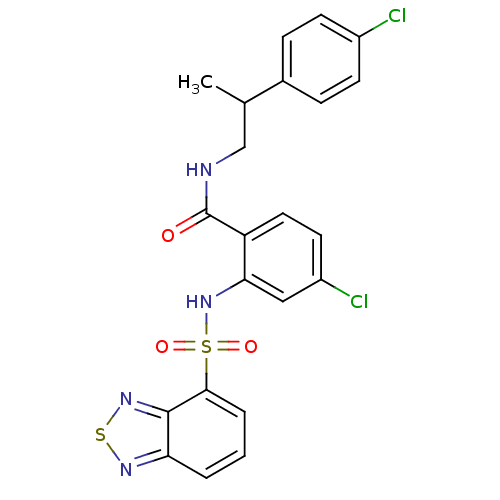
(CHEMBL566360)Show SMILES CC(CNC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H18Cl2N4O3S2/c1-13(14-5-7-15(23)8-6-14)12-25-22(29)17-10-9-16(24)11-19(17)28-33(30,31)20-4-2-3-18-21(20)27-32-26-18/h2-11,13,28H,12H2,1H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415077
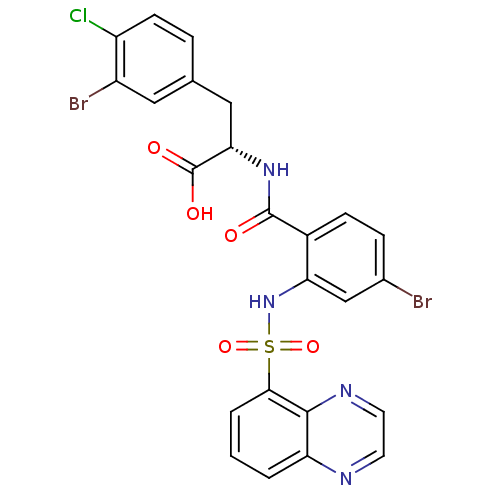
(CHEMBL584525)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H17Br2ClN4O5S/c25-14-5-6-15(23(32)30-20(24(33)34)11-13-4-7-17(27)16(26)10-13)19(12-14)31-37(35,36)21-3-1-2-18-22(21)29-9-8-28-18/h1-10,12,20,31H,11H2,(H,30,32)(H,33,34)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415085
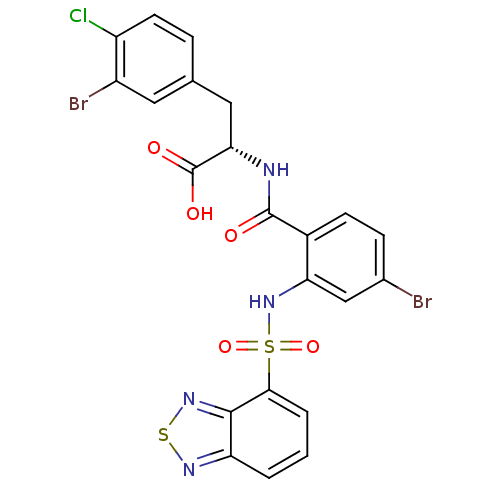
(CHEMBL570520)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Br2ClN4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-15(25)14(24)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50202107
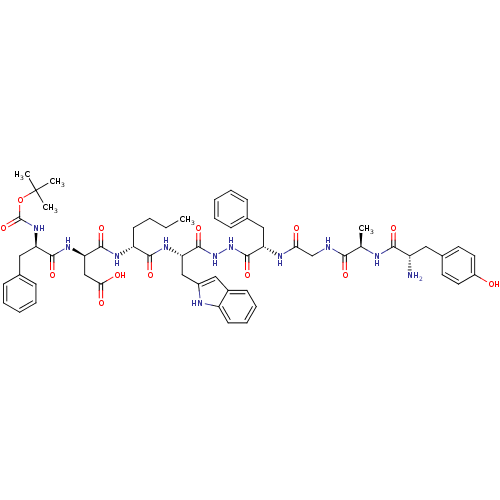
(CHEMBL218600 | H-Tyr-D-Ala-Gly-Phe-NH-NH-Trp-D-Nle...)Show SMILES CCCC[C@@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](Cc1cc2ccccc2[nH]1)C(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C58H73N11O13/c1-6-7-21-43(64-54(78)47(32-49(72)73)66-53(77)44(28-35-16-10-8-11-17-35)67-57(81)82-58(3,4)5)52(76)65-46(31-39-30-38-20-14-15-22-42(38)62-39)56(80)69-68-55(79)45(29-36-18-12-9-13-19-36)63-48(71)33-60-50(74)34(2)61-51(75)41(59)27-37-23-25-40(70)26-24-37/h8-20,22-26,30,34,41,43-47,62,70H,6-7,21,27-29,31-33,59H2,1-5H3,(H,60,74)(H,61,75)(H,63,71)(H,64,78)(H,65,76)(H,66,77)(H,67,81)(H,68,79)(H,69,80)(H,72,73)/t34-,41+,43-,44-,45+,46+,47-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cells |
J Med Chem 50: 165-8 (2007)
Article DOI: 10.1021/jm061268p
BindingDB Entry DOI: 10.7270/Q2TD9X17 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50202107

(CHEMBL218600 | H-Tyr-D-Ala-Gly-Phe-NH-NH-Trp-D-Nle...)Show SMILES CCCC[C@@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](Cc1cc2ccccc2[nH]1)C(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C58H73N11O13/c1-6-7-21-43(64-54(78)47(32-49(72)73)66-53(77)44(28-35-16-10-8-11-17-35)67-57(81)82-58(3,4)5)52(76)65-46(31-39-30-38-20-14-15-22-42(38)62-39)56(80)69-68-55(79)45(29-36-18-12-9-13-19-36)63-48(71)33-60-50(74)34(2)61-51(75)41(59)27-37-23-25-40(70)26-24-37/h8-20,22-26,30,34,41,43-47,62,70H,6-7,21,27-29,31-33,59H2,1-5H3,(H,60,74)(H,61,75)(H,63,71)(H,64,78)(H,65,76)(H,66,77)(H,67,81)(H,68,79)(H,69,80)(H,72,73)/t34-,41+,43-,44-,45+,46+,47-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cells |
J Med Chem 50: 165-8 (2007)
Article DOI: 10.1021/jm061268p
BindingDB Entry DOI: 10.7270/Q2TD9X17 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415084
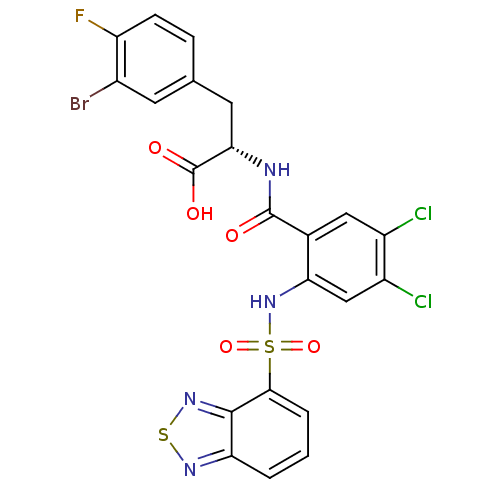
(CHEMBL565324)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1cc(Cl)c(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H14BrCl2FN4O5S2/c23-12-6-10(4-5-15(12)26)7-18(22(32)33)27-21(31)11-8-13(24)14(25)9-17(11)30-37(34,35)19-3-1-2-16-20(19)29-36-28-16/h1-6,8-9,18,30H,7H2,(H,27,31)(H,32,33)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415083

(CHEMBL566574)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1cc(Cl)c(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H16BrCl2FN4O5S/c25-14-8-12(4-5-17(14)28)9-20(24(34)35)31-23(33)13-10-15(26)16(27)11-19(13)32-38(36,37)21-3-1-2-18-22(21)30-7-6-29-18/h1-8,10-11,20,32H,9H2,(H,31,33)(H,34,35)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415082

(CHEMBL583035)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(I)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15BrFIN4O5S2/c23-14-8-11(4-7-15(14)24)9-18(22(31)32)26-21(30)13-6-5-12(25)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415059
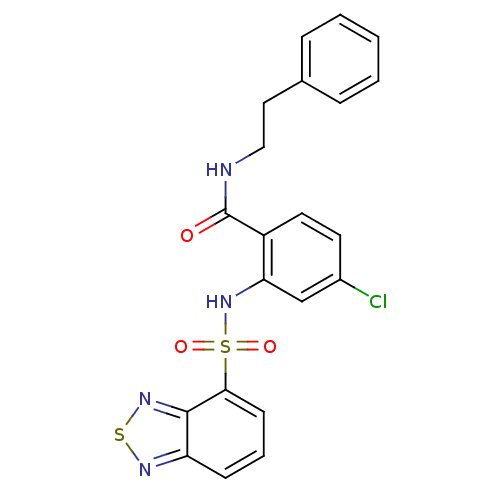
(CHEMBL569616)Show SMILES Clc1ccc(C(=O)NCCc2ccccc2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C21H17ClN4O3S2/c22-15-9-10-16(21(27)23-12-11-14-5-2-1-3-6-14)18(13-15)26-31(28,29)19-8-4-7-17-20(19)25-30-24-17/h1-10,13,26H,11-12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-1] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50413477

(CHEMBL475226)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(I)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C23H19IN4O2/c1-28-19-13-6-5-12-18(19)20(15-8-3-2-4-9-15)26-21(22(28)29)27-23(30)25-17-11-7-10-16(24)14-17/h2-14,21H,1H3,(H2,25,27,30)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I](S)-1-(3-iodophenyl)-3-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)urea from human CCK1 receptor express... |
J Med Chem 52: 2138-47 (2009)
Article DOI: 10.1021/jm801439x
BindingDB Entry DOI: 10.7270/Q22B908P |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415076
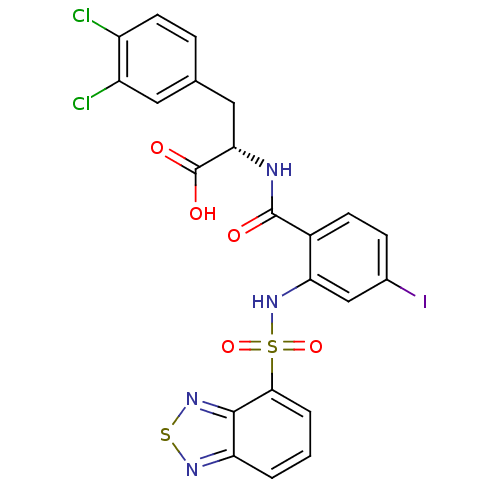
(CHEMBL583344)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)c1ccc(I)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Cl2IN4O5S2/c23-14-7-4-11(8-15(14)24)9-18(22(31)32)26-21(30)13-6-5-12(25)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415075
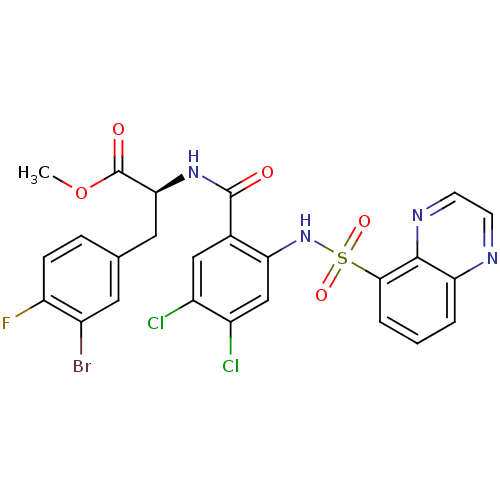
(CHEMBL566177)Show SMILES COC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1cc(Cl)c(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C25H18BrCl2FN4O5S/c1-38-25(35)21(10-13-5-6-18(29)15(26)9-13)32-24(34)14-11-16(27)17(28)12-20(14)33-39(36,37)22-4-2-3-19-23(22)31-8-7-30-19/h2-9,11-12,21,33H,10H2,1H3,(H,32,34)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415068
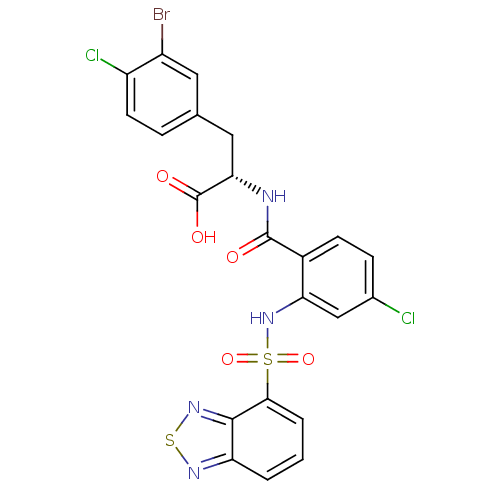
(CHEMBL569470)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15BrCl2N4O5S2/c23-14-8-11(4-7-15(14)25)9-18(22(31)32)26-21(30)13-6-5-12(24)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from CCK1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6373-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.064
BindingDB Entry DOI: 10.7270/Q2222W1W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data