 Found 68 hits of kd data for polymerid = 2135
Found 68 hits of kd data for polymerid = 2135 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50053929
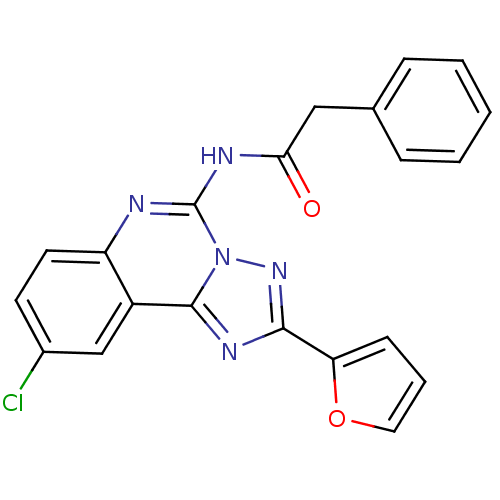
(CHEMBL88147 | N-(9-Chloro-2-furan-2-yl-[1,2,4]tria...)Show SMILES Clc1ccc2nc(NC(=O)Cc3ccccc3)n3nc(nc3c2c1)-c1ccco1 Show InChI InChI=1S/C21H14ClN5O2/c22-14-8-9-16-15(12-14)20-25-19(17-7-4-10-29-17)26-27(20)21(23-16)24-18(28)11-13-5-2-1-3-6-13/h1-10,12H,11H2,(H,23,24,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.0776 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50053929

(CHEMBL88147 | N-(9-Chloro-2-furan-2-yl-[1,2,4]tria...)Show SMILES Clc1ccc2nc(NC(=O)Cc3ccccc3)n3nc(nc3c2c1)-c1ccco1 Show InChI InChI=1S/C21H14ClN5O2/c22-14-8-9-16-15(12-14)20-25-19(17-7-4-10-29-17)26-27(20)21(23-16)24-18(28)11-13-5-2-1-3-6-13/h1-10,12H,11H2,(H,23,24,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.0977 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50397727
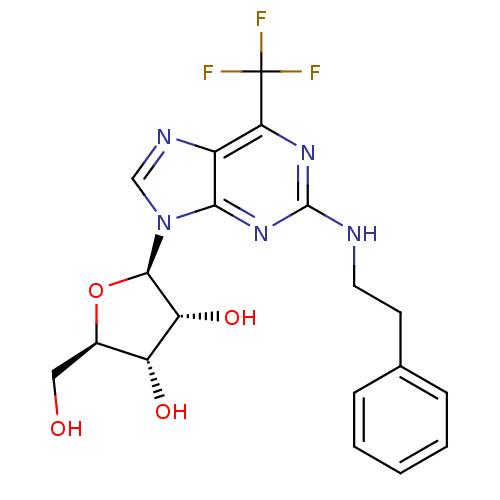
(CHEMBL2181968)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(nc(NCCc3ccccc3)nc12)C(F)(F)F |r| Show InChI InChI=1S/C19H20F3N5O4/c20-19(21,22)15-12-16(26-18(25-15)23-7-6-10-4-2-1-3-5-10)27(9-24-12)17-14(30)13(29)11(8-28)31-17/h1-5,9,11,13-14,17,28-30H,6-8H2,(H,23,25,26)/t11-,13-,14-,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant adenosine A3 receptor expressed in HEK293 cells |
J Med Chem 55: 5676-703 (2012)
Article DOI: 10.1021/jm300087j
BindingDB Entry DOI: 10.7270/Q25H7HDK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50423868
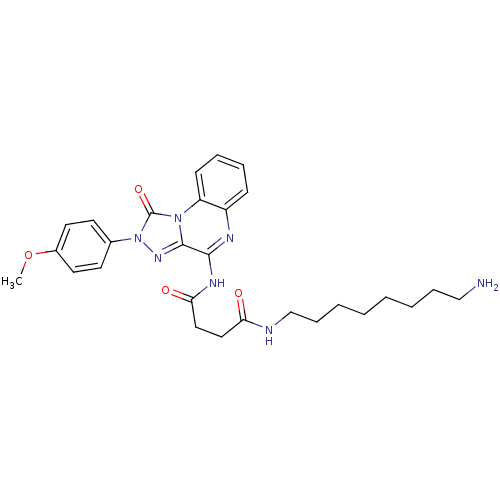
(CHEMBL2023738)Show SMILES COc1ccc(cc1)-n1nc2c(NC(=O)CCC(=O)NCCCCCCCCN)nc3ccccc3n2c1=O Show InChI InChI=1S/C28H35N7O4/c1-39-21-14-12-20(13-15-21)35-28(38)34-23-11-7-6-10-22(23)31-26(27(34)33-35)32-25(37)17-16-24(36)30-19-9-5-3-2-4-8-18-29/h6-7,10-15H,2-5,8-9,16-19,29H2,1H3,(H,30,36)(H,31,32,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.331 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor expressed in forskolin-stimulated CHO cells assessed as inhibition of NECA-induced CRE-SPAP gene t... |
J Med Chem 55: 1771-82 (2012)
Article DOI: 10.1021/jm201722y
BindingDB Entry DOI: 10.7270/Q2ST7R4K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50423871
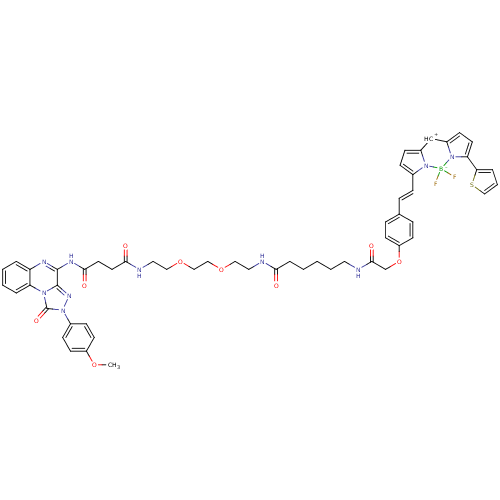
(CHEMBL2024149)Show SMILES COc1ccc(cc1)-n1nc2c(NC(=O)CCC(=O)NCCOCCOCCNC(=O)CCCCCNC(=O)COc3ccc([CH+]\C=c4\ccc5=Cc6ccc(-c7cccs7)n6[B-](F)(F)n45)cc3)nc3ccccc3n2c1=O |t:51| Show InChI InChI=1S/C55H57BF2N10O9S/c1-74-43-23-18-40(19-24-43)68-55(73)65-46-9-5-4-8-45(46)62-53(54(65)64-68)63-51(71)27-26-50(70)61-30-32-76-34-33-75-31-29-60-49(69)11-3-2-6-28-59-52(72)37-77-44-21-13-38(14-22-44)12-15-39-16-17-41-36-42-20-25-47(48-10-7-35-78-48)67(42)56(57,58)66(39)41/h4-5,7-10,12-25,35-36H,2-3,6,11,26-34,37H2,1H3,(H,59,72)(H,60,69)(H,61,70)(H,62,63,71)/b39-15- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.437 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor expressed in forskolin-stimulated CHO cells assessed as inhibition of NECA-induced CRE-SPAP gene t... |
J Med Chem 55: 1771-82 (2012)
Article DOI: 10.1021/jm201722y
BindingDB Entry DOI: 10.7270/Q2ST7R4K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591172
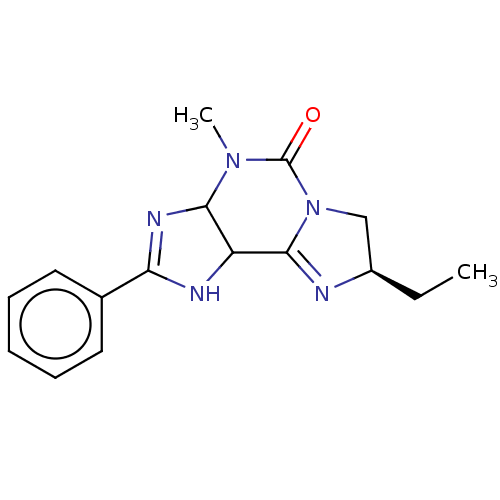
(CHEMBL5178790)Show SMILES CC[C@@H]1CN2C(=N1)C1NC(=NC1N(C)C2=O)c1ccccc1 |r,c:5,10| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50397728
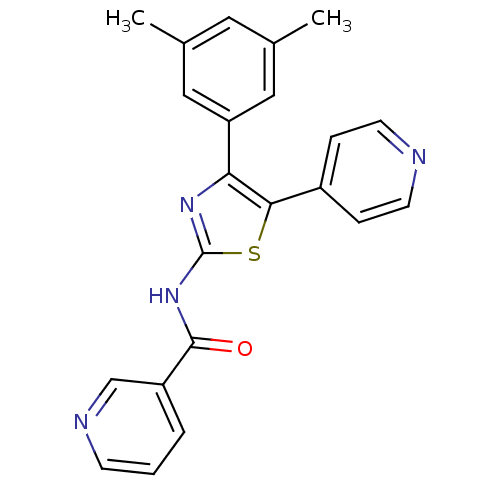
(CHEMBL2181976)Show SMILES Cc1cc(C)cc(c1)-c1nc(NC(=O)c2cccnc2)sc1-c1ccncc1 Show InChI InChI=1S/C22H18N4OS/c1-14-10-15(2)12-18(11-14)19-20(16-5-8-23-9-6-16)28-22(25-19)26-21(27)17-4-3-7-24-13-17/h3-13H,1-2H3,(H,25,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human adenosine A3 receptor expressed in HEK293 cells measured by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00685
BindingDB Entry DOI: 10.7270/Q2930Z2X |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM85618
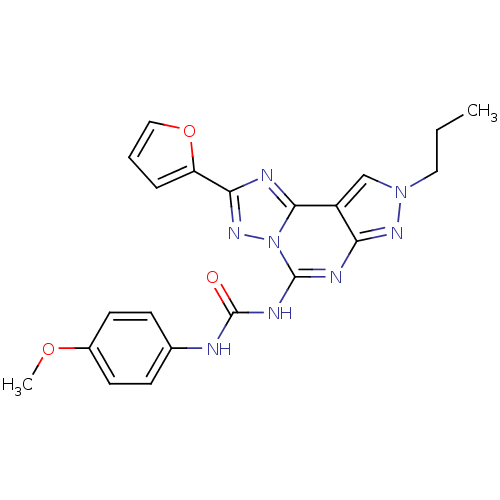
(CHEMBL302765 | J1.251.181G | MRE 3008F20)Show SMILES CCCn1cc2c(n1)nc(NC(=O)Nc1ccc(OC)cc1)n1nc(nc21)-c1ccco1 Show InChI InChI=1S/C21H20N8O3/c1-3-10-28-12-15-17(26-28)24-20(25-21(30)22-13-6-8-14(31-2)9-7-13)29-19(15)23-18(27-29)16-5-4-11-32-16/h4-9,11-12H,3,10H2,1-2H3,(H2,22,24,25,26,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50085371
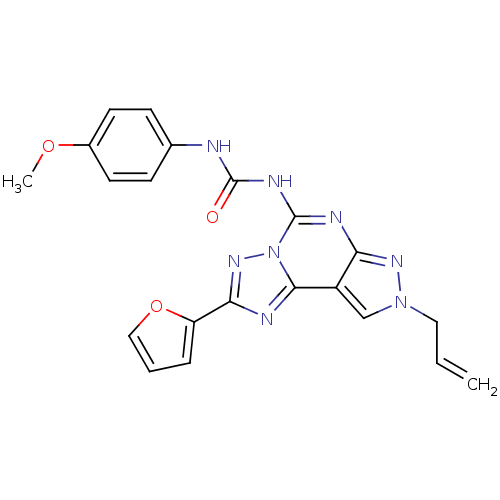
(1-(8-Allyl-2-furan-2-yl-8H-pyrazolo[4,3-e][1,2,4]t...)Show SMILES COc1ccc(NC(=O)Nc2nc3nn(CC=C)cc3c3nc(nn23)-c2ccco2)cc1 Show InChI InChI=1S/C21H18N8O3/c1-3-10-28-12-15-17(26-28)24-20(25-21(30)22-13-6-8-14(31-2)9-7-13)29-19(15)23-18(27-29)16-5-4-11-32-16/h3-9,11-12H,1,10H2,2H3,(H2,22,24,25,26,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a |
Università di Ferrara
Curated by ChEMBL
| Assay Description
Binding affinity against human Adenosine A3 receptor in CHO cells |
Bioorg Med Chem Lett 10: 209-11 (2000)
BindingDB Entry DOI: 10.7270/Q2QV3KQB |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM214689
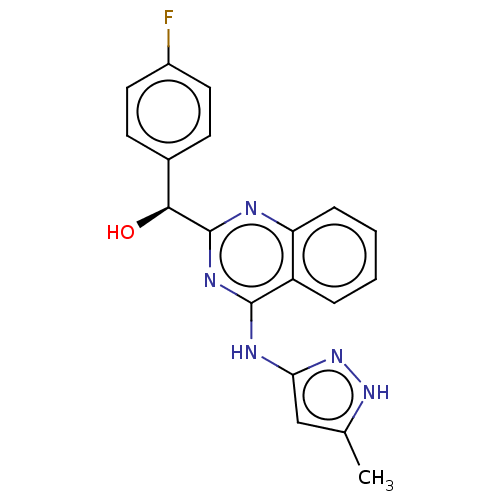
(US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...)Show SMILES Cc1cc(Nc2nc(nc3ccccc23)[C@@H](O)c2ccc(F)cc2)n[nH]1 Show InChI InChI=1S/C19H16FN5O/c1-11-10-16(25-24-11)22-18-14-4-2-3-5-15(14)21-19(23-18)17(26)12-6-8-13(20)9-7-12/h2-10,17,26H,1H3,(H2,21,22,23,24,25)/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 1.42 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Corporation
US Patent
| Assay Description
.(R)- and (S)-(4-Fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol was tested in a radioligand binding assay, according to th... |
US Patent US9295672 (2016)
BindingDB Entry DOI: 10.7270/Q2ZP44ZX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50423869
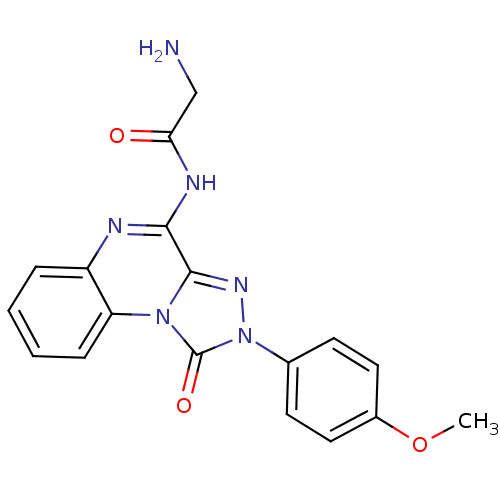
(CHEMBL2023739)Show SMILES COc1ccc(cc1)-n1nc2c(NC(=O)CN)nc3ccccc3n2c1=O Show InChI InChI=1S/C18H16N6O3/c1-27-12-8-6-11(7-9-12)24-18(26)23-14-5-3-2-4-13(14)20-16(17(23)22-24)21-15(25)10-19/h2-9H,10,19H2,1H3,(H,20,21,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor expressed in forskolin-stimulated CHO cells assessed as inhibition of NECA-induced CRE-SPAP gene t... |
J Med Chem 55: 1771-82 (2012)
Article DOI: 10.1021/jm201722y
BindingDB Entry DOI: 10.7270/Q2ST7R4K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50423872
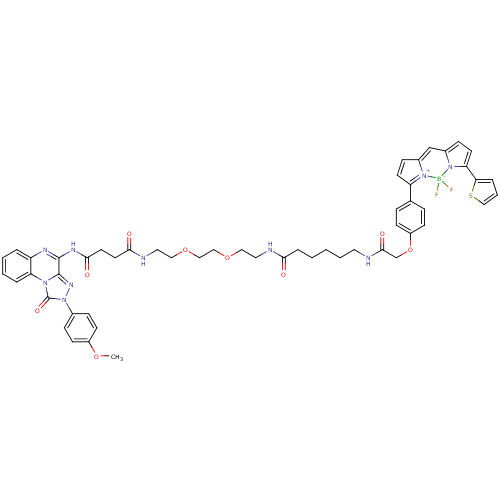
(CHEMBL2024150)Show SMILES COc1ccc(cc1)-n1nc2c(NC(=O)CCC(=O)NCCOCCOCCNC(=O)CCCCCNC(=O)COc3ccc(cc3)C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)nc3ccccc3n2c1=O |c:52,54,t:49| Show InChI InChI=1S/C53H55BF2N10O9S/c1-72-40-20-14-37(15-21-40)66-53(71)63-44-9-5-4-8-42(44)60-51(52(63)62-66)61-49(69)25-24-48(68)59-28-30-74-32-31-73-29-27-58-47(67)11-3-2-6-26-57-50(70)35-75-41-18-12-36(13-19-41)43-22-16-38-34-39-17-23-45(46-10-7-33-76-46)65(39)54(55,56)64(38)43/h4-5,7-10,12-23,33-34H,2-3,6,11,24-32,35H2,1H3,(H,57,70)(H,58,67)(H,59,68)(H,60,61,69) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor expressed in forskolin-stimulated CHO cells assessed as inhibition of NECA-induced CRE-SPAP gene t... |
J Med Chem 55: 1771-82 (2012)
Article DOI: 10.1021/jm201722y
BindingDB Entry DOI: 10.7270/Q2ST7R4K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50348175
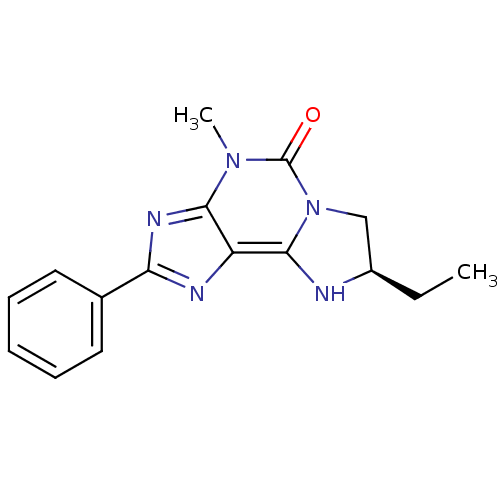
(CHEMBL1800865)Show SMILES CC[C@@H]1Cn2c(N1)c1nc(nc1n(C)c2=O)-c1ccccc1 |r| Show InChI InChI=1S/C16H17N5O/c1-3-11-9-21-15(17-11)12-14(20(2)16(21)22)19-13(18-12)10-7-5-4-6-8-10/h4-8,11,17H,3,9H2,1-2H3/t11-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant A3 adenosine receptor expressed in CHO cells |
J Med Chem 54: 5205-20 (2011)
Article DOI: 10.1021/jm2004738
BindingDB Entry DOI: 10.7270/Q21N81G6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50150766
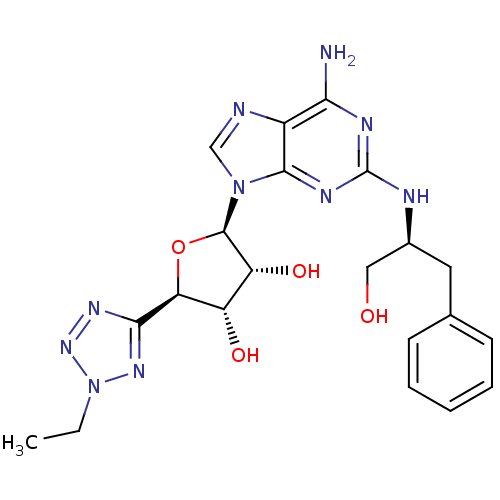
((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@H](CO)Cc3ccccc3)nc12 Show InChI InChI=1S/C21H26N10O4/c1-2-31-28-18(27-29-31)16-14(33)15(34)20(35-16)30-10-23-13-17(22)25-21(26-19(13)30)24-12(9-32)8-11-6-4-3-5-7-11/h3-7,10,12,14-16,20,32-34H,2,8-9H2,1H3,(H3,22,24,25,26)/t12-,14-,15+,16-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor expressed in CHO cells assessed as inhibition of Cl-IB-MECA-mediated cyclic AMP production |
J Med Chem 55: 5676-703 (2012)
Article DOI: 10.1021/jm300087j
BindingDB Entry DOI: 10.7270/Q25H7HDK |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50423874
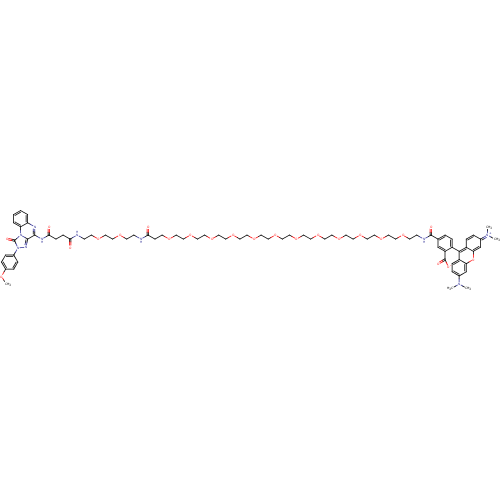
(CHEMBL2024152)Show SMILES COc1ccc(cc1)-n1nc2c(NC(=O)CCC(=O)NCCOCCOCCNC(=O)CCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCNC(=O)c3ccc(c(c3)C([O-])=O)-c3c4ccc(cc4oc4cc(ccc34)=[N+](C)C)N(C)C)nc3ccccc3n2c1=O |(5.15,-2.54,;4.37,-1.21,;2.83,-1.22,;2.07,-2.56,;.53,-2.57,;-.24,-1.24,;.51,.1,;2.05,.11,;-1.78,-1.25,;-2.69,-.01,;-4.15,-.5,;-5.49,.27,;-5.5,1.81,;-4.17,2.58,;-4.17,4.12,;-2.83,1.82,;-1.5,2.6,;-.16,1.83,;-.15,.3,;1.17,2.61,;2.51,1.85,;3.84,2.63,;5.17,1.86,;6.5,2.64,;7.84,1.88,;9.17,2.65,;10.51,1.89,;11.84,2.67,;13.18,1.9,;14.51,2.68,;14.5,4.22,;15.84,1.92,;17.17,2.69,;18.51,1.93,;19.84,2.71,;21.18,1.95,;22.51,2.72,;23.84,1.96,;25.17,2.74,;26.51,1.97,;27.84,2.75,;29.18,1.99,;30.51,2.76,;31.85,2,;33.18,2.78,;34.51,2.01,;35.84,2.79,;37.18,2.03,;38.51,2.8,;39.85,2.04,;41.18,2.82,;42.52,2.06,;43.85,2.83,;45.18,2.07,;46.51,2.85,;47.85,2.08,;49.18,2.86,;50.52,2.1,;51.85,2.87,;53.19,2.11,;54.52,2.89,;55.85,2.12,;57.18,2.9,;58.52,2.14,;59.85,2.91,;61.19,2.15,;62.52,2.93,;63.85,2.17,;65.18,2.94,;66.52,2.18,;67.85,2.96,;67.84,4.5,;69.19,2.19,;69.19,.65,;70.53,-.11,;71.86,.67,;71.85,2.21,;70.51,2.97,;71.84,3.76,;73.16,4.54,;70.5,4.52,;73.2,-.1,;73.21,-1.63,;71.89,-2.4,;71.9,-3.93,;73.23,-4.69,;74.54,-3.92,;74.53,-2.4,;75.87,-1.62,;75.86,-.08,;77.19,.69,;77.19,2.24,;75.84,3,;74.51,2.22,;74.52,.69,;78.52,3.01,;79.86,2.25,;78.51,4.55,;73.24,-6.23,;71.91,-7.01,;74.58,-6.99,;-6.81,-.5,;-6.8,-2.03,;-8.13,-2.8,;-8.13,-4.34,;-6.8,-5.12,;-5.46,-4.34,;-5.47,-2.8,;-4.14,-2.03,;-2.68,-2.5,;-2.19,-3.96,)| Show InChI InChI=1S/C78H104N10O23/c1-85(2)59-13-18-63-68(55-59)111-69-56-60(86(3)4)14-19-64(69)73(63)62-17-10-57(54-65(62)77(93)94)76(92)81-25-29-100-33-35-102-37-39-104-41-43-106-45-47-108-49-51-110-53-52-109-50-48-107-46-44-105-42-40-103-38-36-101-34-30-97-26-22-71(90)80-24-28-99-32-31-98-27-23-79-70(89)20-21-72(91)83-74-75-84-88(58-11-15-61(96-5)16-12-58)78(95)87(75)67-9-7-6-8-66(67)82-74/h6-19,54-56H,20-53H2,1-5H3,(H4-,79,80,81,82,83,89,90,91,92,93,94) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor expressed in forskolin-stimulated CHO cells assessed as inhibition of NECA-induced CRE-SPAP gene t... |
J Med Chem 55: 1771-82 (2012)
Article DOI: 10.1021/jm201722y
BindingDB Entry DOI: 10.7270/Q2ST7R4K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50583648
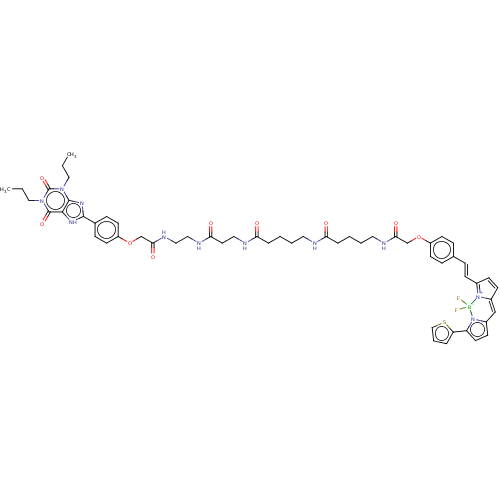
(CHEMBL5092175)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCNC(=O)CCNC(=O)CCCCNC(=O)CCCCNC(=O)COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)cc1 |c:63,65,t:60,(15.68,-14.66,;15.67,-13.11,;14.33,-12.34,;14.33,-10.79,;15.68,-10.02,;17.15,-10.49,;18.04,-9.24,;17.13,-8,;15.67,-8.48,;14.33,-7.7,;14.33,-6.15,;12.99,-8.48,;11.65,-7.71,;11.65,-6.16,;10.3,-5.39,;12.99,-10.02,;11.65,-10.8,;19.58,-9.22,;20.35,-10.54,;21.88,-10.53,;22.64,-9.19,;24.18,-9.17,;24.96,-10.5,;26.5,-10.48,;27.26,-9.14,;27.29,-11.81,;28.83,-11.79,;29.61,-13.11,;31.15,-13.09,;31.94,-14.42,;31.18,-15.76,;33.48,-14.4,;34.26,-15.73,;35.8,-15.71,;36.59,-17.03,;35.83,-18.38,;38.13,-17.02,;38.91,-18.34,;40.45,-18.32,;41.24,-19.65,;42.78,-19.63,;43.56,-20.95,;42.81,-22.3,;45.1,-20.94,;45.89,-22.26,;47.43,-22.24,;48.21,-23.57,;49.75,-23.55,;50.54,-24.88,;49.78,-26.22,;52.08,-24.86,;52.86,-26.18,;54.4,-26.17,;55.18,-27.5,;56.72,-27.48,;57.47,-26.14,;59.01,-26.12,;59.8,-27.44,;61.34,-27.42,;62.84,-27.77,;62.98,-29.31,;61.55,-29.92,;60.54,-28.75,;64.38,-29.97,;65.65,-29.08,;67.16,-29.42,;67.95,-28.09,;66.94,-26.93,;67.28,-25.43,;68.71,-24.81,;68.57,-23.27,;67.06,-22.92,;66.26,-24.25,;65.51,-27.54,;64.09,-26.87,;63.01,-25.79,;65.01,-25.63,;56.68,-24.81,;55.15,-24.83,;21.85,-7.86,;20.32,-7.89,)| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at N-terminal NLuc tagged human A3 adenosine receptor |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50240839
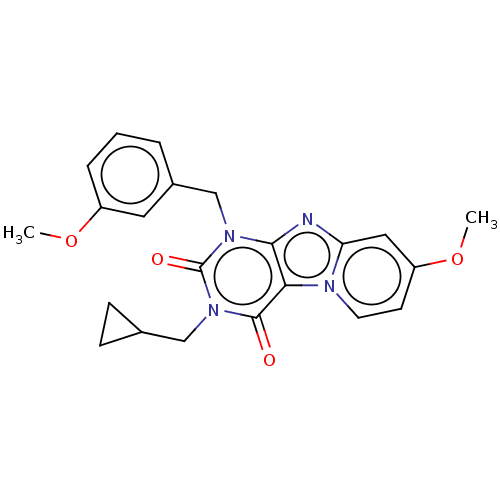
(CHEMBL4062581)Show SMILES COc1cccc(Cn2c3nc4cc(OC)ccn4c3c(=O)n(CC3CC3)c2=O)c1 Show InChI InChI=1S/C22H22N4O4/c1-29-16-5-3-4-15(10-16)13-25-20-19(21(27)26(22(25)28)12-14-6-7-14)24-9-8-17(30-2)11-18(24)23-20/h3-5,8-11,14H,6-7,12-13H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591158
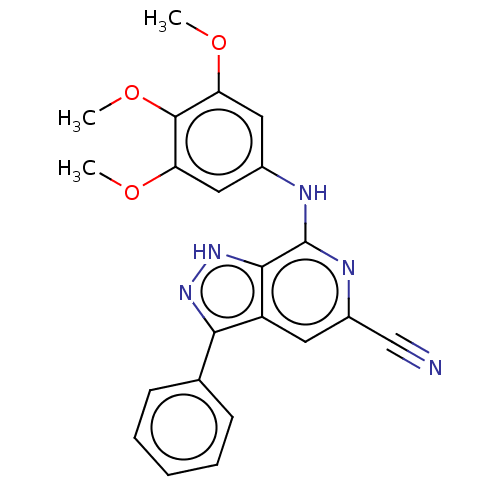
(CHEMBL5181728)Show SMILES COc1cc(Nc2nc(cc3c(n[nH]c23)-c2ccccc2)C#N)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591158

(CHEMBL5181728)Show SMILES COc1cc(Nc2nc(cc3c(n[nH]c23)-c2ccccc2)C#N)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50423866
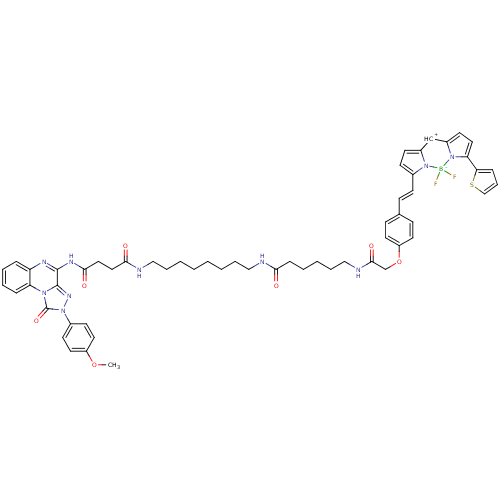
(CHEMBL2024148)Show SMILES COc1ccc(cc1)-n1nc2c(NC(=O)CCC(=O)NCCCCCCCCNC(=O)CCCCCNC(=O)COc3ccc([CH+]\C=c4\ccc5=Cc6ccc(-c7cccs7)n6[B-](F)(F)n45)cc3)nc3ccccc3n2c1=O |t:51| Show InChI InChI=1S/C57H61BF2N10O7S/c1-76-45-29-24-42(25-30-45)70-57(75)67-48-15-9-8-14-47(48)64-55(56(67)66-70)65-53(73)33-32-52(72)62-35-11-5-3-2-4-10-34-61-51(71)17-7-6-12-36-63-54(74)39-77-46-27-19-40(20-28-46)18-21-41-22-23-43-38-44-26-31-49(50-16-13-37-78-50)69(44)58(59,60)68(41)43/h8-9,13-16,18-31,37-38H,2-7,10-12,17,32-36,39H2,1H3,(H,61,71)(H,62,72)(H,63,74)(H,64,65,73)/b41-21- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor expressed in forskolin-stimulated CHO cells assessed as inhibition of NECA-induced CRE-SPAP gene t... |
J Med Chem 55: 1771-82 (2012)
Article DOI: 10.1021/jm201722y
BindingDB Entry DOI: 10.7270/Q2ST7R4K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591161
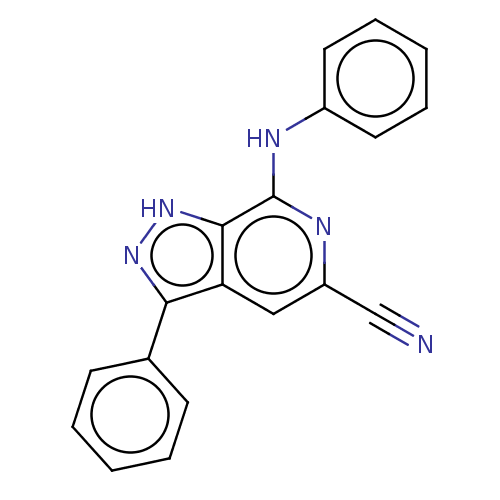
(CHEMBL5180268) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50423867

(CHEMBL2023737)Show SMILES COc1ccc(cc1)-n1nc2c(NC(=O)CCC(=O)NCCOCCOCCN)nc3ccccc3n2c1=O Show InChI InChI=1S/C26H31N7O6/c1-37-19-8-6-18(7-9-19)33-26(36)32-21-5-3-2-4-20(21)29-24(25(32)31-33)30-23(35)11-10-22(34)28-13-15-39-17-16-38-14-12-27/h2-9H,10-17,27H2,1H3,(H,28,34)(H,29,30,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor expressed in forskolin-stimulated CHO cells assessed as inhibition of NECA-induced CRE-SPAP gene t... |
J Med Chem 55: 1771-82 (2012)
Article DOI: 10.1021/jm201722y
BindingDB Entry DOI: 10.7270/Q2ST7R4K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591161

(CHEMBL5180268) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM214690
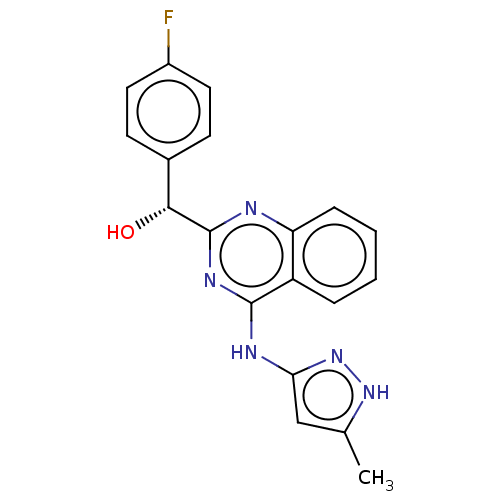
(US9295672, (R)-(4-fluorophenyl)(4-((5-methyl-1H-py...)Show SMILES Cc1cc(Nc2nc(nc3ccccc23)[C@H](O)c2ccc(F)cc2)n[nH]1 Show InChI InChI=1S/C19H16FN5O/c1-11-10-16(25-24-11)22-18-14-4-2-3-5-15(14)21-19(23-18)17(26)12-6-8-13(20)9-7-12/h2-10,17,26H,1H3,(H2,21,22,23,24,25)/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 28.5 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Corporation
US Patent
| Assay Description
.(R)- and (S)-(4-Fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol was tested in a radioligand binding assay, according to th... |
US Patent US9295672 (2016)
BindingDB Entry DOI: 10.7270/Q2ZP44ZX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50583647

(CHEMBL5081913)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)-c1ccc(OCC(=O)NCCNC(=O)CCCCNC(=O)COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)cc1 |c:51,53,t:48,(40.32,-39.99,;40.32,-38.44,;38.98,-37.67,;38.97,-36.12,;40.32,-35.35,;41.79,-35.82,;42.68,-34.57,;41.77,-33.33,;40.31,-33.81,;38.97,-33.03,;38.97,-31.49,;37.64,-33.81,;36.3,-33.04,;36.29,-31.5,;34.95,-30.73,;37.64,-35.36,;36.3,-36.13,;44.21,-34.55,;44.99,-35.87,;46.52,-35.86,;47.27,-34.52,;48.81,-34.5,;49.6,-35.83,;51.13,-35.81,;51.89,-34.47,;51.92,-37.13,;53.46,-37.12,;54.24,-38.44,;55.78,-38.42,;56.56,-39.75,;55.81,-41.09,;58.1,-39.73,;58.89,-41.05,;60.43,-41.03,;61.21,-42.36,;62.75,-42.34,;63.53,-43.66,;62.78,-45,;65.07,-43.65,;65.85,-44.97,;67.39,-44.95,;68.17,-46.28,;69.71,-46.26,;70.46,-44.92,;72,-44.9,;72.79,-46.22,;74.32,-46.2,;75.82,-46.55,;75.96,-48.09,;74.54,-48.7,;73.52,-47.53,;77.36,-48.75,;78.63,-47.86,;80.13,-48.2,;80.93,-46.88,;79.91,-45.71,;80.26,-44.21,;81.68,-43.6,;81.54,-42.06,;80.03,-41.71,;79.24,-43.04,;78.49,-46.32,;77.07,-45.65,;75.99,-44.57,;77.99,-44.41,;69.67,-43.59,;68.14,-43.62,;46.48,-33.2,;44.95,-33.22,)| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at N-terminal NLuc tagged human A3 adenosine receptor |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50423870
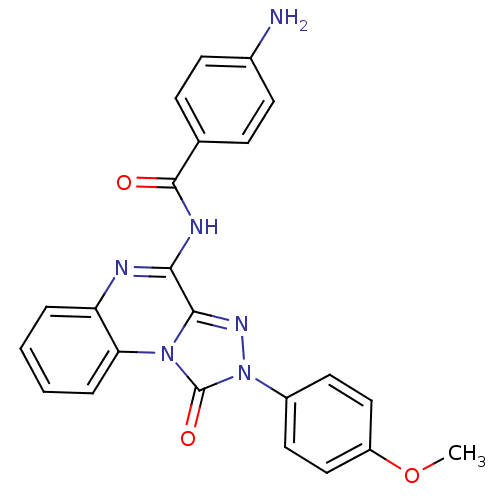
(CHEMBL2024147)Show SMILES COc1ccc(cc1)-n1nc2c(NC(=O)c3ccc(N)cc3)nc3ccccc3n2c1=O Show InChI InChI=1S/C23H18N6O3/c1-32-17-12-10-16(11-13-17)29-23(31)28-19-5-3-2-4-18(19)25-20(21(28)27-29)26-22(30)14-6-8-15(24)9-7-14/h2-13H,24H2,1H3,(H,25,26,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor expressed in forskolin-stimulated CHO cells assessed as inhibition of NECA-induced CRE-SPAP gene t... |
J Med Chem 55: 1771-82 (2012)
Article DOI: 10.1021/jm201722y
BindingDB Entry DOI: 10.7270/Q2ST7R4K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50597191
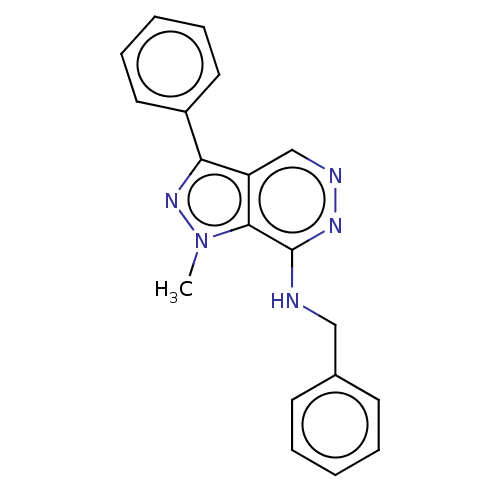
(CHEMBL5195631) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00052
BindingDB Entry DOI: 10.7270/Q20G3Q6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50583644
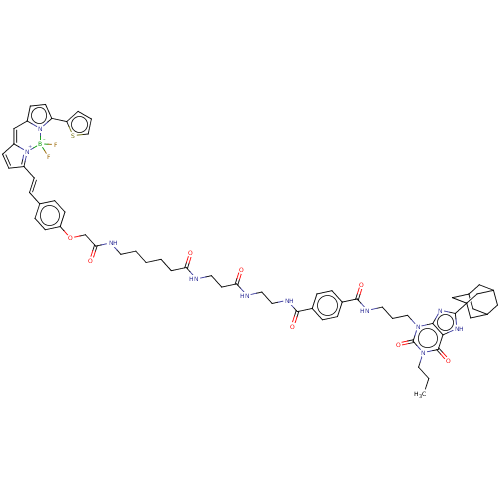
(CHEMBL5080679)Show SMILES CCCn1c(=O)n(CCCNC(=O)c2ccc(cc2)C(=O)NCCNC(=O)CCNC(=O)CCCCCNC(=O)COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)c2nc([nH]c2c1=O)C12CC3CC(CC(C3)C1)C2 |c:52,54,t:49,TLB:79:80:77.78.83:84,THB:81:80:77:83.82.84,81:82:77:85.79.80,79:78:84:85.80.81| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to NLuc human A3 adenosine receptor expressed in HEK293-A cells in presence of 1 uM MRS1220 by NanoBRET binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591163
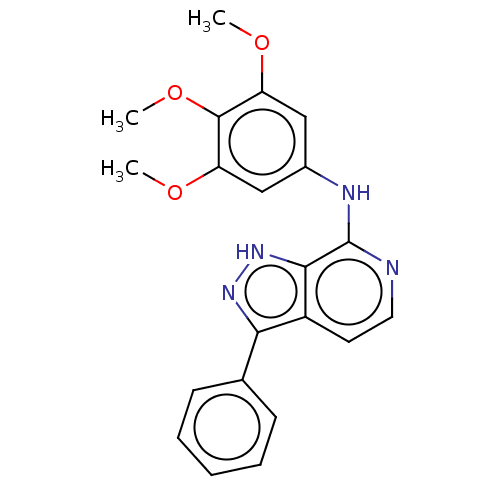
(CHEMBL3929143)Show SMILES COc1cc(Nc2nccc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591168
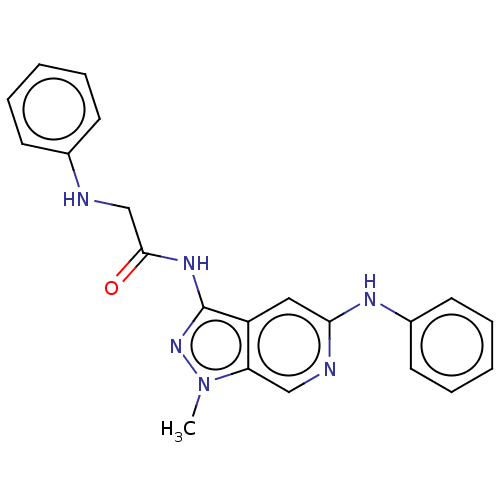
(CHEMBL5180010)Show SMILES Cn1nc(NC(=O)CNc2ccccc2)c2cc(Nc3ccccc3)ncc12 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591162
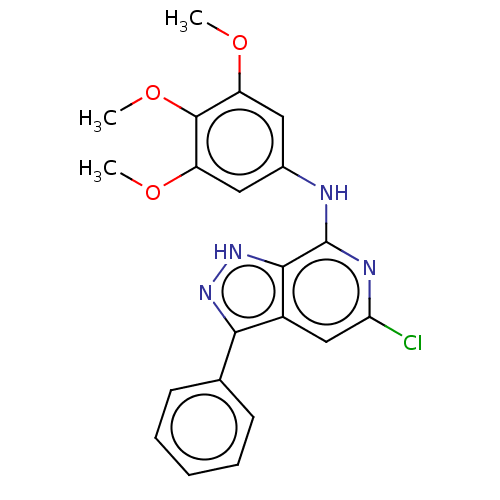
(CHEMBL3929812)Show SMILES COc1cc(Nc2nc(Cl)cc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591162

(CHEMBL3929812)Show SMILES COc1cc(Nc2nc(Cl)cc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591168

(CHEMBL5180010)Show SMILES Cn1nc(NC(=O)CNc2ccccc2)c2cc(Nc3ccccc3)ncc12 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591166
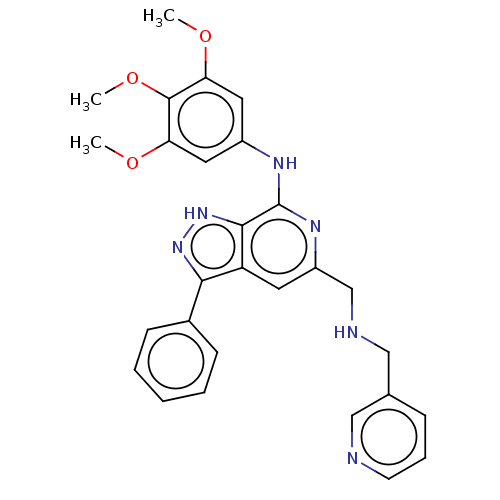
(CHEMBL5189470)Show SMILES COc1cc(Nc2nc(CNCc3cccnc3)cc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591166

(CHEMBL5189470)Show SMILES COc1cc(Nc2nc(CNCc3cccnc3)cc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
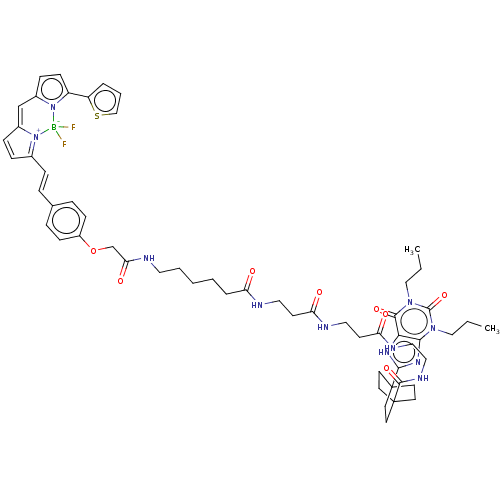
(Homo sapiens (Human)) | BDBM50583640
(CHEMBL5075285)Show SMILES CCCn1c2nc([nH]c2c(=O)n(CCC)c1=O)C12CCC(CC1)(CC2)C(=O)NCCNC(=O)CCNC(=O)CCNC(=O)CCCCCNC(=O)COc1ccc(\C=C\C2=[N+]3C(C=C2)=Cc2ccc(-c4cccs4)n2[B-]3(F)F)cc1 |c:66,68,t:63| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to NLuc human A3 adenosine receptor expressed in HEK293-A cells in presence of 1 uM MRS1220 by NanoBRET binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02067
BindingDB Entry DOI: 10.7270/Q2CV4NM3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50423873
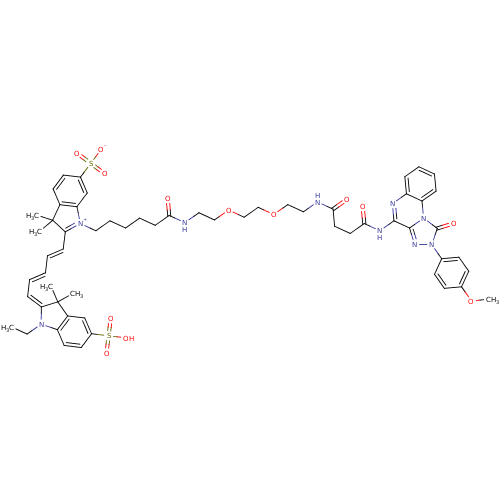
(CHEMBL2024151)Show SMILES CCN1\C(=C\C=C\C=C\C2=[N+](CCCCCC(=O)NCCOCCOCCNC(=O)CCC(=O)Nc3nc4ccccc4n4c3nn(-c3ccc(OC)cc3)c4=O)c3cc(ccc3C2(C)C)S([O-])(=O)=O)C(C)(C)c2cc(ccc12)S(O)(=O)=O |c:9| Show InChI InChI=1S/C59H69N9O13S2/c1-7-65-47-28-26-42(82(73,74)75)38-45(47)59(4,5)50(65)18-10-8-11-19-51-58(2,3)44-27-25-43(83(76,77)78)39-49(44)66(51)33-15-9-12-20-52(69)60-31-34-80-36-37-81-35-32-61-53(70)29-30-54(71)63-55-56-64-68(40-21-23-41(79-6)24-22-40)57(72)67(56)48-17-14-13-16-46(48)62-55/h8,10-11,13-14,16-19,21-28,38-39H,7,9,12,15,20,29-37H2,1-6H3,(H4-,60,61,62,63,69,70,71,73,74,75,76,77,78) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A3 receptor expressed in forskolin-stimulated CHO cells assessed as inhibition of NECA-induced CRE-SPAP gene t... |
J Med Chem 55: 1771-82 (2012)
Article DOI: 10.1021/jm201722y
BindingDB Entry DOI: 10.7270/Q2ST7R4K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591165
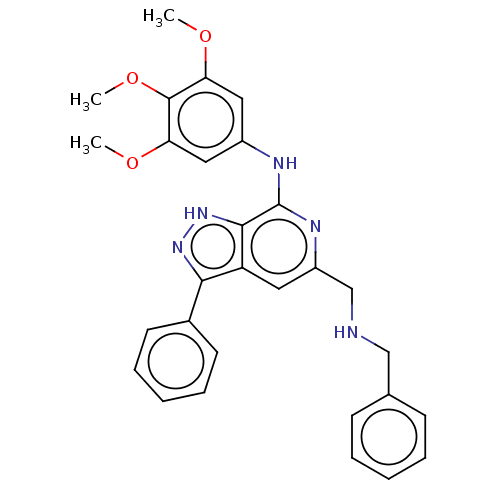
(CHEMBL5185003)Show SMILES COc1cc(Nc2nc(CNCc3ccccc3)cc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591170
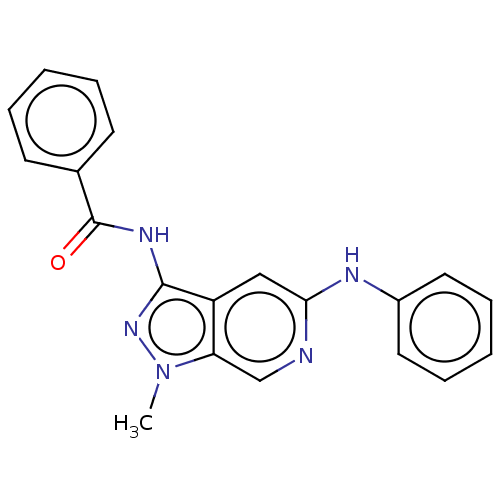
(CHEMBL5186062) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591165

(CHEMBL5185003)Show SMILES COc1cc(Nc2nc(CNCc3ccccc3)cc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591163

(CHEMBL3929143)Show SMILES COc1cc(Nc2nccc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591164
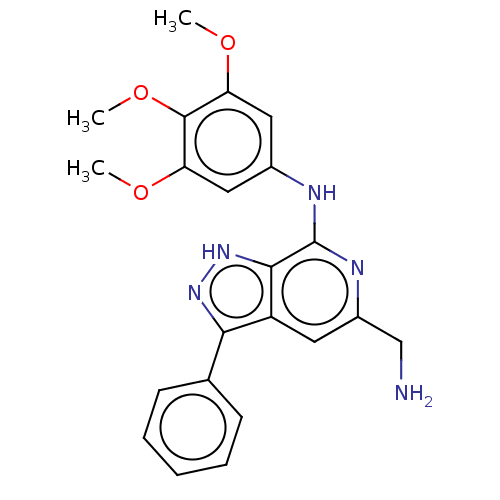
(CHEMBL5196781)Show SMILES COc1cc(Nc2nc(CN)cc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591164

(CHEMBL5196781)Show SMILES COc1cc(Nc2nc(CN)cc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591167
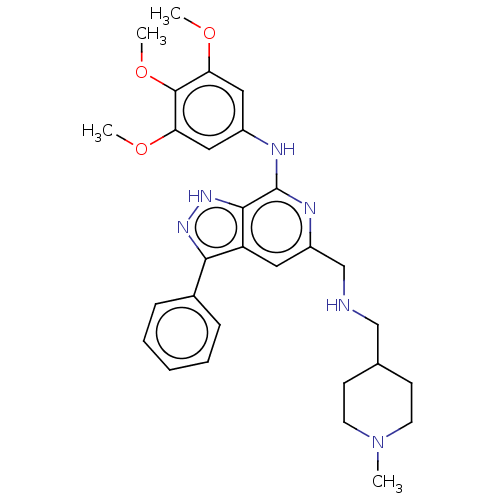
(CHEMBL5189274)Show SMILES COc1cc(Nc2nc(CNCC3CCN(C)CC3)cc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591171
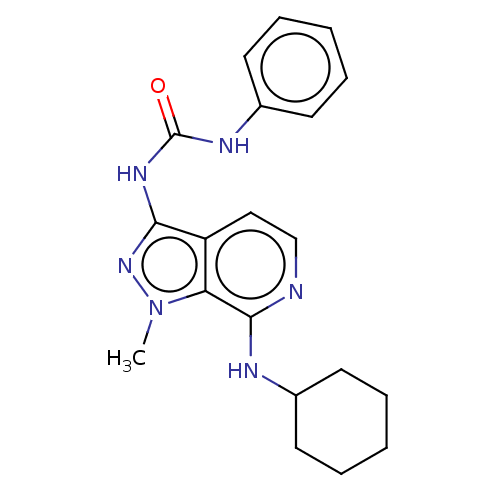
(CHEMBL5174600) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 302 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591169
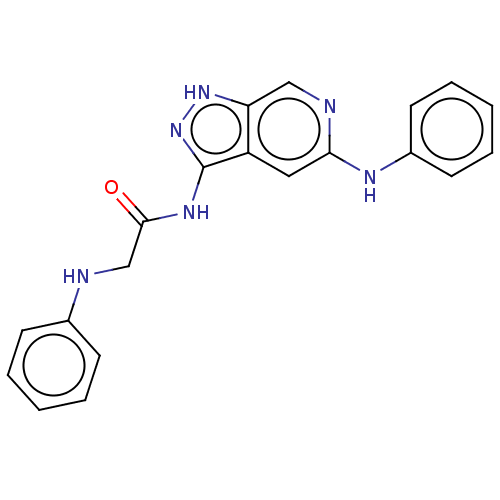
(CHEMBL5201810)Show SMILES O=C(CNc1ccccc1)Nc1n[nH]c2cnc(Nc3ccccc3)cc12 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591160
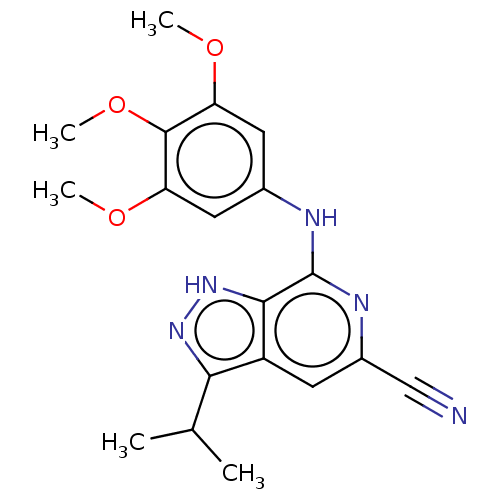
(CHEMBL5173159)Show SMILES COc1cc(Nc2nc(cc3c(n[nH]c23)C(C)C)C#N)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591169

(CHEMBL5201810)Show SMILES O=C(CNc1ccccc1)Nc1n[nH]c2cnc(Nc3ccccc3)cc12 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591159
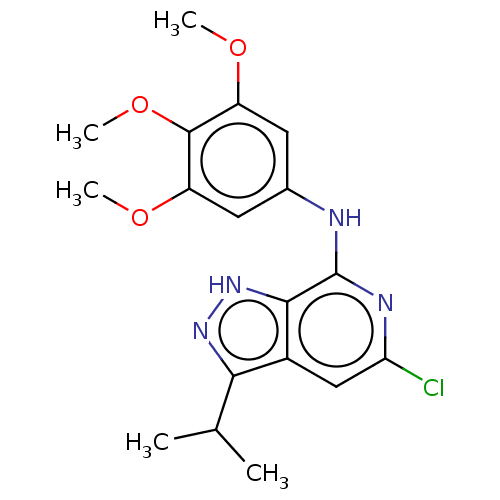
(CHEMBL3927012)Show SMILES COc1cc(Nc2nc(Cl)cc3c(n[nH]c23)C(C)C)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50591167

(CHEMBL5189274)Show SMILES COc1cc(Nc2nc(CNCC3CCN(C)CC3)cc3c(n[nH]c23)-c2ccccc2)cc(OC)c1OC | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 575 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01123
BindingDB Entry DOI: 10.7270/Q2KP8643 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data