 Found 2340 hits of ic50 for UniProtKB: P21554
Found 2340 hits of ic50 for UniProtKB: P21554 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50373120
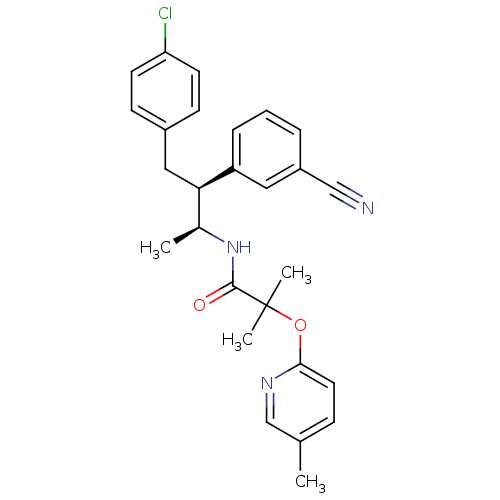
(CHEMBL260977 | MK-0364)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(C)cn1)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C27H28ClN3O2/c1-18-8-13-25(30-17-18)33-27(3,4)26(32)31-19(2)24(15-20-9-11-23(28)12-10-20)22-7-5-6-21(14-22)16-29/h5-14,17,19,24H,15H2,1-4H3,(H,31,32)/t19-,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR-141716 from human wild type CB1R expressed in CHO cells |
J Med Chem 51: 2108-14 (2008)
Article DOI: 10.1021/jm7014974
BindingDB Entry DOI: 10.7270/Q27H1KDR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50306222
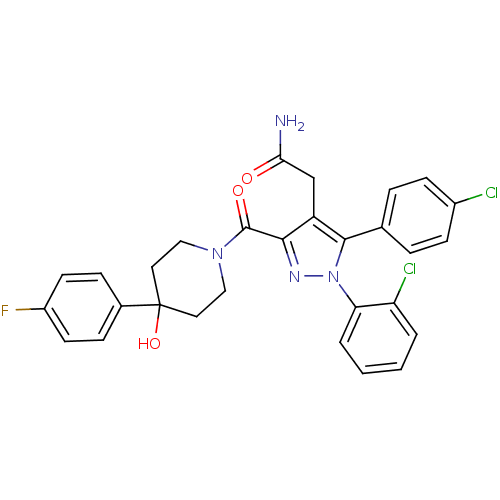
(2-(1-(2-chlorophenyl)-5-(4-chlorophenyl)-3-(4-(4-f...)Show SMILES NC(=O)Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)C(=O)N1CCC(O)(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C29H25Cl2FN4O3/c30-20-9-5-18(6-10-20)27-22(17-25(33)37)26(34-36(27)24-4-2-1-3-23(24)31)28(38)35-15-13-29(39,14-16-35)19-7-11-21(32)12-8-19/h1-12,39H,13-17H2,(H2,33,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human CB1 receptor expressed in CHO-K1 cells by GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 453-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.003
BindingDB Entry DOI: 10.7270/Q2MK6D09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267545

((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)C1CC1 |r| Show InChI InChI=1S/C25H24F6N2O3S/c26-24(27,28)18-5-3-16(4-6-18)21-14-22(21)23(34)32-7-9-33(10-8-32)37(35,36)20-12-17(15-1-2-15)11-19(13-20)25(29,30)31/h3-6,11-13,15,21-22H,1-2,7-10,14H2/t21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50306235
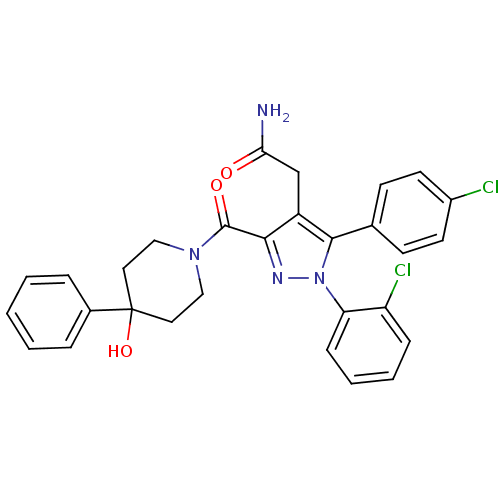
(2-(1-(2-chlorophenyl)-5-(4-chlorophenyl)-3-(4-hydr...)Show SMILES NC(=O)Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)C(=O)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C29H26Cl2N4O3/c30-21-12-10-19(11-13-21)27-22(18-25(32)36)26(33-35(27)24-9-5-4-8-23(24)31)28(37)34-16-14-29(38,15-17-34)20-6-2-1-3-7-20/h1-13,38H,14-18H2,(H2,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human CB1 receptor expressed in CHO-K1 cells by GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 453-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.003
BindingDB Entry DOI: 10.7270/Q2MK6D09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50306234
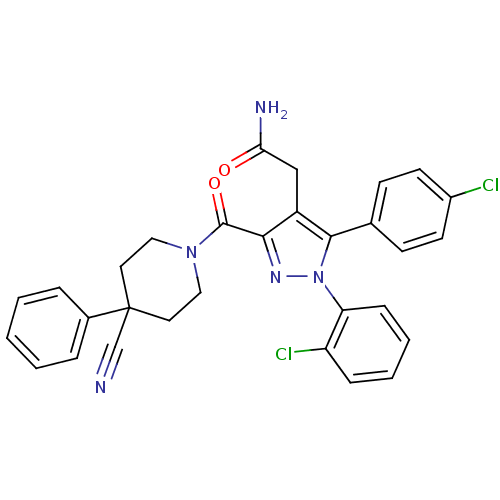
(2-(1-(2-chlorophenyl)-5-(4-chlorophenyl)-3-(4-cyan...)Show SMILES NC(=O)Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)C(=O)N1CCC(CC1)(C#N)c1ccccc1 Show InChI InChI=1S/C30H25Cl2N5O2/c31-22-12-10-20(11-13-22)28-23(18-26(34)38)27(35-37(28)25-9-5-4-8-24(25)32)29(39)36-16-14-30(19-33,15-17-36)21-6-2-1-3-7-21/h1-13H,14-18H2,(H2,34,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human CB1 receptor expressed in CHO-K1 cells by GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 453-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.003
BindingDB Entry DOI: 10.7270/Q2MK6D09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human cannabinoid CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation af... |
Eur J Med Chem 45: 1133-9 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.018
BindingDB Entry DOI: 10.7270/Q2K937P0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50306222

(2-(1-(2-chlorophenyl)-5-(4-chlorophenyl)-3-(4-(4-f...)Show SMILES NC(=O)Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)C(=O)N1CCC(O)(CC1)c1ccc(F)cc1 Show InChI InChI=1S/C29H25Cl2FN4O3/c30-20-9-5-18(6-10-20)27-22(17-25(33)37)26(34-36(27)24-4-2-1-3-23(24)31)28(38)35-15-13-29(39,14-16-35)19-7-11-21(32)12-8-19/h1-12,39H,13-17H2,(H2,33,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP55940-induced GTPgammaS binding |
Bioorg Med Chem Lett 20: 453-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.003
BindingDB Entry DOI: 10.7270/Q2MK6D09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267907
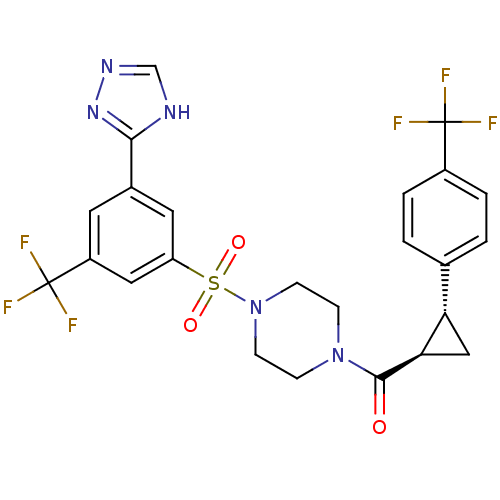
((4-(3-(4H-1,2,4-triazol-3-yl)-5-(trifluoromethyl)p...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)-c1nnc[nH]1 |r| Show InChI InChI=1S/C24H21F6N5O3S/c25-23(26,27)16-3-1-14(2-4-16)19-12-20(19)22(36)34-5-7-35(8-6-34)39(37,38)18-10-15(21-31-13-32-33-21)9-17(11-18)24(28,29)30/h1-4,9-11,13,19-20H,5-8,12H2,(H,31,32,33)/t19-,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50320187
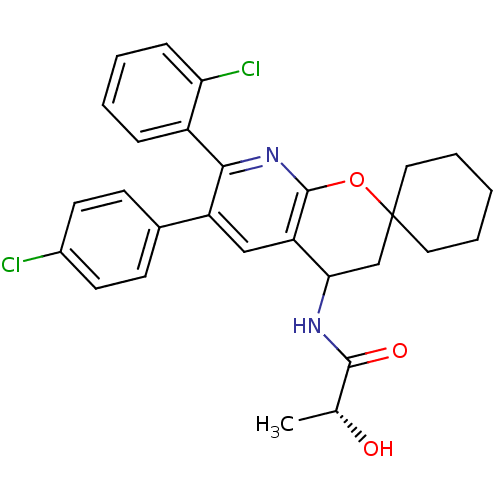
((2R)-N-(7'-(2-CHLOROPHENYL)-6'-(4-CHLOROPHENYL)-3'...)Show SMILES C[C@@H](O)C(=O)NC1CC2(CCCCC2)Oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H28Cl2N2O3/c1-17(33)26(34)31-24-16-28(13-5-2-6-14-28)35-27-22(24)15-21(18-9-11-19(29)12-10-18)25(32-27)20-7-3-4-8-23(20)30/h3-4,7-12,15,17,24,33H,2,5-6,13-14,16H2,1H3,(H,31,34)/t17-,24?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316529
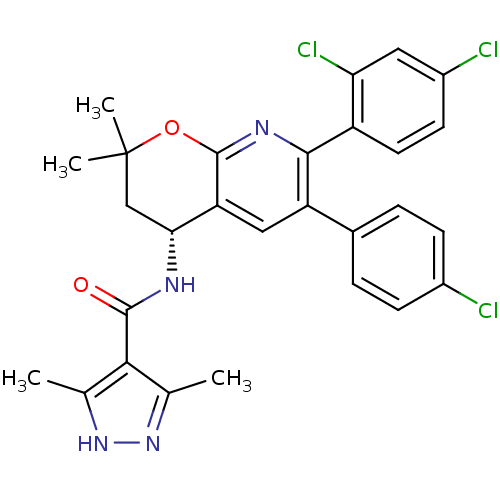
(CHEMBL1095151 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES Cc1n[nH]c(C)c1C(=O)N[C@@H]1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H25Cl3N4O2/c1-14-24(15(2)35-34-14)26(36)32-23-13-28(3,4)37-27-21(23)12-20(16-5-7-17(29)8-6-16)25(33-27)19-10-9-18(30)11-22(19)31/h5-12,23H,13H2,1-4H3,(H,32,36)(H,34,35)/t23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316524
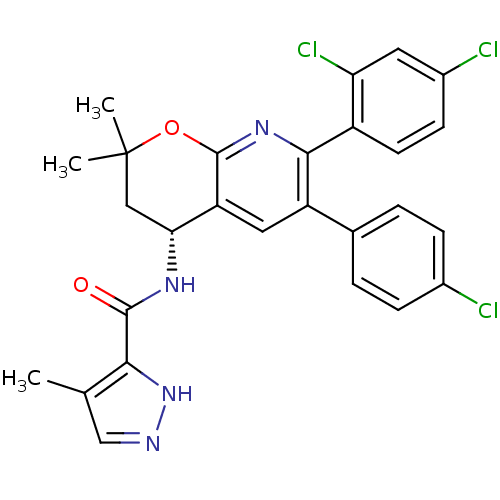
(CHEMBL1095158 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES Cc1cn[nH]c1C(=O)N[C@@H]1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H23Cl3N4O2/c1-14-13-31-34-23(14)25(35)32-22-12-27(2,3)36-26-20(22)11-19(15-4-6-16(28)7-5-15)24(33-26)18-9-8-17(29)10-21(18)30/h4-11,13,22H,12H2,1-3H3,(H,31,34)(H,32,35)/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316527
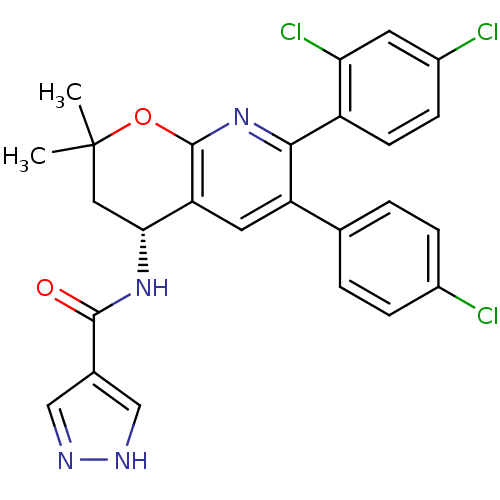
(CHEMBL1097841 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES CC1(C)C[C@@H](NC(=O)c2cn[nH]c2)c2cc(-c3ccc(Cl)cc3)c(nc2O1)-c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H21Cl3N4O2/c1-26(2)11-22(32-24(34)15-12-30-31-13-15)20-10-19(14-3-5-16(27)6-4-14)23(33-25(20)35-26)18-8-7-17(28)9-21(18)29/h3-10,12-13,22H,11H2,1-2H3,(H,30,31)(H,32,34)/t22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50305440
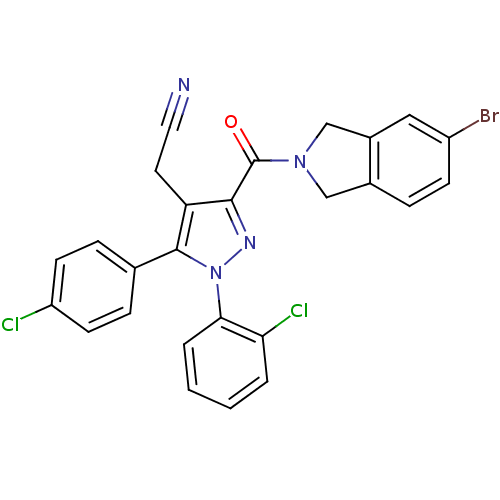
(2-(3-(5-bromoisoindoline-2-carbonyl)-1-(2-chloroph...)Show SMILES Clc1ccc(cc1)-c1c(CC#N)c(nn1-c1ccccc1Cl)C(=O)N1Cc2ccc(Br)cc2C1 Show InChI InChI=1S/C26H17BrCl2N4O/c27-19-8-5-17-14-32(15-18(17)13-19)26(34)24-21(11-12-30)25(16-6-9-20(28)10-7-16)33(31-24)23-4-2-1-3-22(23)29/h1-10,13H,11,14-15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 20: 26-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.047
BindingDB Entry DOI: 10.7270/Q28G8KS9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50306234

(2-(1-(2-chlorophenyl)-5-(4-chlorophenyl)-3-(4-cyan...)Show SMILES NC(=O)Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)C(=O)N1CCC(CC1)(C#N)c1ccccc1 Show InChI InChI=1S/C30H25Cl2N5O2/c31-22-12-10-20(11-13-22)28-23(18-26(34)38)27(35-37(28)25-9-5-4-8-24(25)32)29(39)36-16-14-30(19-33,15-17-36)21-6-2-1-3-7-21/h1-13H,14-18H2,(H2,34,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP55940-induced GTPgammaS binding |
Bioorg Med Chem Lett 20: 453-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.003
BindingDB Entry DOI: 10.7270/Q2MK6D09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50306235

(2-(1-(2-chlorophenyl)-5-(4-chlorophenyl)-3-(4-hydr...)Show SMILES NC(=O)Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)C(=O)N1CCC(O)(CC1)c1ccccc1 Show InChI InChI=1S/C29H26Cl2N4O3/c30-21-12-10-19(11-13-21)27-22(18-25(32)36)26(33-35(27)24-9-5-4-8-23(24)31)28(37)34-16-14-29(38,15-17-34)20-6-2-1-3-7-20/h1-13,38H,14-18H2,(H2,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP55940-induced GTPgammaS binding |
Bioorg Med Chem Lett 20: 453-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.003
BindingDB Entry DOI: 10.7270/Q2MK6D09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cells |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50389939
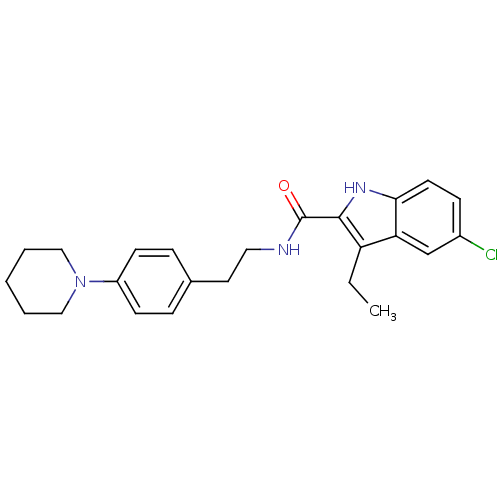
(CHEMBL1553629)Show SMILES CCc1c([nH]c2ccc(Cl)cc12)C(=O)NCCc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C24H28ClN3O/c1-2-20-21-16-18(25)8-11-22(21)27-23(20)24(29)26-13-12-17-6-9-19(10-7-17)28-14-4-3-5-15-28/h6-11,16,27H,2-5,12-15H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Positive allosteric modulatory activity at C-terminal GFP-tagged human CB1R expressed in HEK293 cell membrane after 90 mins in presence of [3H]CP5594... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00655
BindingDB Entry DOI: 10.7270/Q2DN48RK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CB1 receptor |
Bioorg Med Chem Lett 20: 1448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.065
BindingDB Entry DOI: 10.7270/Q2028RPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316528
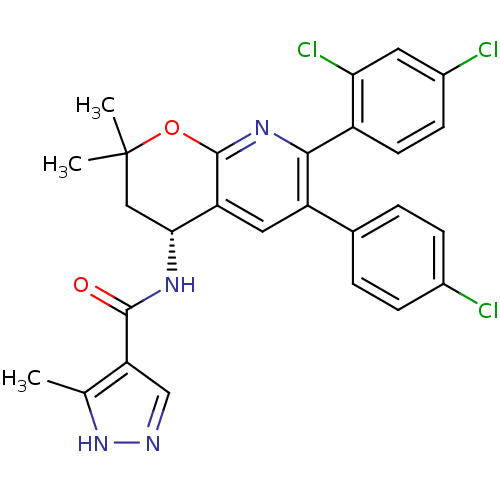
(CHEMBL1095150 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES Cc1[nH]ncc1C(=O)N[C@@H]1CC(C)(C)Oc2nc(-c3ccc(Cl)cc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H23Cl3N4O2/c1-14-21(13-31-34-14)25(35)32-23-12-27(2,3)36-26-20(23)11-19(15-4-6-16(28)7-5-15)24(33-26)18-9-8-17(29)10-22(18)30/h4-11,13,23H,12H2,1-3H3,(H,31,34)(H,32,35)/t23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cannabinoid CB1 receptor |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50320184
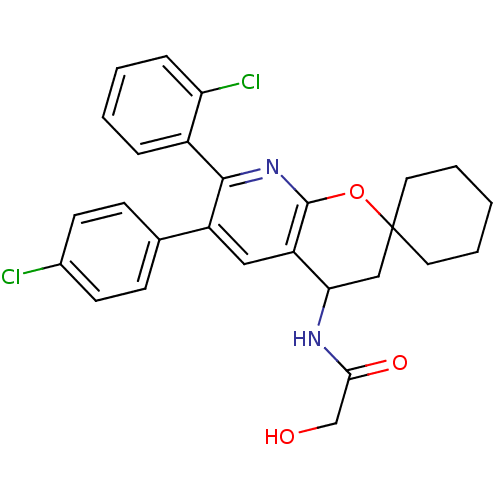
(CHEMBL1086494 | rac-N-(7'-(2-chlorophenyl)-6'-(4-c...)Show SMILES OCC(=O)NC1CC2(CCCCC2)Oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H26Cl2N2O3/c28-18-10-8-17(9-11-18)20-14-21-23(30-24(33)16-32)15-27(12-4-1-5-13-27)34-26(21)31-25(20)19-6-2-3-7-22(19)29/h2-3,6-11,14,23,32H,1,4-5,12-13,15-16H2,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]CP-55940 binding to human recombinant CB1 receptor in CHO cells |
J Med Chem 49: 7584-7 (2006)
Article DOI: 10.1021/jm060996+
BindingDB Entry DOI: 10.7270/Q2QN67KG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of radiolabeled CP5549 binding to human Cannabinoid receptor 1 expressed in CHO cells by competition assay |
J Med Chem 63: 6276-6302 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00917
BindingDB Entry DOI: 10.7270/Q2TT4VHX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50205166

(CHEMBL231636 | N-((2S,3S)-4-(4-chlorophenyl)-3-(3-...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1cncc(c1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-23-13-21(15-33-16-23)27(29,30)31)24(12-18-7-9-22(28)10-8-18)20-6-4-5-19(11-20)14-32/h4-11,13,15-17,24H,12H2,1-3H3,(H,34,35)/t17-,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2184-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.087
BindingDB Entry DOI: 10.7270/Q2HM584G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inverse agonist activity at cannabinoid CB1 receptor (unknown origin) |
Bioorg Med Chem Lett 19: 2591-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.005
BindingDB Entry DOI: 10.7270/Q2PK0G1X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267775
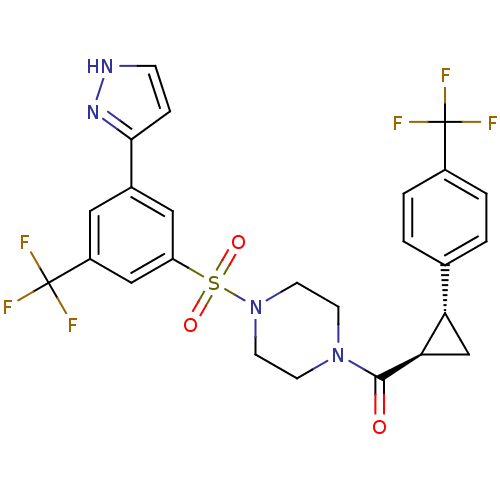
((4-(3-(1H-pyrazol-5-yl)-5-(trifluoromethyl)phenyls...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)-c1cc[nH]n1 |r| Show InChI InChI=1S/C25H22F6N4O3S/c26-24(27,28)17-3-1-15(2-4-17)20-14-21(20)23(36)34-7-9-35(10-8-34)39(37,38)19-12-16(22-5-6-32-33-22)11-18(13-19)25(29,30)31/h1-6,11-13,20-21H,7-10,14H2,(H,32,33)/t20-,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267730
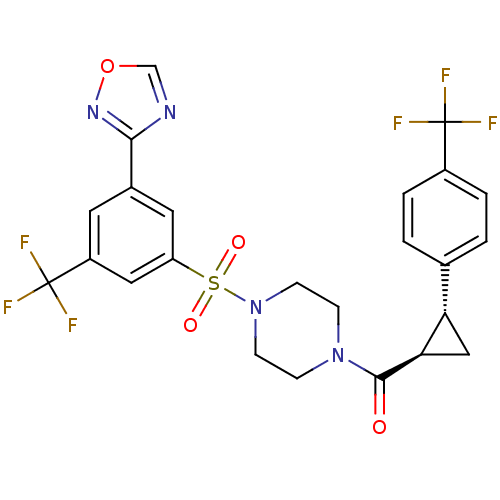
((4-(3-(1,2,4-oxadiazol-3-yl)-5-(trifluoromethyl)ph...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)-c1ncon1 |r| Show InChI InChI=1S/C24H20F6N4O4S/c25-23(26,27)16-3-1-14(2-4-16)19-12-20(19)22(35)33-5-7-34(8-6-33)39(36,37)18-10-15(21-31-13-38-32-21)9-17(11-18)24(28,29)30/h1-4,9-11,13,19-20H,5-8,12H2/t19-,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267585
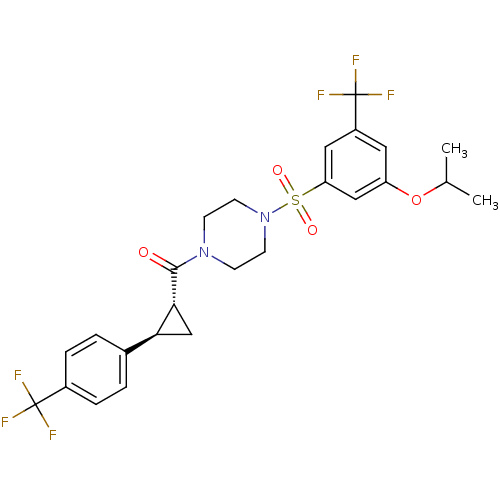
((4-(3-isopropoxy-5-(trifluoromethyl)phenylsulfonyl...)Show SMILES CC(C)Oc1cc(cc(c1)S(=O)(=O)N1CCN(CC1)C(=O)[C@@H]1C[C@H]1c1ccc(cc1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H26F6N2O4S/c1-15(2)37-19-11-18(25(29,30)31)12-20(13-19)38(35,36)33-9-7-32(8-10-33)23(34)22-14-21(22)16-3-5-17(6-4-16)24(26,27)28/h3-6,11-13,15,21-22H,7-10,14H2,1-2H3/t21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 5195-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.046
BindingDB Entry DOI: 10.7270/Q2028RMH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50306225
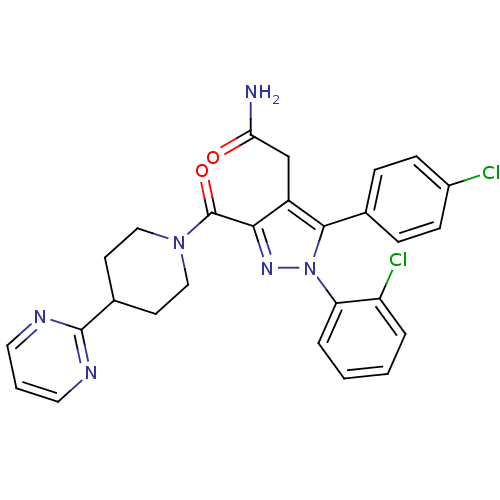
(2-(1-(2-chlorophenyl)-5-(4-chlorophenyl)-3-(4-(pyr...)Show SMILES NC(=O)Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)C(=O)N1CCC(CC1)c1ncccn1 Show InChI InChI=1S/C27H24Cl2N6O2/c28-19-8-6-17(7-9-19)25-20(16-23(30)36)24(33-35(25)22-5-2-1-4-21(22)29)27(37)34-14-10-18(11-15-34)26-31-12-3-13-32-26/h1-9,12-13,18H,10-11,14-16H2,(H2,30,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human CB1 receptor expressed in CHO-K1 cells by GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 453-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.003
BindingDB Entry DOI: 10.7270/Q2MK6D09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Antagonist activity against human recombinant cannabinoid-1 receptor |
Bioorg Med Chem Lett 19: 2990-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.037
BindingDB Entry DOI: 10.7270/Q2CN7543 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50008022
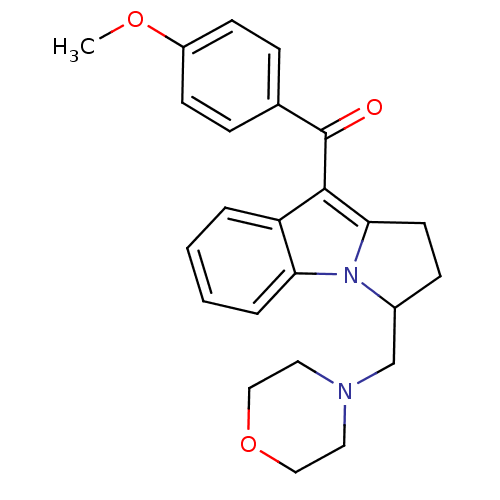
((4-Methoxy-phenyl)-(3-morpholin-4-ylmethyl-2,3-dih...)Show SMILES COc1ccc(cc1)C(=O)c1c2CCC(CN3CCOCC3)n2c2ccccc12 Show InChI InChI=1S/C24H26N2O3/c1-28-19-9-6-17(7-10-19)24(27)23-20-4-2-3-5-21(20)26-18(8-11-22(23)26)16-25-12-14-29-15-13-25/h2-7,9-10,18H,8,11-16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Agonist activity at CB1 receptor (unknown origin) |
Eur J Med Chem 43: 513-39 (2008)
Article DOI: 10.1016/j.ejmech.2007.04.007
BindingDB Entry DOI: 10.7270/Q2G73DGR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21279
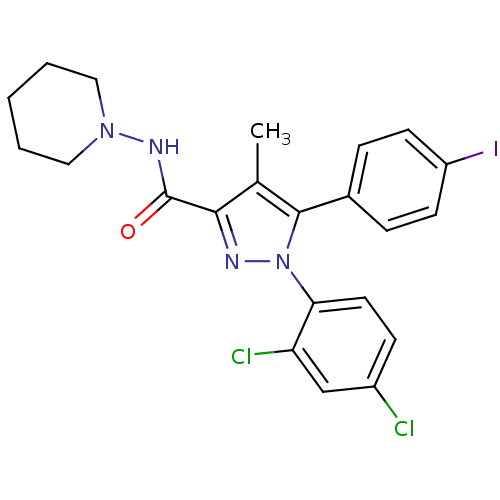
(1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...)Show SMILES Cc1c(nn(c1-c1ccc(I)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl2IN4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-7-16(23)13-18(19)24)21(14)15-5-8-17(25)9-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR-141716 from human wild type CB1R expressed in CHO cells |
J Med Chem 51: 2108-14 (2008)
Article DOI: 10.1021/jm7014974
BindingDB Entry DOI: 10.7270/Q27H1KDR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50306232

(4-(2-amino-2-oxoethyl)-1-(2-chlorophenyl)-5-(4-chl...)Show SMILES NC(=O)Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)C(=O)NCC1CCN(CC1)C#N Show InChI InChI=1S/C25H24Cl2N6O2/c26-18-7-5-17(6-8-18)24-19(13-22(29)34)23(31-33(24)21-4-2-1-3-20(21)27)25(35)30-14-16-9-11-32(15-28)12-10-16/h1-8,16H,9-14H2,(H2,29,34)(H,30,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in COS7 cells |
Bioorg Med Chem Lett 20: 453-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.003
BindingDB Entry DOI: 10.7270/Q2MK6D09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50314119
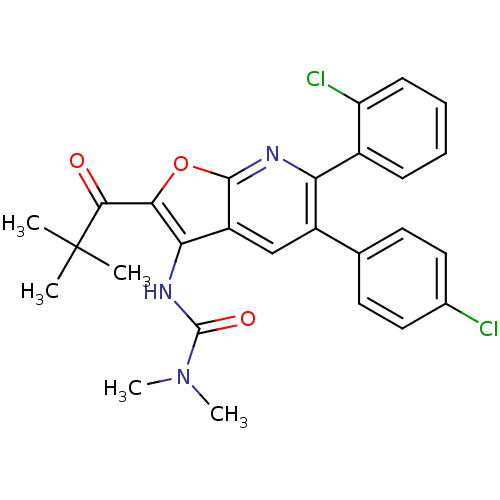
(3-(6-(2-chlorophenyl)-5-(4-chlorophenyl)-2-pivaloy...)Show SMILES CN(C)C(=O)Nc1c(oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1)C(=O)C(C)(C)C Show InChI InChI=1S/C27H25Cl2N3O3/c1-27(2,3)24(33)23-22(31-26(34)32(4)5)19-14-18(15-10-12-16(28)13-11-15)21(30-25(19)35-23)17-8-6-7-9-20(17)29/h6-14H,1-5H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CB1 receptor |
Bioorg Med Chem Lett 20: 1448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.065
BindingDB Entry DOI: 10.7270/Q2028RPD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50320186
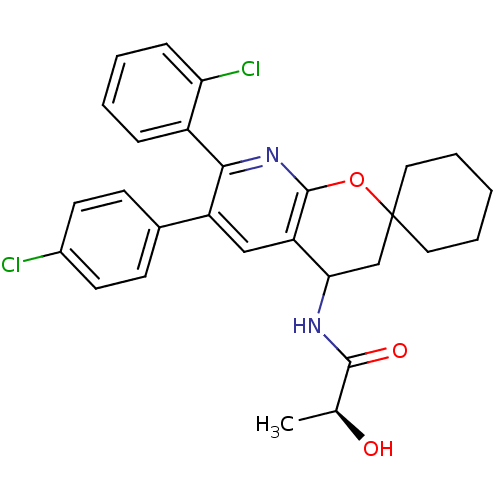
((2S)-N-(7'-(2-CHLOROPHENYL)-6'-(4-CHLOROPHENYL)-3'...)Show SMILES C[C@H](O)C(=O)NC1CC2(CCCCC2)Oc2nc(-c3ccccc3Cl)c(cc12)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H28Cl2N2O3/c1-17(33)26(34)31-24-16-28(13-5-2-6-14-28)35-27-22(24)15-21(18-9-11-19(29)12-10-18)25(32-27)20-7-3-4-8-23(20)30/h3-4,7-12,15,17,24,33H,2,5-6,13-14,16H2,1H3,(H,31,34)/t17-,24?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor |
Bioorg Med Chem Lett 20: 3750-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.071
BindingDB Entry DOI: 10.7270/Q22F7NNR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50314122
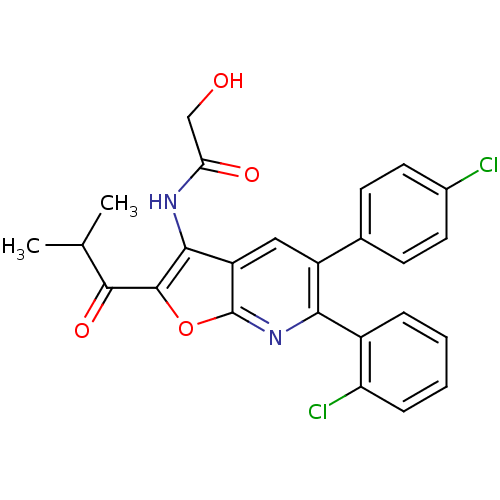
(CHEMBL1091832 | N-(6-(2-chlorophenyl)-5-(4-chlorop...)Show SMILES CC(C)C(=O)c1oc2nc(-c3ccccc3Cl)c(cc2c1NC(=O)CO)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H20Cl2N2O4/c1-13(2)23(32)24-22(28-20(31)12-30)18-11-17(14-7-9-15(26)10-8-14)21(29-25(18)33-24)16-5-3-4-6-19(16)27/h3-11,13,30H,12H2,1-2H3,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CB1 receptor |
Bioorg Med Chem Lett 20: 1448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.065
BindingDB Entry DOI: 10.7270/Q2028RPD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50306215
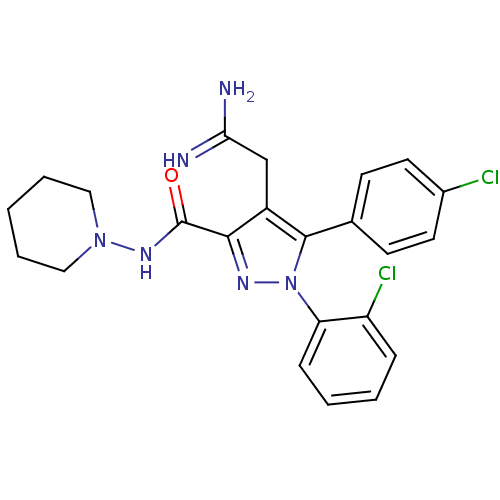
(4-(2-amino-2-iminoethyl)-1-(2-chlorophenyl)-5-(4-c...)Show SMILES NC(=N)Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H24Cl2N6O/c24-16-10-8-15(9-11-16)22-17(14-20(26)27)21(23(32)29-30-12-4-1-5-13-30)28-31(22)19-7-3-2-6-18(19)25/h2-3,6-11H,1,4-5,12-14H2,(H3,26,27)(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human CB1 receptor expressed in CHO-K1 cells by GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 453-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.003
BindingDB Entry DOI: 10.7270/Q2MK6D09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50305415
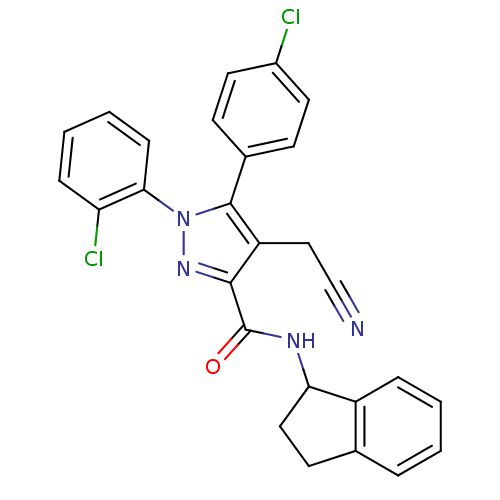
(1-(2-chlorophenyl)-5-(4-chlorophenyl)-4-(cyanometh...)Show SMILES Clc1ccc(cc1)-c1c(CC#N)c(nn1-c1ccccc1Cl)C(=O)NC1CCc2ccccc12 Show InChI InChI=1S/C27H20Cl2N4O/c28-19-12-9-18(10-13-19)26-21(15-16-30)25(32-33(26)24-8-4-3-7-22(24)29)27(34)31-23-14-11-17-5-1-2-6-20(17)23/h1-10,12-13,23H,11,14-15H2,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 20: 26-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.047
BindingDB Entry DOI: 10.7270/Q28G8KS9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50217220

(CHEMBL226590 | N-{(1S,2S)-2-(3-cyanophenyl)-3-[4-(...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1cc(C)ccn1)[C@@H](Cc1ccc(OCCF)cc1)c1cccc(c1)C#N Show InChI InChI=1S/C29H32FN3O3/c1-20-12-14-32-27(16-20)36-29(3,4)28(34)33-21(2)26(24-7-5-6-23(17-24)19-31)18-22-8-10-25(11-9-22)35-15-13-30/h5-12,14,16-17,21,26H,13,15,18H2,1-4H3,(H,33,34)/t21-,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 50: 3427-30 (2007)
Article DOI: 10.1021/jm070131b
BindingDB Entry DOI: 10.7270/Q2Z037VJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50305422
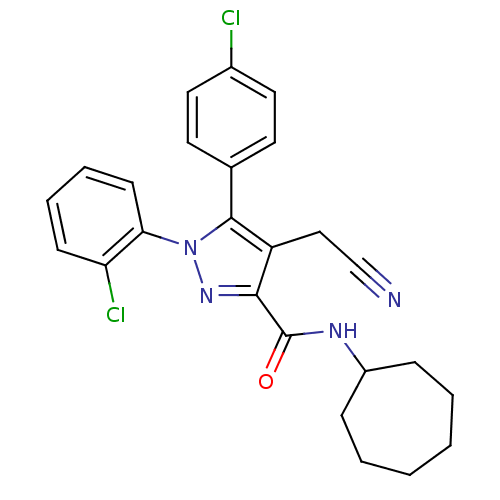
(1-(2-chlorophenyl)-5-(4-chlorophenyl)-4-(cyanometh...)Show SMILES Clc1ccc(cc1)-c1c(CC#N)c(nn1-c1ccccc1Cl)C(=O)NC1CCCCCC1 Show InChI InChI=1S/C25H24Cl2N4O/c26-18-13-11-17(12-14-18)24-20(15-16-28)23(25(32)29-19-7-3-1-2-4-8-19)30-31(24)22-10-6-5-9-21(22)27/h5-6,9-14,19H,1-4,7-8,15H2,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CB1 receptor expressed in CHO-K1 cells assessed as inhibition of CP55940-stimulated GTPgammaS binding |
Bioorg Med Chem Lett 20: 26-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.047
BindingDB Entry DOI: 10.7270/Q28G8KS9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50306232

(4-(2-amino-2-oxoethyl)-1-(2-chlorophenyl)-5-(4-chl...)Show SMILES NC(=O)Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccccc1Cl)C(=O)NCC1CCN(CC1)C#N Show InChI InChI=1S/C25H24Cl2N6O2/c26-18-7-5-17(6-8-18)24-19(13-22(29)34)23(31-33(24)21-4-2-1-3-20(21)27)25(35)30-14-16-9-11-32(15-28)12-10-16/h1-8,16H,9-14H2,(H2,29,34)(H,30,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human CB1 receptor expressed in CHO-K1 cells by GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 453-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.003
BindingDB Entry DOI: 10.7270/Q2MK6D09 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50316525
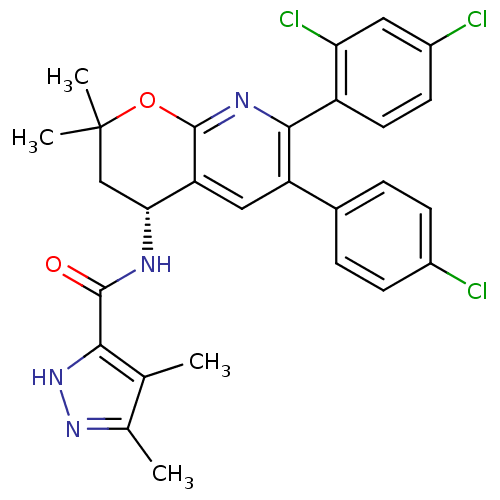
(CHEMBL1095476 | N-[(4R)-6-(4-Chlorophenyl)-7-(2,4-...)Show SMILES Cc1n[nH]c(C(=O)N[C@@H]2CC(C)(C)Oc3nc(-c4ccc(Cl)cc4Cl)c(cc23)-c2ccc(Cl)cc2)c1C |r| Show InChI InChI=1S/C28H25Cl3N4O2/c1-14-15(2)34-35-24(14)26(36)32-23-13-28(3,4)37-27-21(23)12-20(16-5-7-17(29)8-6-16)25(33-27)19-10-9-18(30)11-22(19)31/h5-12,23H,13H2,1-4H3,(H,32,36)(H,34,35)/t23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant cannabinoid CB1 receptor expressed in CHO cells |
J Med Chem 53: 4028-37 (2010)
Article DOI: 10.1021/jm100023j
BindingDB Entry DOI: 10.7270/Q2TQ61QD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50200833
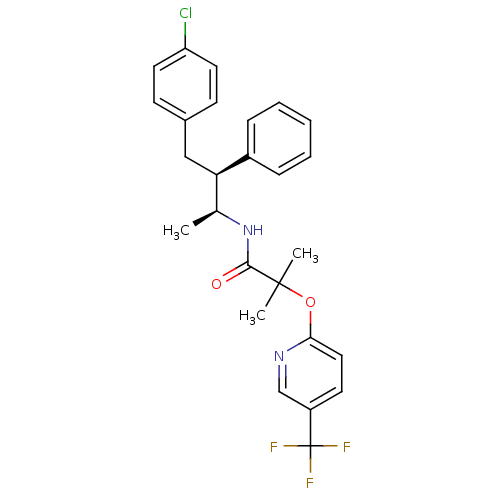
(CHEMBL373626 | N-((2S,3S)-4-(4-chlorophenyl)-3-phe...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1ccccc1 |r| Show InChI InChI=1S/C26H26ClF3N2O2/c1-17(22(19-7-5-4-6-8-19)15-18-9-12-21(27)13-10-18)32-24(33)25(2,3)34-23-14-11-20(16-31-23)26(28,29)30/h4-14,16-17,22H,15H2,1-3H3,(H,32,33)/t17-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]CP-55940 binding to human recombinant CB1 receptor in CHO cells |
J Med Chem 49: 7584-7 (2006)
Article DOI: 10.1021/jm060996+
BindingDB Entry DOI: 10.7270/Q2QN67KG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267545

((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...)Show SMILES FC(F)(F)c1ccc(cc1)[C@@H]1C[C@H]1C(=O)N1CCN(CC1)S(=O)(=O)c1cc(cc(c1)C(F)(F)F)C1CC1 |r| Show InChI InChI=1S/C25H24F6N2O3S/c26-24(27,28)18-5-3-16(4-6-18)21-14-22(21)23(34)32-7-9-33(10-8-32)37(35,36)20-12-17(15-1-2-15)11-19(13-20)25(29,30)31/h3-6,11-13,15,21-22H,1-2,7-10,14H2/t21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267487
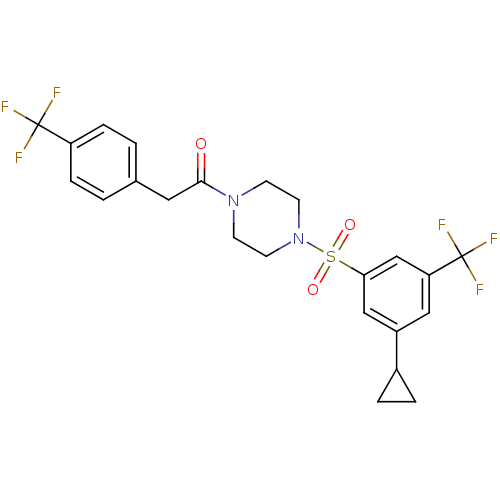
(1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...)Show SMILES FC(F)(F)c1ccc(CC(=O)N2CCN(CC2)S(=O)(=O)c2cc(cc(c2)C(F)(F)F)C2CC2)cc1 Show InChI InChI=1S/C23H22F6N2O3S/c24-22(25,26)18-5-1-15(2-6-18)11-21(32)30-7-9-31(10-8-30)35(33,34)20-13-17(16-3-4-16)12-19(14-20)23(27,28)29/h1-2,5-6,12-14,16H,3-4,7-11H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50267584
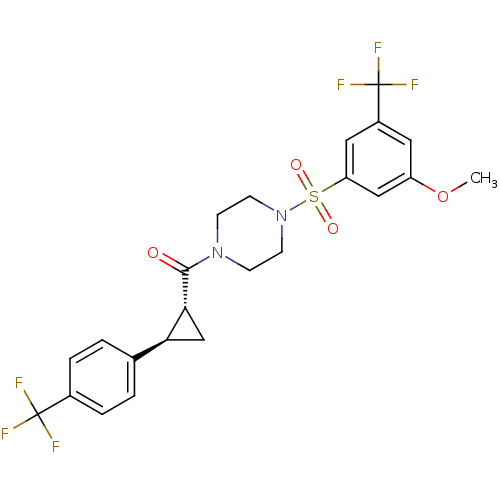
((4-(3-methoxy-5-(trifluoromethyl)phenylsulfonyl)pi...)Show SMILES COc1cc(cc(c1)S(=O)(=O)N1CCN(CC1)C(=O)[C@@H]1C[C@H]1c1ccc(cc1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H22F6N2O4S/c1-35-17-10-16(23(27,28)29)11-18(12-17)36(33,34)31-8-6-30(7-9-31)21(32)20-13-19(20)14-2-4-15(5-3-14)22(24,25)26/h2-5,10-12,19-20H,6-9,13H2,1H3/t19-,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells |
J Med Chem 52: 2550-8 (2009)
Article DOI: 10.1021/jm900063x
BindingDB Entry DOI: 10.7270/Q2028RF8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392283
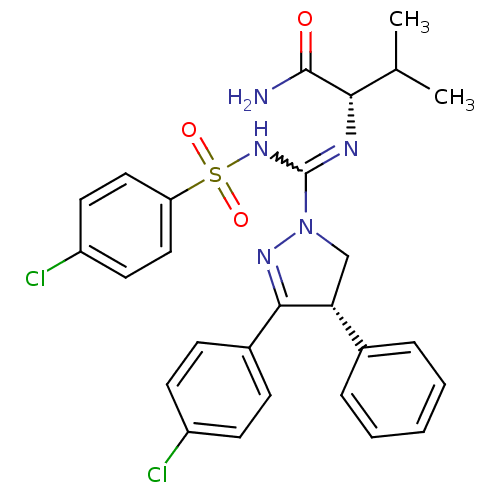
(CHEMBL2153670)Show SMILES CC(C)[C@H](N=C(NS(=O)(=O)c1ccc(Cl)cc1)N1C[C@@H](C(=N1)c1ccc(Cl)cc1)c1ccccc1)C(N)=O |r,w:5.5,c:21| Show InChI InChI=1S/C27H27Cl2N5O3S/c1-17(2)24(26(30)35)31-27(33-38(36,37)22-14-12-21(29)13-15-22)34-16-23(18-6-4-3-5-7-18)25(32-34)19-8-10-20(28)11-9-19/h3-15,17,23-24H,16H2,1-2H3,(H2,30,35)(H,31,33)/t23-,24+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jenrin Discovery
Curated by ChEMBL
| Assay Description
Displacement of 3[H]ligand from recombinant human CB1 receptor |
Bioorg Med Chem Lett 22: 6173-80 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.004
BindingDB Entry DOI: 10.7270/Q2BV7HR2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data