

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341928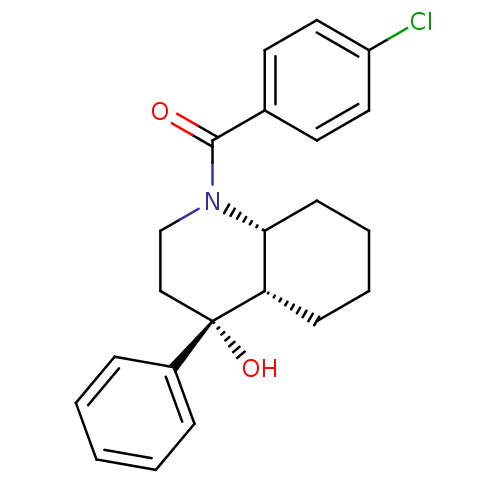 (CHEMBL1765160 | cis-(4-chlorophenyl)((4R,4aS,8aR)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491839 (CHEMBL2387183) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491838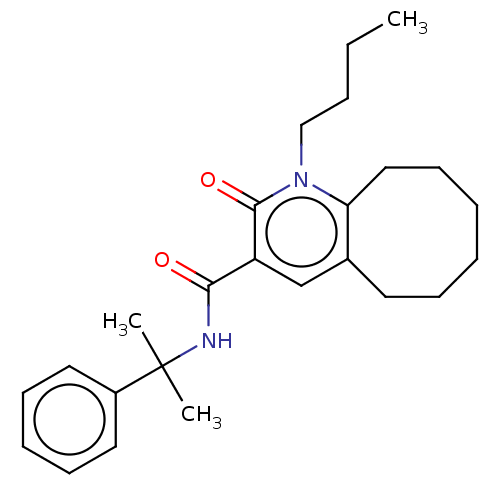 (CHEMBL2387185) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50379694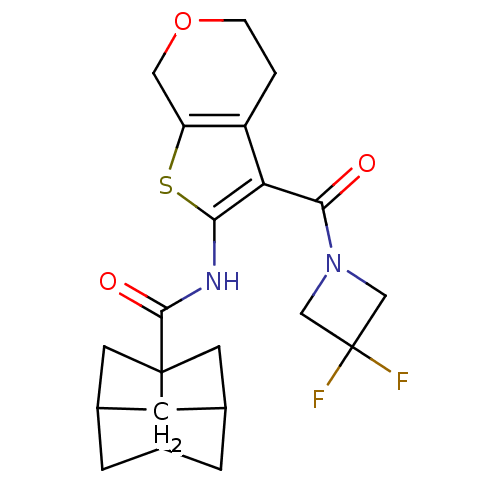 (CHEMBL2010900) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 20 min... | Bioorg Med Chem Lett 22: 2604-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.121 BindingDB Entry DOI: 10.7270/Q2FN1765 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340312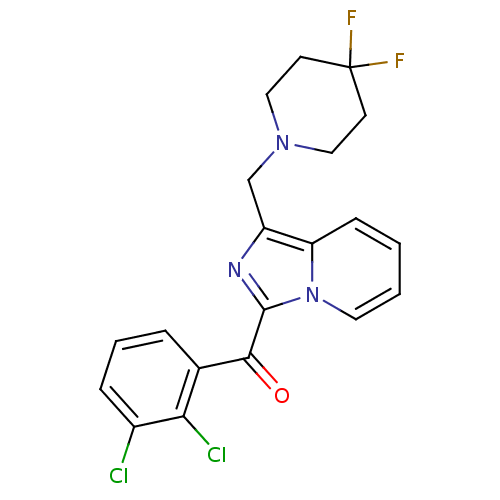 ((2,3-dichlorophenyl)(1-((4,4-difluoropiperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176622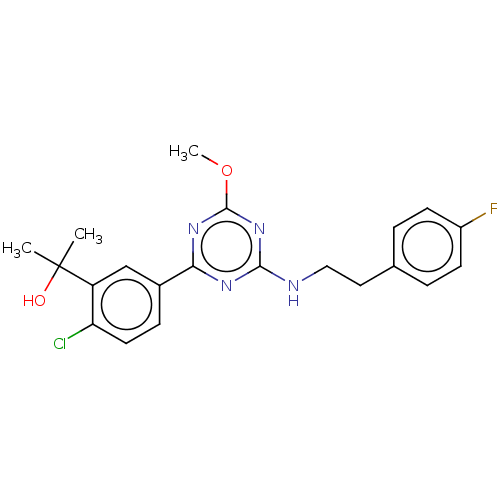 (US9115121, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50222831 (CHEMBL401132 | N-(5-tert-butyl-3-(cyclopropylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 form human CB2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6299-304 (2007) Article DOI: 10.1016/j.bmcl.2007.09.004 BindingDB Entry DOI: 10.7270/Q2PG1SJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50222845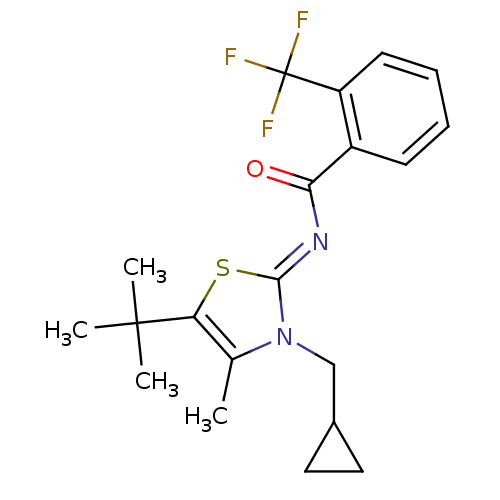 (CHEMBL247830 | N-(5-tert-butyl-3-(cyclopropylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 form human CB2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6299-304 (2007) Article DOI: 10.1016/j.bmcl.2007.09.004 BindingDB Entry DOI: 10.7270/Q2PG1SJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341934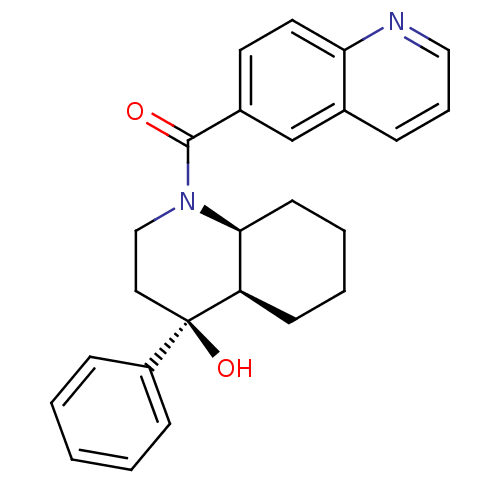 (CHEMBL1765259 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341920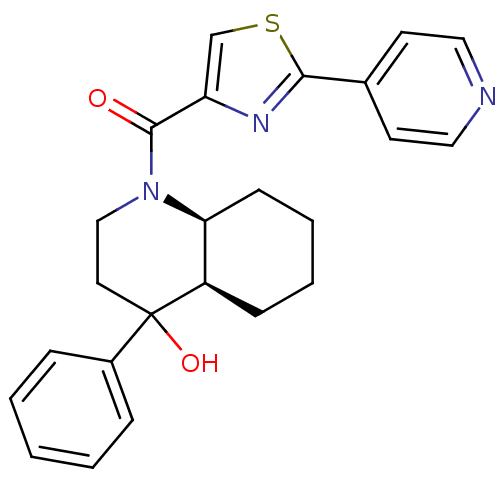 (((4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-1(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341920 (((4aR,8aS)-4-hydroxy-4-phenyloctahydroquinolin-1(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176622 (US9115121, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 24: 283-7 (2014) Article DOI: 10.1016/j.bmcl.2013.11.023 BindingDB Entry DOI: 10.7270/Q2ZC85VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM590790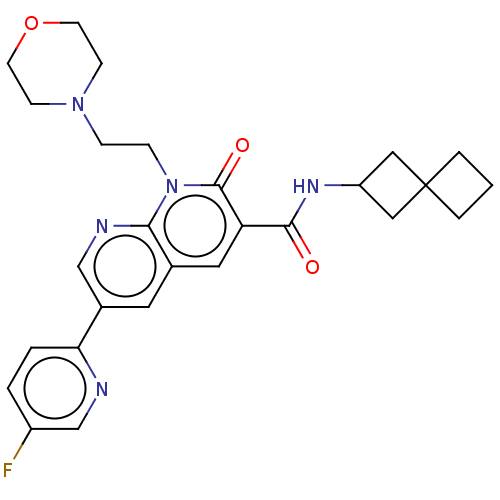 (6-(5-fluoropyridin-2-yl)-1-(2- morpholinoethyl)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4H60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM590808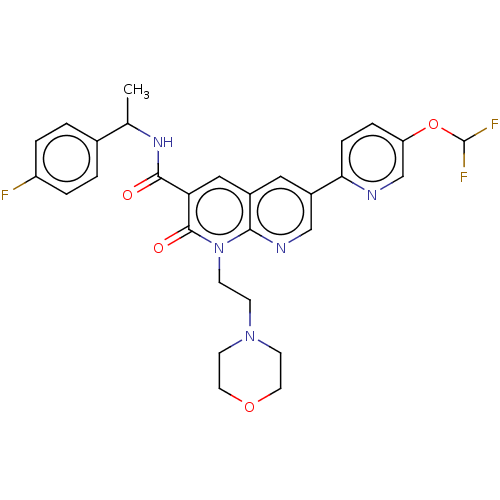 (6-(5-(difluoromethoxy)pyridin-2-yl)-N-(1-(4- fluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4H60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341930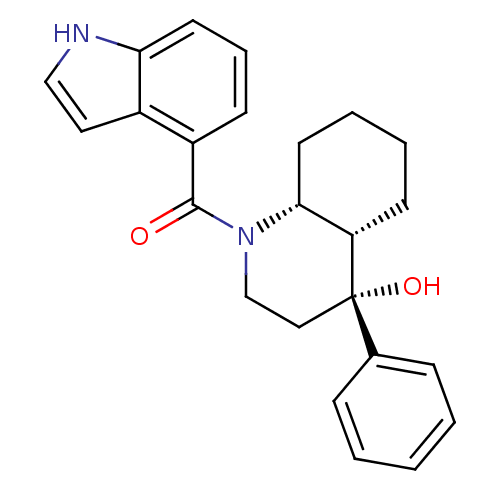 (CHEMBL1765246 | cis-((4R,4aS,8aR)-4-hydroxy-4-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50308537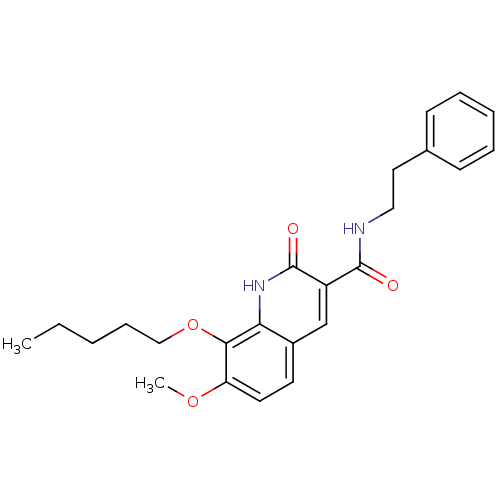 (7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kuopio Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S binding to human CB2 receptor expressed in CHO cells | J Med Chem 49: 2022-7 (2006) Article DOI: 10.1021/jm050879z BindingDB Entry DOI: 10.7270/Q21J9C3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM590781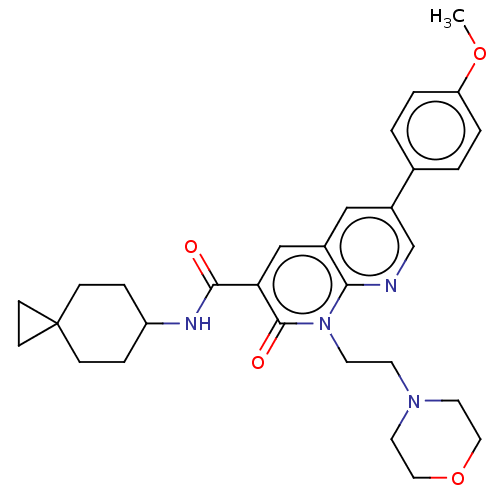 (6-(4-methoxyphenyl)-1-(2- morpholinoethyl)-2-oxo-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4H60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50340311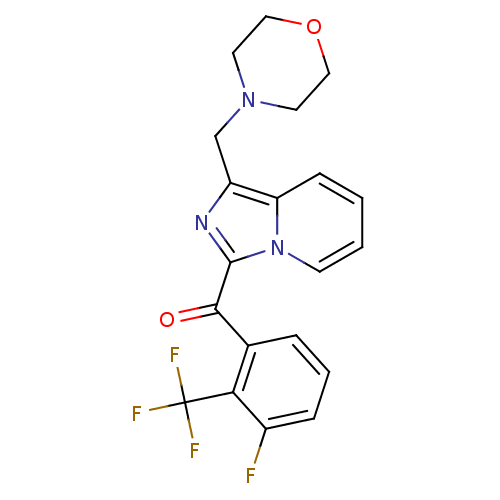 ((3-fluoro-2-(trifluoromethyl)phenyl)(1-(morpholino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP level | Bioorg Med Chem Lett 21: 2354-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.082 BindingDB Entry DOI: 10.7270/Q2C829MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029958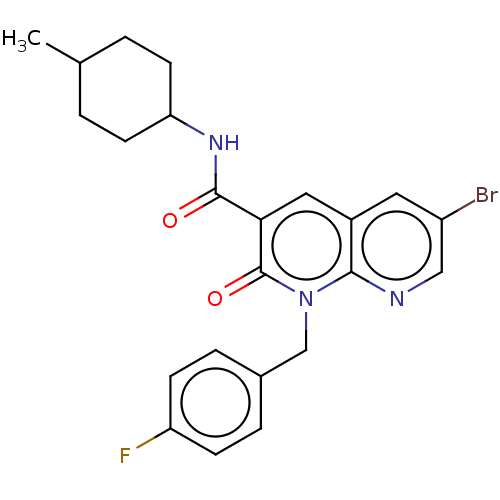 (CHEMBL3353439) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21311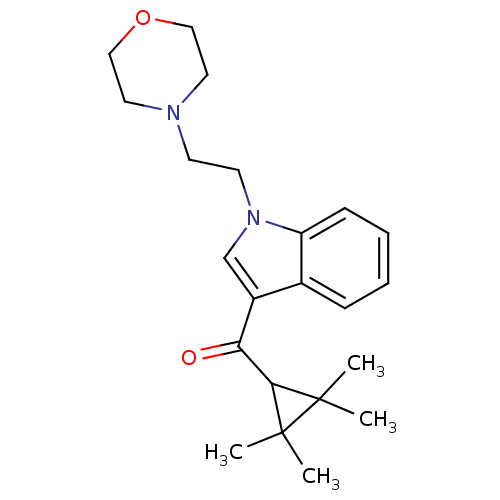 (4-(2-{3-[(2,2,3,3-tetramethylcyclopropyl)carbonyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor | Bioorg Med Chem 16: 1111-24 (2008) Article DOI: 10.1016/j.bmc.2007.10.087 BindingDB Entry DOI: 10.7270/Q208665C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21311 (4-(2-{3-[(2,2,3,3-tetramethylcyclopropyl)carbonyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor | J Med Chem 52: 369-78 (2009) Article DOI: 10.1021/jm801044g BindingDB Entry DOI: 10.7270/Q2VQ33M2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50308539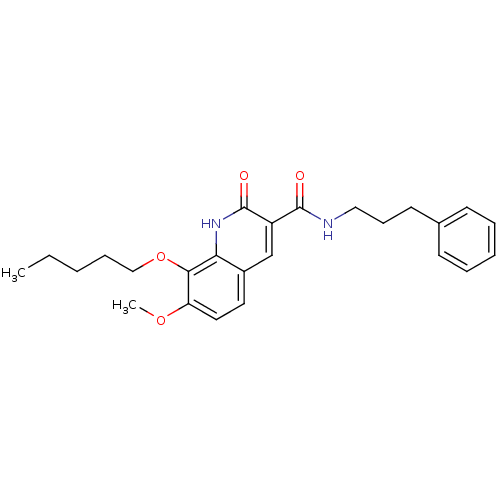 (7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kuopio Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S binding to human CB2 receptor expressed in CHO cells | J Med Chem 49: 2022-7 (2006) Article DOI: 10.1021/jm050879z BindingDB Entry DOI: 10.7270/Q21J9C3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM590785 (6-(4-fluorophenyl)-1-(2-morpholinoethyl)-2- oxo-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4H60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 form human CB2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6299-304 (2007) Article DOI: 10.1016/j.bmcl.2007.09.004 BindingDB Entry DOI: 10.7270/Q2PG1SJQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in CHO cells | Bioorg Med Chem 16: 1111-24 (2008) Article DOI: 10.1016/j.bmc.2007.10.087 BindingDB Entry DOI: 10.7270/Q208665C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341929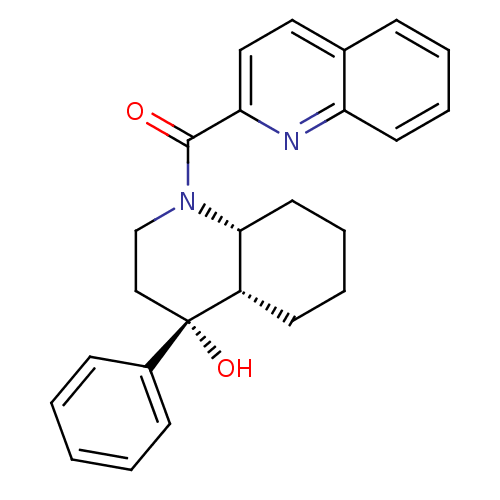 (CHEMBL1765161 | cis-((4R,4aS,8aR)-4-hydroxy-4-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50379693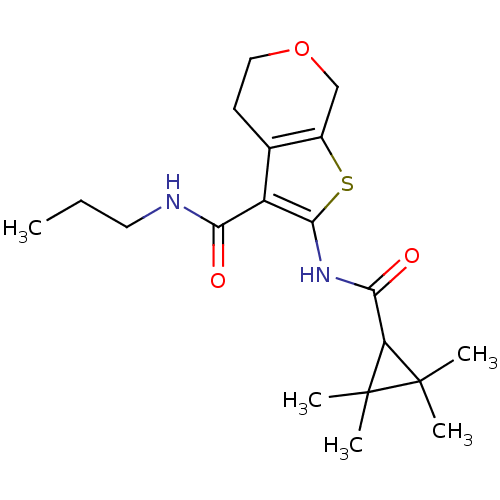 (CHEMBL2010905) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 20 min... | Bioorg Med Chem Lett 22: 2604-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.121 BindingDB Entry DOI: 10.7270/Q2FN1765 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176645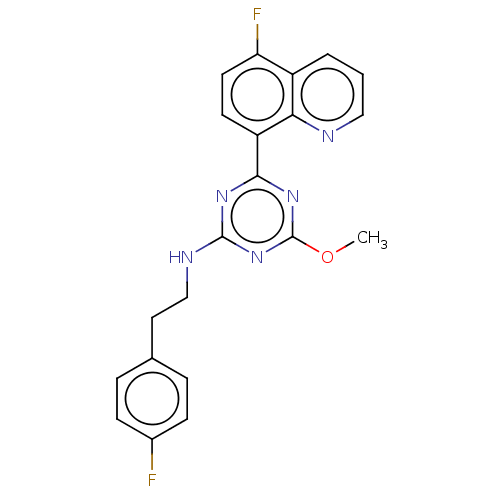 (US9115121, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176668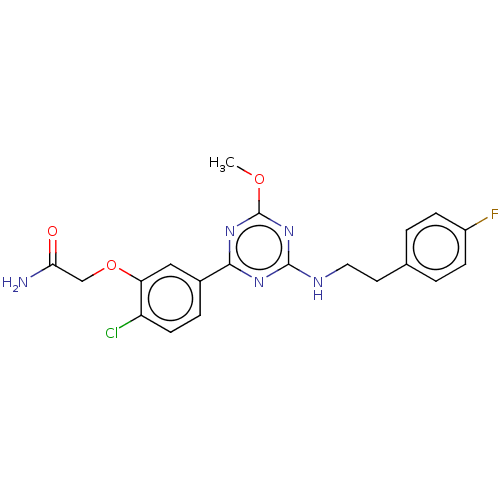 (US9115121, 65) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176606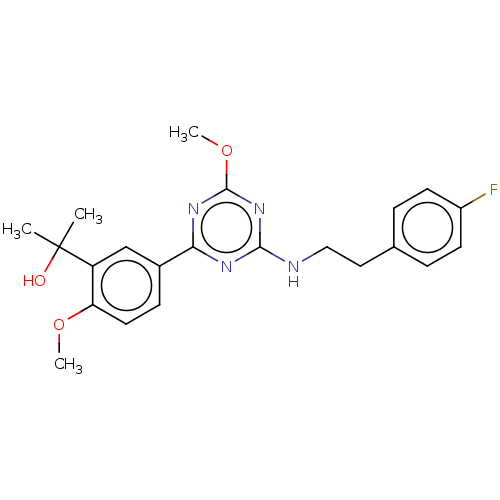 (US9115121, 58) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50380894 (CHEMBL2019078) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP release after 45 mins | Bioorg Med Chem Lett 22: 2803-6 (2012) Article DOI: 10.1016/j.bmcl.2012.02.072 BindingDB Entry DOI: 10.7270/Q2DB82VV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM590787 (1-(2-morpholinoethyl)-2-oxo-6- (pyridin-2-yl)-N-(s...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4H60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human CB2 in CHO cells assessed as inhibition of cAMP production | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50375384 (CHEMBL263913) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in CHO cells | Bioorg Med Chem 16: 1111-24 (2008) Article DOI: 10.1016/j.bmc.2007.10.087 BindingDB Entry DOI: 10.7270/Q208665C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50222834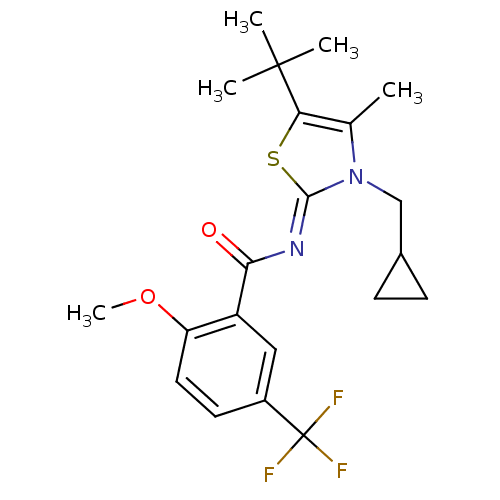 (CHEMBL398713 | N-(5-tert-butyl-3-(cyclopropylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 form human CB2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6299-304 (2007) Article DOI: 10.1016/j.bmcl.2007.09.004 BindingDB Entry DOI: 10.7270/Q2PG1SJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM590783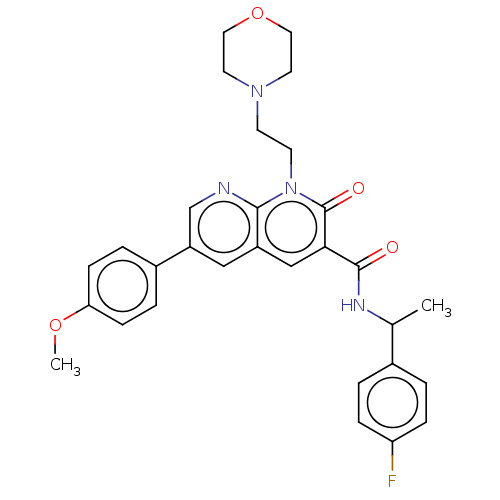 (N-(1-(4-fluorophenyl)ethyl)-6-(4-methoxyphenyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4H60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM590792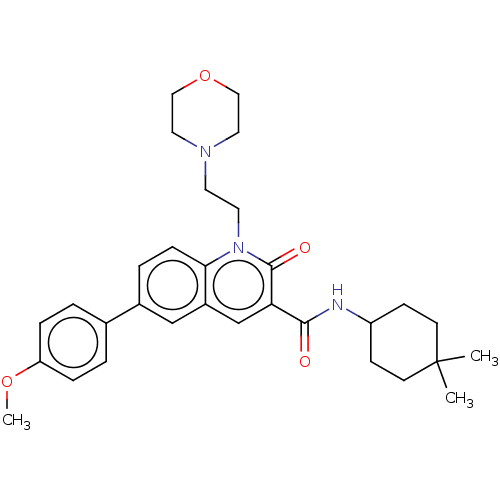 (N-(4,4-dimethylcyclohexyl)-6- (4-methoxyphenyl)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4H60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM590782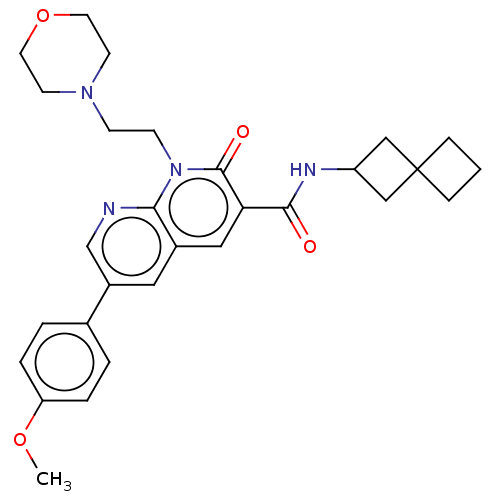 (6-(4-methoxyphenyl)-1-(2-morpholinoethyl)-2- oxo-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description CB1R binding protocol involves the use of the same solution buffer used for both incubation and washing reaction (Tris-HCl, 50 mM; EDTA, 2.5 mM; MgCl... | Citation and Details BindingDB Entry DOI: 10.7270/Q2VM4H60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50600961 (CHEMBL5198892) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00256 BindingDB Entry DOI: 10.7270/Q2Z89HGZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50222841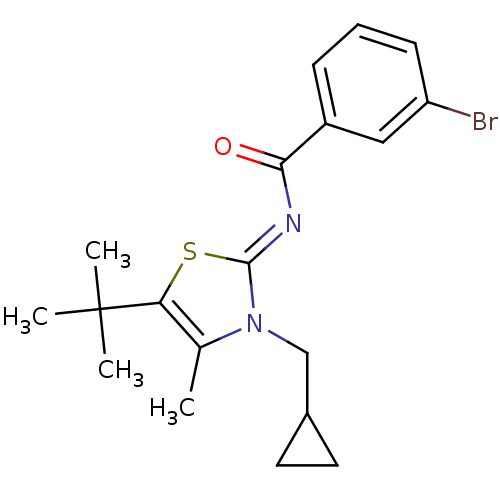 ((Z)-3-bromo-N-(5-tert-butyl-3-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 form human CB2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6299-304 (2007) Article DOI: 10.1016/j.bmcl.2007.09.004 BindingDB Entry DOI: 10.7270/Q2PG1SJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM176604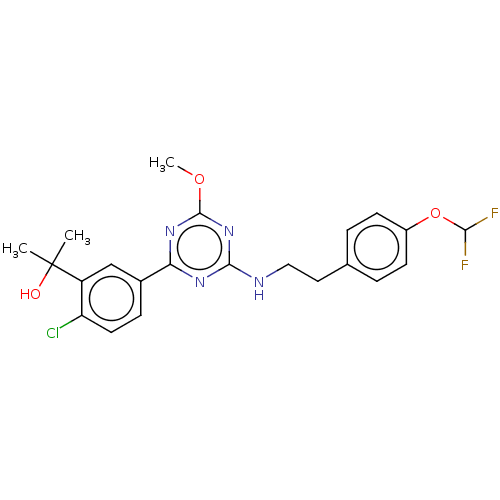 (US9115121, 46) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US Patent | Assay Description Affinity binding tests were performed according to the experimental conditions described by M. Rinaldi-Carmona in J. Pharmacol. Exp. Therap. 1998, 28... | US Patent US9115121 (2015) BindingDB Entry DOI: 10.7270/Q23N225X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50341936 (CHEMBL1765261 | cis-rac-((4S,4aR,8aS)-4-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP production treated 15 mins before fo... | Bioorg Med Chem Lett 21: 2359-64 (2011) Article DOI: 10.1016/j.bmcl.2011.02.078 BindingDB Entry DOI: 10.7270/Q2M90901 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50494951 (CHEMBL3099054) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor expressed in CHO cells | Bioorg Med Chem Lett 24: 283-7 (2014) Article DOI: 10.1016/j.bmcl.2013.11.023 BindingDB Entry DOI: 10.7270/Q2ZC85VD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491842 (CHEMBL2387078) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258652 (CHEMBL466651 | N-(4-Methylcyclohexyl)-1-(p-fluorob...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50249585 (CHEMBL3099055) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Farmacia-Scienze del Farmaco, UniversitÓ degli Studi di Bari Aldo Moro , Via Orabona 4, 70125, Bari, Italy. Curated by ChEMBL | Assay Description Inhibition of CB2 receptor (unknown origin) | J Med Chem 60: 9913-9931 (2017) Article DOI: 10.1021/acs.jmedchem.7b00155 BindingDB Entry DOI: 10.7270/Q2639S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50395162 (CHEMBL2163935) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB2 receptor expressed in CHO cell membranes assessed as inhibition of forskolin-induced cAMP accumulation | J Med Chem 55: 6608-23 (2012) Article DOI: 10.1021/jm300763w BindingDB Entry DOI: 10.7270/Q2WS8VCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50495626 (CHEMBL3114182) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inverse agonist activity at human CB2 receptor expressed in CHO membranes by [35S]GTPgammaS binding assay | Eur J Med Chem 74: 524-32 (2014) Article DOI: 10.1016/j.ejmech.2013.10.070 BindingDB Entry DOI: 10.7270/Q21V5HXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491840 (CHEMBL2387186) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi Pharmaceutical Research Center Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP production | Bioorg Med Chem 21: 3154-63 (2013) Article DOI: 10.1016/j.bmc.2013.03.030 BindingDB Entry DOI: 10.7270/Q22R3VKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1814 total ) | Next | Last >> |