 Found 2961 hits of ic50 data for polymerid = 2148
Found 2961 hits of ic50 data for polymerid = 2148 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM143355
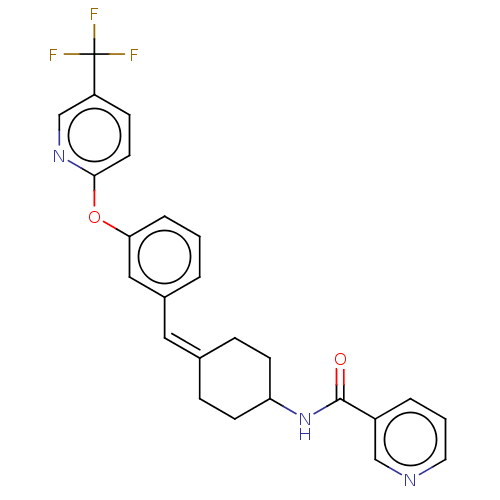
(US9682953, 20.A-1)Show SMILES CCOC(=O)Cn1nc2C(=O)N(C(c2c1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C Show InChI InChI=1S/C25H22F3N3O2/c26-25(27,28)20-8-11-23(30-16-20)33-22-5-1-3-18(14-22)13-17-6-9-21(10-7-17)31-24(32)19-4-2-12-29-15-19/h1-5,8,11-16,21H,6-7,9-10H2,(H,31,32)/b17-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH cofactor addition and me... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50136037
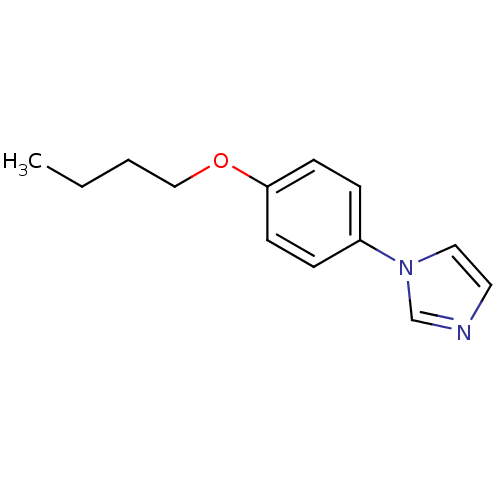
(1-(4-Butoxy-phenyl)-1H-imidazole | CHEMBL112532)Show InChI InChI=1S/C13H16N2O/c1-2-3-10-16-13-6-4-12(5-7-13)15-9-8-14-11-15/h4-9,11H,2-3,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Concentration required to inhibit cytochrome P450 19A1. |
J Med Chem 46: 5416-27 (2003)
Article DOI: 10.1021/jm020557k
BindingDB Entry DOI: 10.7270/Q2QF8S96 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50136037

(1-(4-Butoxy-phenyl)-1H-imidazole | CHEMBL112532)Show InChI InChI=1S/C13H16N2O/c1-2-3-10-16-13-6-4-12(5-7-13)15-9-8-14-11-15/h4-9,11H,2-3,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50317865

(2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...)Show InChI InChI=1S/C15H20N2S/c1-15(2,3)12-4-6-14(7-5-12)18-9-8-13-10-16-11-17-13/h4-7,10-11H,8-9H2,1-3H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 53: 6445-56 (2010)
Article DOI: 10.1021/jm100643t
BindingDB Entry DOI: 10.7270/Q2QF8T3X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50317865

(2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...)Show InChI InChI=1S/C15H20N2S/c1-15(2,3)12-4-6-14(7-5-12)18-9-8-13-10-16-11-17-13/h4-7,10-11H,8-9H2,1-3H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 expressed in insect cell microsome after 30 mins by fluorescence assay |
J Med Chem 53: 3840-4 (2010)
Article DOI: 10.1021/jm901890s
BindingDB Entry DOI: 10.7270/Q21Z44KC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM143359
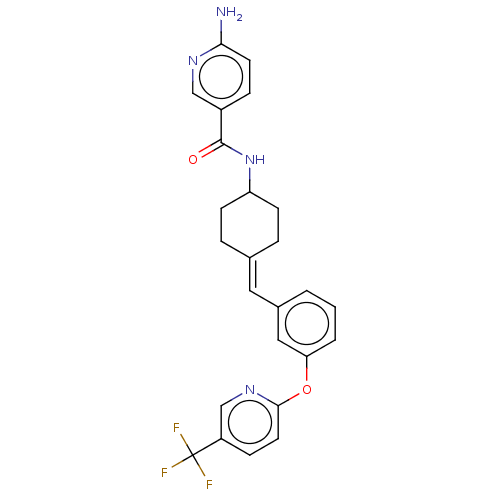
(US9682953, 20.A-3)Show SMILES Nc1ccc(cn1)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(12.65,-1.17,;11.31,-1.94,;11.31,-3.48,;9.98,-4.25,;8.65,-3.48,;8.65,-1.94,;9.98,-1.17,;7.31,-4.25,;7.31,-5.79,;5.98,-3.48,;4.65,-4.25,;4.65,-5.79,;3.31,-6.56,;1.98,-5.79,;1.98,-4.25,;3.31,-3.48,;.65,-6.56,;-.69,-5.79,;-.69,-4.25,;-2.02,-3.48,;-3.36,-4.25,;-3.36,-5.79,;-4.69,-6.56,;-6.02,-5.79,;-6.02,-4.25,;-7.36,-3.48,;-8.69,-4.25,;-8.69,-5.79,;-7.36,-6.56,;-10.02,-3.48,;-11.36,-4.25,;-10.02,-1.94,;-10.02,-5.02,;-2.02,-6.56,)| Show InChI InChI=1S/C25H23F3N4O2/c26-25(27,28)19-7-11-23(31-15-19)34-21-3-1-2-17(13-21)12-16-4-8-20(9-5-16)32-24(33)18-6-10-22(29)30-14-18/h1-3,6-7,10-15,20H,4-5,8-9H2,(H2,29,30)(H,32,33)/b16-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH cofactor addition and me... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM143373

(US9682953, 20.A-10 | US9682953, 20.A-9)Show SMILES Nc1ncc(cn1)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(11.34,2.69,;10,1.93,;10,.38,;8.67,-.38,;7.34,.38,;7.34,1.93,;8.67,2.69,;6,-.38,;6,-1.93,;4.67,.38,;3.33,-.38,;3.33,-1.93,;2,-2.69,;.67,-1.93,;.67,-.38,;2,.38,;-.67,-2.69,;-2,-1.93,;-2,-.38,;-3.33,.38,;-4.67,-.38,;-4.67,-1.93,;-6,-2.69,;-7.34,-1.93,;-7.34,-.38,;-8.67,.38,;-10,-.38,;-10,-1.93,;-8.67,-2.69,;-11.34,.38,;-12.67,-.39,;-11.34,1.93,;-11.34,-1.16,;-3.33,-2.69,)| Show InChI InChI=1S/C24H22F3N5O2/c25-24(26,27)18-6-9-21(29-14-18)34-20-3-1-2-16(11-20)10-15-4-7-19(8-5-15)32-22(33)17-12-30-23(28)31-13-17/h1-3,6,9-14,19H,4-5,7-8H2,(H,32,33)(H2,28,30,31)/b15-10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >13 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH cofactor addition and me... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM143362
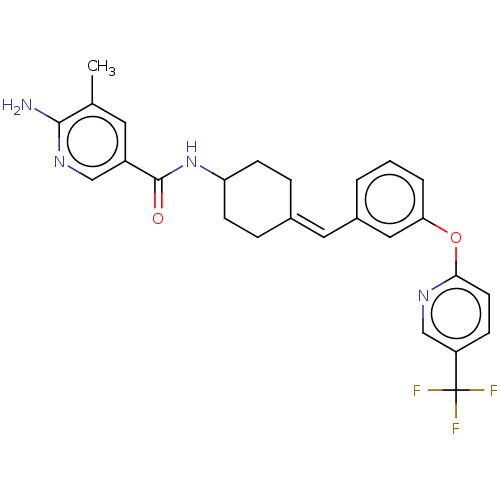
(US9682953, 20.A-5)Show SMILES Cc1cc(cnc1N)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(11.34,-.39,;10,.38,;8.67,-.38,;7.34,.38,;7.34,1.93,;8.67,2.69,;10,1.93,;11.34,2.69,;6,-.38,;6,-1.93,;4.67,.38,;3.33,-.38,;3.33,-1.93,;2,-2.69,;.67,-1.93,;.67,-.38,;2,.38,;-.67,-2.69,;-2,-1.93,;-2,-.38,;-3.33,.38,;-4.67,-.38,;-4.67,-1.93,;-6,-2.69,;-7.34,-1.93,;-7.34,-.38,;-8.67,.38,;-10,-.38,;-10,-1.93,;-8.67,-2.69,;-11.34,.38,;-12.67,-.39,;-11.34,1.93,;-11.34,-1.16,;-3.33,-2.69,)| Show InChI InChI=1S/C26H25F3N4O2/c1-16-11-19(14-32-24(16)30)25(34)33-21-8-5-17(6-9-21)12-18-3-2-4-22(13-18)35-23-10-7-20(15-31-23)26(27,28)29/h2-4,7,10-15,21H,5-6,8-9H2,1H3,(H2,30,32)(H,33,34)/b17-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >13 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH cofactor addition and me... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50460773
(CHEMBL4227082)Show SMILES Cc1cn2c(cnc2c(Nc2cc(Cc3ccccn3)ns2)n1)-c1cn[nH]c1 Show InChI InChI=1S/C19H16N8S/c1-12-11-27-16(13-8-22-23-9-13)10-21-19(27)18(24-12)25-17-7-15(26-28-17)6-14-4-2-3-5-20-14/h2-5,7-11H,6H2,1H3,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes |
Bioorg Med Chem Lett 28: 1397-1403 (2018)
Article DOI: 10.1016/j.bmcl.2018.02.037
BindingDB Entry DOI: 10.7270/Q2K35X91 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50201124
(3-(6-(1H-imidazol-1-yl)-4-methyl-1H-benzo[d]imidaz...)Show SMILES Cc1cc(cc2nc([nH]c12)-c1c(NCC(O)c2cccc(Cl)c2)cc[nH]c1=O)-n1ccnc1 Show InChI InChI=1S/C24H21ClN6O2/c1-14-9-17(31-8-7-26-13-31)11-19-22(14)30-23(29-19)21-18(5-6-27-24(21)33)28-12-20(32)15-3-2-4-16(25)10-15/h2-11,13,20,32H,12H2,1H3,(H,29,30)(H2,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Concentration required to inhibit cytochrome P450 isozyme CYP2C19 in vitro by 50% |
J Med Chem 48: 5639-43 (2005)
Article DOI: 10.1021/jm050392q
BindingDB Entry DOI: 10.7270/Q2MW2GPP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM247369
(US9447092, 2)Show SMILES Cc1nn(C)cc1-c1nc2c(N3CCN(Cc4ncon4)CC3)c(Cl)cnc2[nH]1 Show InChI InChI=1S/C18H20ClN9O/c1-11-12(8-26(2)24-11)17-22-15-16(13(19)7-20-18(15)23-17)28-5-3-27(4-6-28)9-14-21-10-29-25-14/h7-8,10H,3-6,9H2,1-2H3,(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... |
US Patent US9447092 (2016)
BindingDB Entry DOI: 10.7270/Q2PV6J9C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM247371
(US9447092, 1)Show SMILES Cc1nn(C)cc1-c1nc2c(N3CCN(Cc4ccc(Cl)cc4)CC3)c(Cl)cnc2[nH]1 Show InChI InChI=1S/C22H23Cl2N7/c1-14-17(13-29(2)28-14)21-26-19-20(18(24)11-25-22(19)27-21)31-9-7-30(8-10-31)12-15-3-5-16(23)6-4-15/h3-6,11,13H,7-10,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... |
US Patent US9447092 (2016)
BindingDB Entry DOI: 10.7270/Q2PV6J9C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM247368
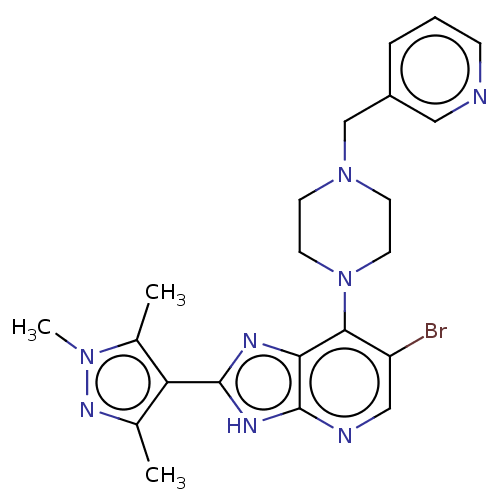
(US9447092, Comparator 2, Example 57)Show SMILES Cc1nn(C)c(C)c1-c1nc2c(N3CCN(Cc4cccnc4)CC3)c(Br)cnc2[nH]1 Show InChI InChI=1S/C22H25BrN8/c1-14-18(15(2)29(3)28-14)21-26-19-20(17(23)12-25-22(19)27-21)31-9-7-30(8-10-31)13-16-5-4-6-24-11-16/h4-6,11-12H,7-10,13H2,1-3H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... |
US Patent US9447092 (2016)
BindingDB Entry DOI: 10.7270/Q2PV6J9C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50552388
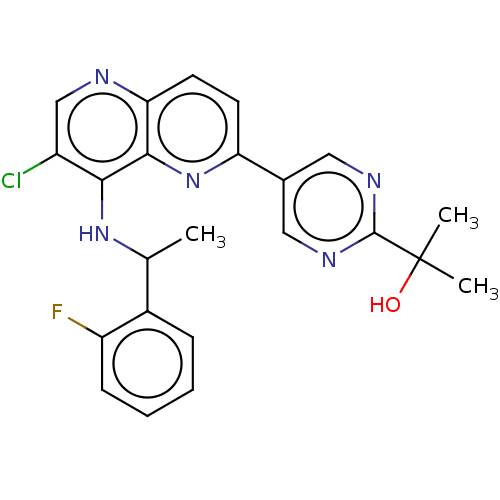
(CHEMBL4742065)Show SMILES CC(Nc1c(Cl)cnc2ccc(nc12)-c1cnc(nc1)C(C)(C)O)c1ccccc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01732
BindingDB Entry DOI: 10.7270/Q2S46WM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM247367
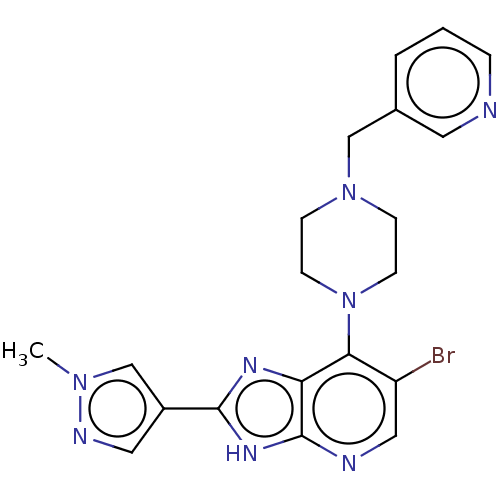
(US9447092, Comparator 1, Example 56)Show SMILES Cn1cc(cn1)-c1nc2c(N3CCN(Cc4cccnc4)CC3)c(Br)cnc2[nH]1 Show InChI InChI=1S/C20H21BrN8/c1-27-13-15(10-24-27)19-25-17-18(16(21)11-23-20(17)26-19)29-7-5-28(6-8-29)12-14-3-2-4-22-9-14/h2-4,9-11,13H,5-8,12H2,1H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... |
US Patent US9447092 (2016)
BindingDB Entry DOI: 10.7270/Q2PV6J9C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50552393

(CHEMBL4779059)Show SMILES Cc1ccc(C)c(Nc2c(Cl)cnc3ccc(cc23)-c2cnc(nc2)C(C)(C)O)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant CYP2C19 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01732
BindingDB Entry DOI: 10.7270/Q2S46WM7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50352206
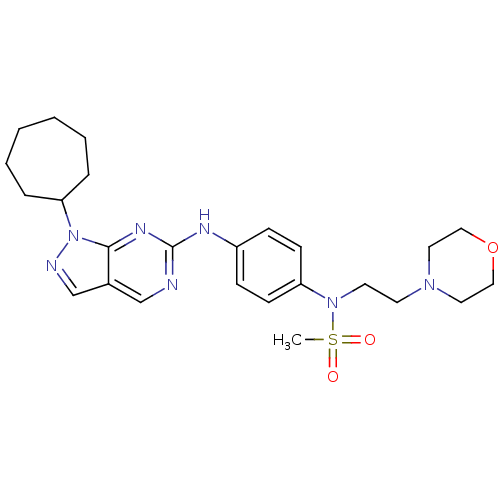
(CHEMBL1825089)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H35N7O3S/c1-36(33,34)31(13-12-30-14-16-35-17-15-30)22-10-8-21(9-11-22)28-25-26-18-20-19-27-32(24(20)29-25)23-6-4-2-3-5-7-23/h8-11,18-19,23H,2-7,12-17H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM155255
(US10098888, Compound 105 | US11642348, Compound 10...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)C#CC1CCN(C)CC1 Show InChI InChI=1S/C31H42N4O3/c1-6-35(26-11-15-38-16-12-26)29-19-25(8-7-24-9-13-34(5)14-10-24)18-27(23(29)4)30(36)32-20-28-21(2)17-22(3)33-31(28)37/h17-19,24,26H,6,9-16,20H2,1-5H3,(H,32,36)(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 33.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc.
US Patent
| Assay Description
The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... |
US Patent US10098888 (2018)
BindingDB Entry DOI: 10.7270/Q2BK1FDQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50384464
(CHEMBL2036210)Show SMILES CCC(=O)N(Cc1ccc(cc1)C(F)(F)F)c1cc(F)cc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C18H15F4N5O/c1-2-16(28)27(10-11-3-5-13(6-4-11)18(20,21)22)15-8-12(7-14(19)9-15)17-23-25-26-24-17/h3-9H,2,10H2,1H3,(H,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 938-942 (2011)
Article DOI: 10.1021/ml200223s
BindingDB Entry DOI: 10.7270/Q2Z89DGQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50294698
(4-((2-chlorobenzyl)(1-(1-methyl-1H-1,2,3-triazol-4...)Show SMILES CC(N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1)c1cn(C)nn1 Show InChI InChI=1S/C19H17Cl2N5/c1-13(19-12-25(2)24-23-19)26(11-15-5-3-4-6-17(15)20)16-8-7-14(10-22)18(21)9-16/h3-9,12-13H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 19: 2637-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.146
BindingDB Entry DOI: 10.7270/Q2FX79GM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM30130

(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) using luciferin tagged substrate preincubated for 10 mins before substrate addition |
Eur J Med Chem 103: 210-22 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.001
BindingDB Entry DOI: 10.7270/Q2DR2XBB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50380531

(CHEMBL2018917)Show InChI InChI=1S/C18H20N2OS/c1-12(2)14-6-4-13(5-7-14)11-22-18-19-16-9-8-15(21-3)10-17(16)20-18/h4-10,12H,11H2,1-3H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mpheytoin as substrate preincubated for 5 mins by LC-MS/MS analysis |
J Med Chem 55: 1205-14 (2012)
Article DOI: 10.1021/jm201346g
BindingDB Entry DOI: 10.7270/Q2QV3NHG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50113978
(CHEMBL3605543)Show SMILES CCn1c(nc2ccc(cc12)C(F)(F)F)[C@@H](C)NS(=O)(=O)c1ccncc1 |r| Show InChI InChI=1S/C17H17F3N4O2S/c1-3-24-15-10-12(17(18,19)20)4-5-14(15)22-16(24)11(2)23-27(25,26)13-6-8-21-9-7-13/h4-11,23H,3H2,1-2H3/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method |
J Med Chem 58: 7057-75 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01078
BindingDB Entry DOI: 10.7270/Q2Z89F6J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138237

(3-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-3-15(13-18-9-1)4-2-12-21-17-7-5-16(6-8-17)20-11-10-19-14-20/h1,3,5-11,13-14H,2,4,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138232
(2-(4-Imidazol-1-yl-phenoxymethyl)-pyridine | CHEMB...)Show InChI InChI=1S/C15H13N3O/c1-2-8-17-13(3-1)11-19-15-6-4-14(5-7-15)18-10-9-16-12-18/h1-10,12H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138233

(2-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-2-10-19-15(4-1)5-3-13-21-17-8-6-16(7-9-17)20-12-11-18-14-20/h1-2,4,6-12,14H,3,5,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138236
(2-Hexyloxy-5-imidazol-1-yl-pyridine | CHEMBL320105)Show InChI InChI=1S/C14H19N3O/c1-2-3-4-5-10-18-14-7-6-13(11-16-14)17-9-8-15-12-17/h6-9,11-12H,2-5,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50136059

(5-(4-Butoxy-phenyl)-1H-imidazole | CHEMBL151170)Show InChI InChI=1S/C13H16N2O/c1-2-3-8-16-12-6-4-11(5-7-12)13-9-14-10-15-13/h4-7,9-10H,2-3,8H2,1H3,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Concentration required to inhibit cytochrome P450 19A1. |
J Med Chem 46: 5416-27 (2003)
Article DOI: 10.1021/jm020557k
BindingDB Entry DOI: 10.7270/Q2QF8S96 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138238

(4-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1(2-15-7-9-18-10-8-15)13-21-17-5-3-16(4-6-17)20-12-11-19-14-20/h3-12,14H,1-2,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50136043
(4-(4-Butoxy-phenyl)-pyrimidine | CHEMBL151974)Show InChI InChI=1S/C14H16N2O/c1-2-3-10-17-13-6-4-12(5-7-13)14-8-9-15-11-16-14/h4-9,11H,2-3,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Concentration required to inhibit cytochrome P450 19A1. |
J Med Chem 46: 5416-27 (2003)
Article DOI: 10.1021/jm020557k
BindingDB Entry DOI: 10.7270/Q2QF8S96 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50410623
(CHEMBL2113179)Show SMILES OCCCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C25H23ClN4O5S/c26-18-9-11-19(12-10-18)30(36(34,35)20-13-7-17(8-14-20)4-3-15-31)16-23(32)28-29-24-21-5-1-2-6-22(21)27-25(24)33/h1-2,5-14,31H,3-4,15-16H2,(H,28,32)(H,27,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against cytochrome P450 2C19 isoform |
J Med Chem 48: 7882-905 (2005)
Article DOI: 10.1021/jm050645f
BindingDB Entry DOI: 10.7270/Q2QF8TN3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50123453
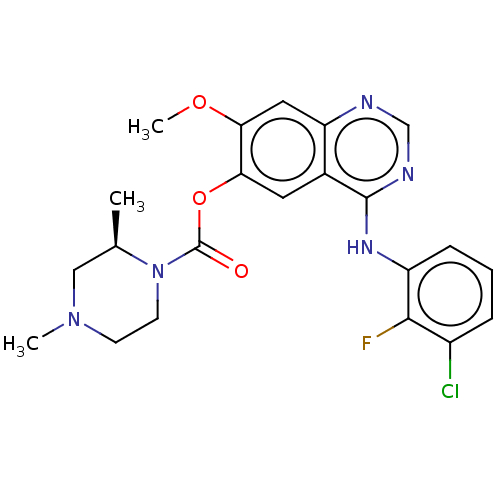
(CHEMBL3623290)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC(=O)N1CCN(C)C[C@H]1C |r| Show InChI InChI=1S/C22H23ClFN5O3/c1-13-11-28(2)7-8-29(13)22(30)32-19-9-14-17(10-18(19)31-3)25-12-26-21(14)27-16-6-4-5-15(23)20(16)24/h4-6,9-10,12-13H,7-8,11H2,1-3H3,(H,25,26,27)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 58: 8200-15 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01073
BindingDB Entry DOI: 10.7270/Q29P33FH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50297385
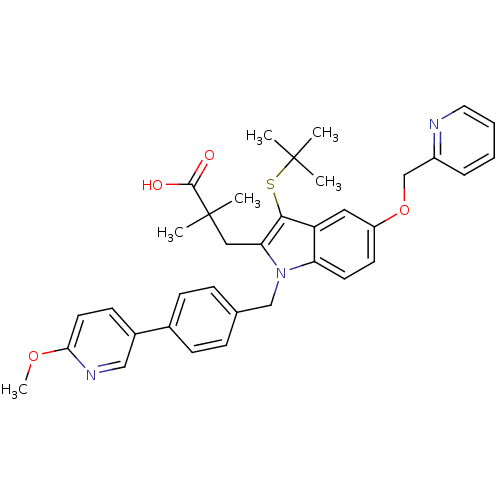
(3-[3-tert-Butylsulfanyl-1-[4-(6-methoxy-pyridin-3-...)Show SMILES COc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccccn4)ccc23)cc1 Show InChI InChI=1S/C36H39N3O4S/c1-35(2,3)44-33-29-19-28(43-23-27-9-7-8-18-37-27)15-16-30(29)39(31(33)20-36(4,5)34(40)41)22-24-10-12-25(13-11-24)26-14-17-32(42-6)38-21-26/h7-19,21H,20,22-23H2,1-6H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >50 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50380515
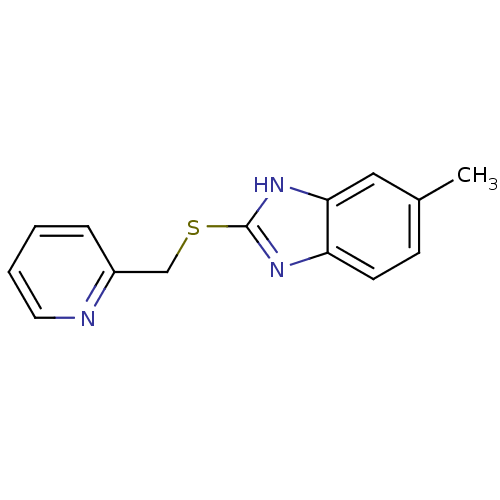
(CHEMBL2019024)Show InChI InChI=1S/C14H13N3S/c1-10-5-6-12-13(8-10)17-14(16-12)18-9-11-4-2-3-7-15-11/h2-8H,9H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mpheytoin as substrate preincubated for 5 mins by LC-MS/MS analysis |
J Med Chem 55: 1205-14 (2012)
Article DOI: 10.1021/jm201346g
BindingDB Entry DOI: 10.7270/Q2QV3NHG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50384467
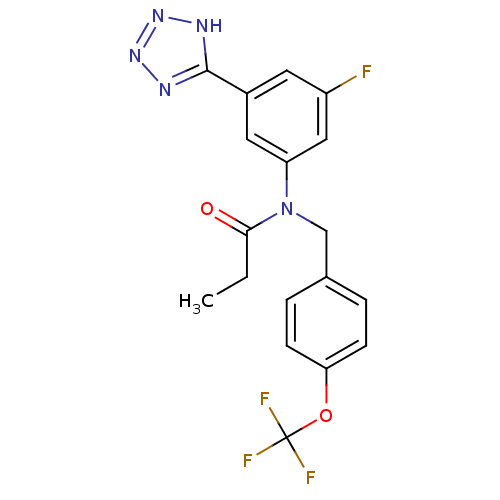
(CHEMBL2036213)Show SMILES CCC(=O)N(Cc1ccc(OC(F)(F)F)cc1)c1cc(F)cc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C18H15F4N5O2/c1-2-16(28)27(10-11-3-5-15(6-4-11)29-18(20,21)22)14-8-12(7-13(19)9-14)17-23-25-26-24-17/h3-9H,2,10H2,1H3,(H,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 938-942 (2011)
Article DOI: 10.1021/ml200223s
BindingDB Entry DOI: 10.7270/Q2Z89DGQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50326289
(4-(1-(1H-Imidazole-4-yl)-2-(4-trifluoromeethylphen...)Show InChI InChI=1S/C17H14F3N3S/c18-17(19,20)13-1-3-14(4-2-13)24-10-15(16-9-22-11-23-16)12-5-7-21-8-6-12/h1-9,11,15H,10H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 53: 6445-56 (2010)
Article DOI: 10.1021/jm100643t
BindingDB Entry DOI: 10.7270/Q2QF8T3X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50446472
(CHEMBL3110021 | US9765054, Compound 41B)Show SMILES ONC(=O)[C@@H]1[C@@H]([C@H]1c1ccc(cc1)-c1cnco1)c1ccccc1 |r| Show InChI InChI=1S/C19H16N2O3/c22-19(21-23)18-16(13-4-2-1-3-5-13)17(18)14-8-6-12(7-9-14)15-10-20-11-24-15/h1-11,16-18,23H,(H,21,22)/t16-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
BioFocus
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
J Med Chem 56: 9934-54 (2013)
Article DOI: 10.1021/jm4011884
BindingDB Entry DOI: 10.7270/Q2DZ09RK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50396441
(CHEMBL2170635)Show SMILES NC(=N)NC(=O)c1ccc(C2CCN(CC2)C(=O)c2ccncc2)c(c1)C(F)(F)F Show InChI InChI=1S/C20H20F3N5O2/c21-20(22,23)16-11-14(17(29)27-19(24)25)1-2-15(16)12-5-9-28(10-6-12)18(30)13-3-7-26-8-4-13/h1-4,7-8,11-12H,5-6,9-10H2,(H4,24,25,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibiton of CYP2C19 in human liver microsomes using 7-ethoxy-3-cyanocoumarin as substrate after 45 mins by LC/MS/MS analysis |
J Med Chem 55: 7114-40 (2012)
Article DOI: 10.1021/jm300601d
BindingDB Entry DOI: 10.7270/Q2FB5430 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50294716
(4-((2-chlorobenzyl)(2-methyl-1-(4-methyl-4H-1,2,4-...)Show SMILES CC(C)C(N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1)c1nncn1C Show InChI InChI=1S/C21H21Cl2N5/c1-14(2)20(21-26-25-13-27(21)3)28(12-16-6-4-5-7-18(16)22)17-9-8-15(11-24)19(23)10-17/h4-10,13-14,20H,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 19: 2637-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.146
BindingDB Entry DOI: 10.7270/Q2FX79GM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM30130

(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126882
BindingDB Entry DOI: 10.7270/Q25Q50MC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50294696
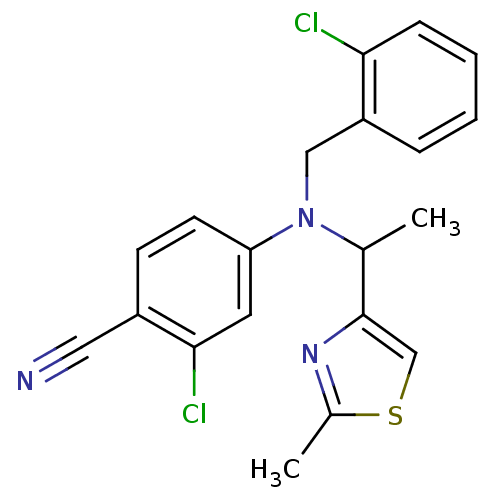
(4-((2-chlorobenzyl)(1-(2-methylthiazol-4-yl)ethyl)...)Show SMILES CC(N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1)c1csc(C)n1 Show InChI InChI=1S/C20H17Cl2N3S/c1-13(20-12-26-14(2)24-20)25(11-16-5-3-4-6-18(16)21)17-8-7-15(10-23)19(22)9-17/h3-9,12-13H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 19: 2637-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.146
BindingDB Entry DOI: 10.7270/Q2FX79GM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50136038
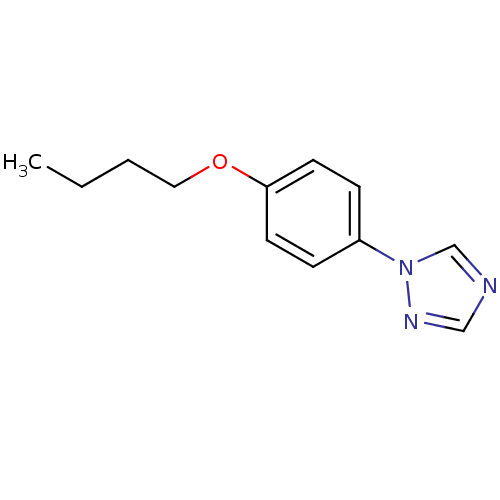
(1-(4-Butoxy-phenyl)-1H-[1,2,4]triazole | CHEMBL345...)Show InChI InChI=1S/C12H15N3O/c1-2-3-8-16-12-6-4-11(5-7-12)15-10-13-9-14-15/h4-7,9-10H,2-3,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Concentration required to inhibit cytochrome P450 19A1. |
J Med Chem 46: 5416-27 (2003)
Article DOI: 10.1021/jm020557k
BindingDB Entry DOI: 10.7270/Q2QF8S96 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50380510

(CHEMBL2018925)Show InChI InChI=1S/C21H18N2O2S/c1-24-17-10-11-19-20(13-17)23-21(22-19)26-14-15-6-5-9-18(12-15)25-16-7-3-2-4-8-16/h2-13H,14H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using (S)-mpheytoin as substrate preincubated for 5 mins by LC-MS/MS analysis |
J Med Chem 55: 1205-14 (2012)
Article DOI: 10.1021/jm201346g
BindingDB Entry DOI: 10.7270/Q2QV3NHG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50026732

(CHEMBL3331506)Show InChI InChI=1S/C16H16ClFN2/c17-13-7-5-12(6-8-13)11-20(14-9-19-10-14)16-4-2-1-3-15(16)18/h1-8,14,19H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C19 incubated for 5 mins by fluorescence assay |
ACS Med Chem Lett 5: 999-1004 (2014)
Article DOI: 10.1021/ml500187a
BindingDB Entry DOI: 10.7270/Q2MW2JRD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM93132
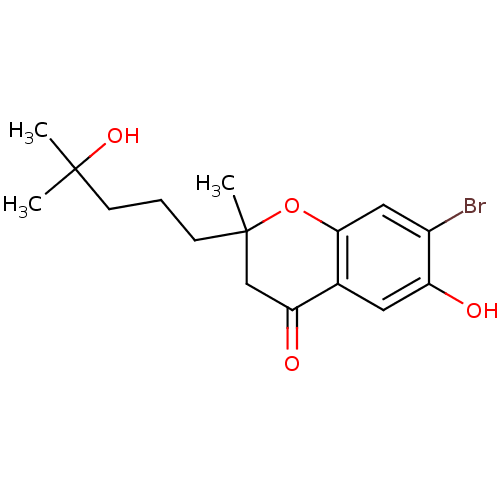
(PBQ1)Show InChI InChI=1S/C16H21BrO4/c1-15(2,20)5-4-6-16(3)9-13(19)10-7-12(18)11(17)8-14(10)21-16/h7-8,18,20H,4-6,9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the West Indies
| Assay Description
The test compounds were evaluated for their ability to inhibit the catlytic activity of human CYP1 enzymes by means of high throughput fluorometric d... |
Org Med Chem Lett 2: 21 (2012)
Article DOI: 10.1186/2191-2858-2-21
BindingDB Entry DOI: 10.7270/Q25H7DWM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50294708

(4-((2-chlorobenzyl)(1-(1-isopropyl-1H-tetrazol-5-y...)Show SMILES CC(C)n1nnnc1C(C)N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1 Show InChI InChI=1S/C20H20Cl2N6/c1-13(2)28-20(24-25-26-28)14(3)27(12-16-6-4-5-7-18(16)21)17-9-8-15(11-23)19(22)10-17/h4-10,13-14H,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 19: 2637-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.146
BindingDB Entry DOI: 10.7270/Q2FX79GM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM155253
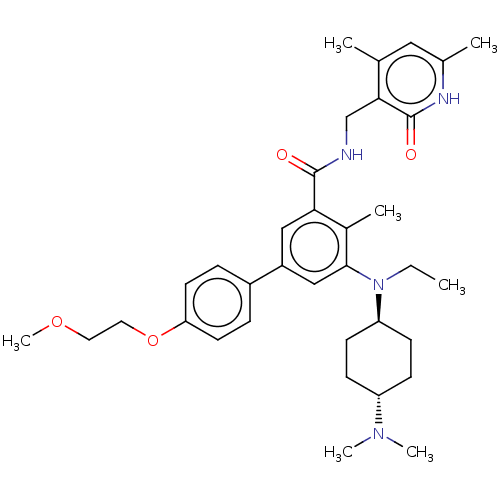
(US10098888, Compound 1 | US9006242, 1)Show SMILES CCN([C@H]1CC[C@@H](CC1)N(C)C)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(OCCOC)cc1 |r,wU:3.2,wD:6.9,(-6.67,1.54,;-5.33,.77,;-4,1.54,;-4,3.08,;-5.33,3.85,;-5.33,5.39,;-4,6.16,;-2.67,5.39,;-2.67,3.85,;-4,7.7,;-5.33,8.47,;-2.67,8.47,;-2.67,.77,;-1.33,1.54,;,.77,;,-.77,;-1.33,-1.54,;-1.33,-3.08,;,-3.85,;-2.67,-3.85,;-2.67,-5.39,;-4,-6.16,;-4,-7.7,;-2.67,-8.47,;-5.33,-8.47,;-6.67,-7.7,;-8,-8.47,;-6.67,-6.16,;-5.33,-5.39,;-5.33,-3.85,;-2.67,-.77,;-4,-1.54,;1.33,1.54,;2.67,.77,;4,1.54,;4,3.08,;5.33,3.85,;6.67,3.08,;6.67,1.54,;8,.77,;8,-.77,;2.67,3.85,;1.33,3.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 89.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc.
US Patent
| Assay Description
The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... |
US Patent US10098888 (2018)
BindingDB Entry DOI: 10.7270/Q2BK1FDQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50294701
(4-((2-chlorobenzyl)(1-(5-methyl-1,3,4-oxadiazol-2-...)Show SMILES CC(N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1)c1nnc(C)o1 Show InChI InChI=1S/C19H16Cl2N4O/c1-12(19-24-23-13(2)26-19)25(11-15-5-3-4-6-17(15)20)16-8-7-14(10-22)18(21)9-16/h3-9,12H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 19: 2637-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.146
BindingDB Entry DOI: 10.7270/Q2FX79GM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138231

(4-[2-(4-Imidazol-1-yl-phenoxy)-ethyl]-morpholine |...)Show InChI InChI=1S/C15H19N3O2/c1-3-15(4-2-14(1)18-6-5-16-13-18)20-12-9-17-7-10-19-11-8-17/h1-6,13H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50301852
(2-(3-methyl-1,2-dioxaspiro[4.5]decan-3-yl)-N-(pyri...)Show InChI InChI=1S/C17H24N2O3/c1-16(13-17(22-21-16)7-3-2-4-8-17)11-15(20)19-12-14-5-9-18-10-6-14/h5-6,9-10H,2-4,7-8,11-13H2,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Infectious Diseases Initiative
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 |
Bioorg Med Chem Lett 19: 5657-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.024
BindingDB Entry DOI: 10.7270/Q22R3RRX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data