 Found 1062 hits of ic50 data for polymerid = 2152,3431,7886
Found 1062 hits of ic50 data for polymerid = 2152,3431,7886 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT7 receptor expressed in CHO cells |
Bioorg Med Chem 24: 1793-810 (2016)
Article DOI: 10.1016/j.bmc.2016.03.006
BindingDB Entry DOI: 10.7270/Q2J67JS7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in CHO cells measured after 120 mins by scintillation counting method |
Bioorg Med Chem 25: 471-482 (2017)
Article DOI: 10.1016/j.bmc.2016.11.014
BindingDB Entry DOI: 10.7270/Q2CF9S3S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT7 receptor after 120 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT7 receptor expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556317
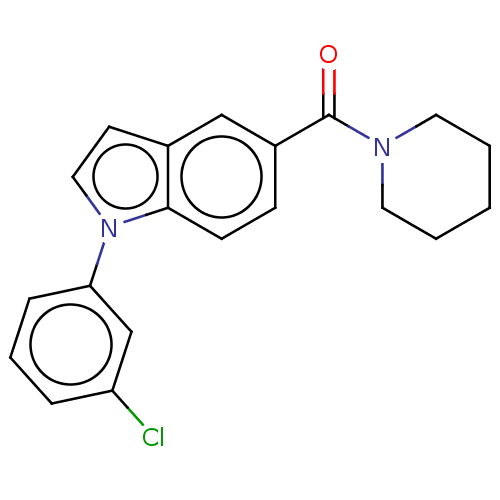
(US11345702, Table 1.35 | US20230390274, Compound A...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556286
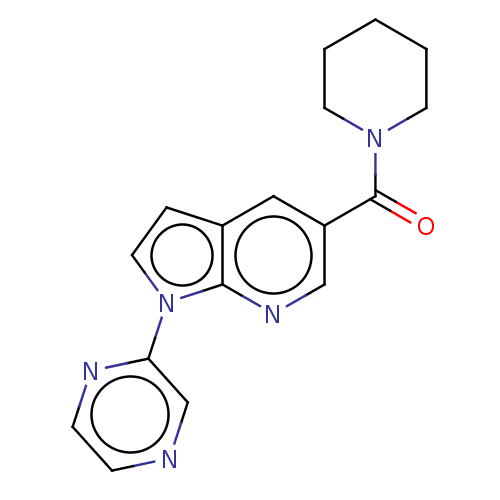
(US11345702, Table 1.4 | US20230390274, Compound A-...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
China State Institute of Pharmaceutical Industry
Curated by ChEMBL
| Assay Description
Antagonist activity at 5HT7R receptor (unknown origin) assessed as forskolin-mediated cAMP accumulation preincubated for 30 mins followed by forskoli... |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126703
BindingDB Entry DOI: 10.7270/Q2DR2ZZT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT7 receptor expressed in HEK293 cells assessed as inhibition of Gs protein-mediated cAMP accumulation by luminescence... |
J Med Chem 61: 7218-7233 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00642
BindingDB Entry DOI: 10.7270/Q2BC424N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT7 receptor expressed in CHO-K1 cells assessed as decrease in serotonin-induced cAMP level after 1 hr by TR-FRET assa... |
Bioorg Med Chem Lett 28: 2039-2049 (2018)
Article DOI: 10.1016/j.bmcl.2018.04.059
BindingDB Entry DOI: 10.7270/Q2CJ8H4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human 5-HT7 receptor expressed in HEK293 cells assessed as reduction in cAMP accumulation incubated for 30 mins by microplate ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01093
BindingDB Entry DOI: 10.7270/Q2833WTS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human 5-HT7 receptor expressed in HEK293 cells assessed as reduction in cAMP accumulation incubated for 30 mins by microplate ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01093
BindingDB Entry DOI: 10.7270/Q2833WTS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.933 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT7 receptor expressed in HEK293 cells assessed as inhibition of Gs protein-mediated cAMP accumulation by luminescence... |
J Med Chem 61: 7218-7233 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00642
BindingDB Entry DOI: 10.7270/Q2BC424N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556311
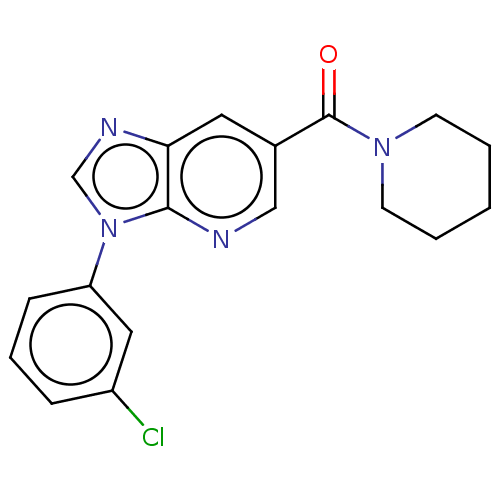
(US11345702, Table 1.29 | US20230390274, Compound A...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Adamed Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant 5-HT7 receptor assessed as inhibition of serotonin-induced cAMP accumulation |
J Med Chem 57: 4543-57 (2014)
Article DOI: 10.1021/jm401895u
BindingDB Entry DOI: 10.7270/Q2N29ZHX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556300
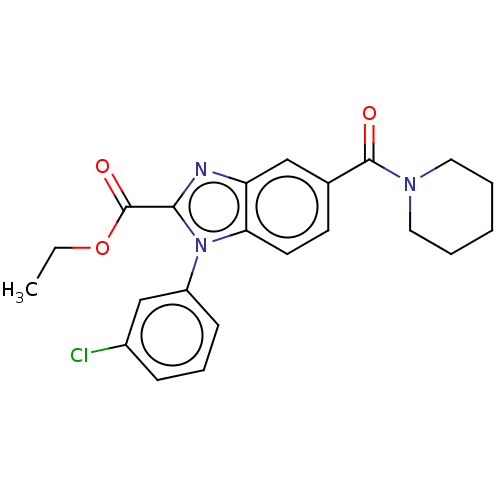
(US11345702, Table 1.18 | US20230390274, Compound A...)Show SMILES CCOC(=O)c1nc2cc(ccc2n1-c1cccc(Cl)c1)C(=O)N1CCCCC1 |$;;O;;;;;;;;;;;;;;;;;;;;;;;;;;$| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50425433
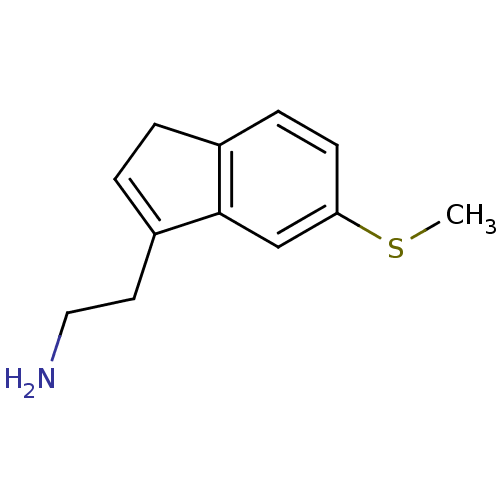
(METHIOTHEPINE)Show InChI InChI=1S/C12H15NS/c1-14-11-5-4-9-2-3-10(6-7-13)12(9)8-11/h3-5,8H,2,6-7,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT7 receptor expressed in HEK293 assessed as inhibition of 5HT-induced cAMP accumulation |
Eur J Med Chem 60: 42-50 (2013)
Article DOI: 10.1016/j.ejmech.2012.11.042
BindingDB Entry DOI: 10.7270/Q21V5G98 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556287
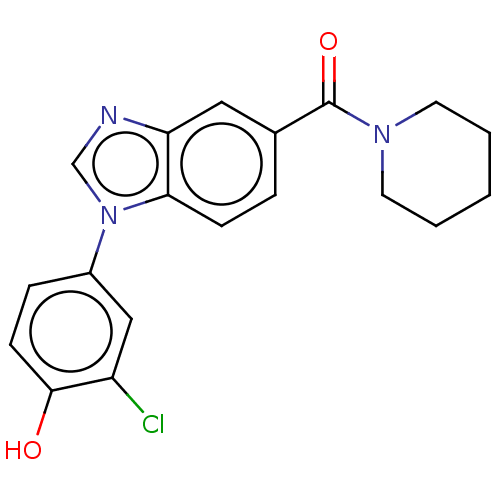
(US11345702, Table 1.5 | US20230390274, Compound A-...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 receptor expressed in CHO cells |
J Nat Prod 66: 535-7 (2003)
Article DOI: 10.1021/np0205102
BindingDB Entry DOI: 10.7270/Q2CZ36WZ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant 5-HT7 receptor expressed in CHOK1 cells measured after 60 mins in the presence of serotonin by LANCE TR-FRET... |
Bioorg Med Chem 27: 4163-4173 (2019)
Article DOI: 10.1016/j.bmc.2019.07.046
BindingDB Entry DOI: 10.7270/Q2VT1WD5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant 5-HT7 receptor expressed in CHOK1 cells measured after 60 mins in the presence of serotonin by LANCE TR-FRET... |
Bioorg Med Chem 27: 4163-4173 (2019)
Article DOI: 10.1016/j.bmc.2019.07.046
BindingDB Entry DOI: 10.7270/Q2VT1WD5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50445521

(CHEMBL3103033 | US11345702, Table 1.21 | US2023039...)Show InChI InChI=1S/C19H18ClN3O/c20-15-5-4-6-16(12-15)23-13-21-17-11-14(7-8-18(17)23)19(24)22-9-2-1-3-10-22/h4-8,11-13H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556325
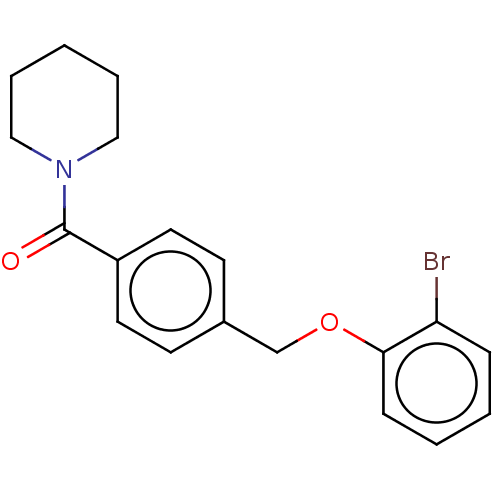
(US11345702, Table 2.8 | US20230390274, Compound A-...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT7 receptor |
Bioorg Med Chem 16: 2570-8 (2008)
Article DOI: 10.1016/j.bmc.2007.11.049
BindingDB Entry DOI: 10.7270/Q2JW8FRT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556313
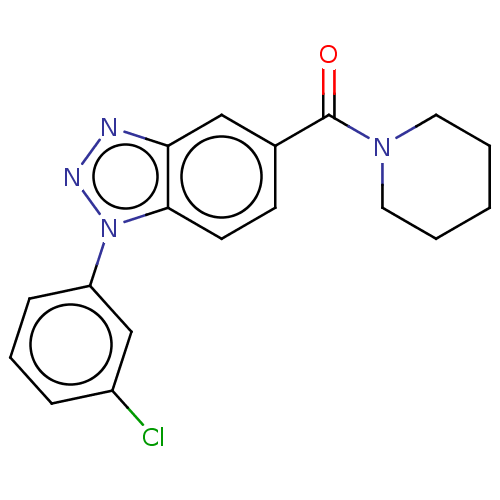
(US11345702, Table 1.31 | US20230390274, Compound A...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556306
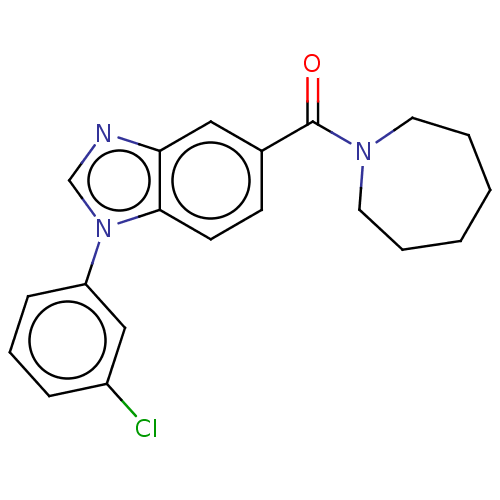
(US11345702, Table 1.24 | US20230390274, Compound A...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT7R expressed in HEK293 cells assessed as inhibition of serotonin stimulated-cAMP levels preincubated for 10 mins fol... |
Eur J Med Chem 123: 180-190 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.029
BindingDB Entry DOI: 10.7270/Q27D2X36 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT7R expressed in HEK293 cells assessed as inhibition of serotonin stimulated-cAMP levels preincubated for 10 mins fol... |
Eur J Med Chem 123: 180-190 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.029
BindingDB Entry DOI: 10.7270/Q27D2X36 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556299
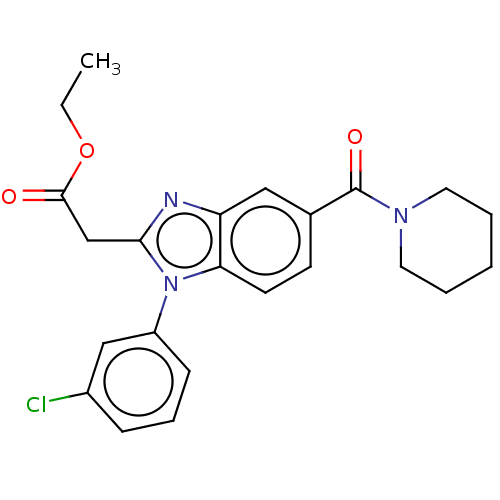
(US11345702, Table 1.17 | US20230390274, Compound A...)Show SMILES CCOC(=O)Cc1nc2cc(ccc2n1-c1cccc(Cl)c1)C(=O)N1CCCCC1 |$;;O;;;;;;;;;;;;;;;;;;;;;;;;;;;$| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556294
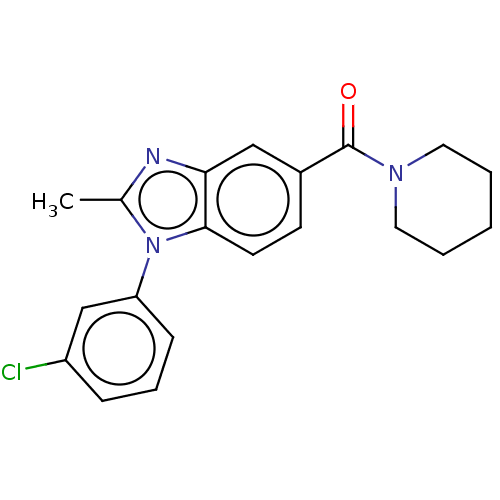
(US11345702, Table 1.12 | US20230390274, Compound A...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556298
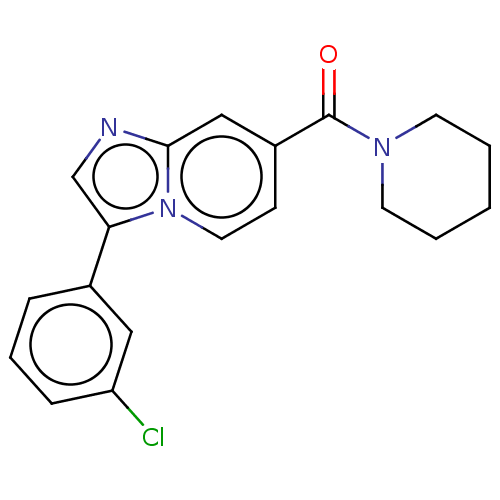
(US11345702, Table 1.16 | US20230390274, Compound A...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556316
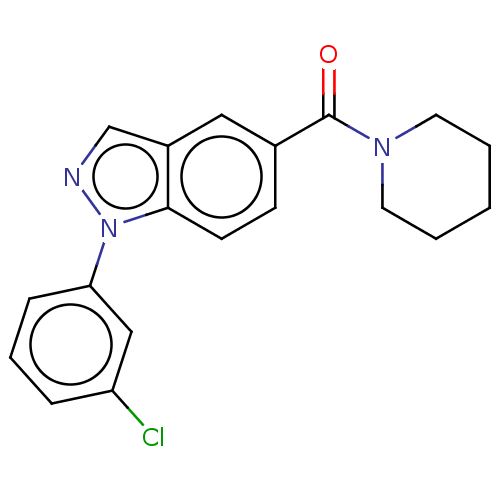
(US11345702, Table 1.34 | US20230390274, Compound A...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM352210
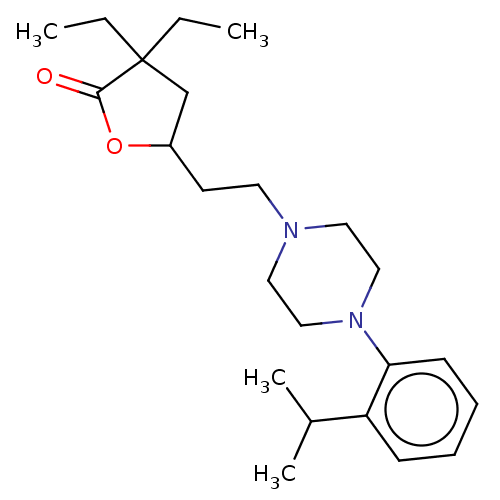
(US10239868, Entry 14 | US10287274, Entry 14 | US10...)Show InChI InChI=1S/C23H36N2O2/c1-5-23(6-2)17-19(27-22(23)26)11-12-24-13-15-25(16-14-24)21-10-8-7-9-20(21)18(3)4/h7-10,18-19H,5-6,11-17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TEMPLE UNIVERSITY OF THE COMMONWEALTH SYSTEM OF HIGHER EDUCATION
US Patent
| Assay Description
A stock concentration of 5 nM [3H]LSD (lysergic acid diethyl amide) is prepared in 50 mM Tris-HCl, 10 mM MgCl2, 1 mM EDTA, pH 7.4 (Assay Buffer). Ali... |
US Patent US9802924 (2017)
BindingDB Entry DOI: 10.7270/Q25Q4Z7N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50306820

(CHEMBL602878 | N-(Cyclopropylmethyl)-N-(3-(4-(3-(t...)Show SMILES FC(F)(F)c1cccc(c1)N1CCN(CCCN(CC2CC2)S(=O)(=O)c2cccc3cccnc23)CC1 Show InChI InChI=1S/C27H31F3N4O2S/c28-27(29,30)23-7-2-8-24(19-23)33-17-15-32(16-18-33)13-4-14-34(20-21-10-11-21)37(35,36)25-9-1-5-22-6-3-12-31-26(22)25/h1-3,5-9,12,19,21H,4,10-11,13-18,20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in CHO cells |
Bioorg Med Chem 18: 1665-75 (2010)
Article DOI: 10.1016/j.bmc.2009.12.067
BindingDB Entry DOI: 10.7270/Q2R78F99 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50445514

(CHEMBL3103040 | US11345702, Table 1.33 | US2023039...)Show InChI InChI=1S/C20H21N3O2/c1-25-17-8-6-16(7-9-17)23-14-21-18-13-15(5-10-19(18)23)20(24)22-11-3-2-4-12-22/h5-10,13-14H,2-4,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM9274

(CHEMBL1822893 | US8835436, Example 8)Show SMILES Cc1nc(C(=O)NCCN2CCN(CC2)c2cccc(Cl)c2Cl)c(C)n1-c1ccccc1 Show InChI InChI=1S/C24H27Cl2N5O/c1-17-23(28-18(2)31(17)19-7-4-3-5-8-19)24(32)27-11-12-29-13-15-30(16-14-29)21-10-6-9-20(25)22(21)26/h3-10H,11-16H2,1-2H3,(H,27,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT7 receptor by competition binding assay |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM427037
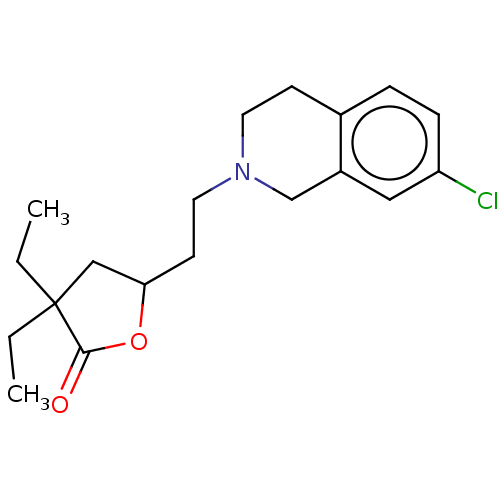
(US10544117, Entry 230070 | US11192871, Example 230...)Show InChI InChI=1S/C19H26ClNO2/c1-3-19(4-2)12-17(23-18(19)22)8-10-21-9-7-14-5-6-16(20)11-15(14)13-21/h5-6,11,17H,3-4,7-10,12-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University—Of The Commonwealth System of Higher Education
US Patent
| Assay Description
A solution of the compound of the disclosure to be tested is prepared as a 1-mg/ml stock in Assay Buffer or DMSO according to its solubility. A simil... |
US Patent US10544117 (2020)
BindingDB Entry DOI: 10.7270/Q2W66P43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556320
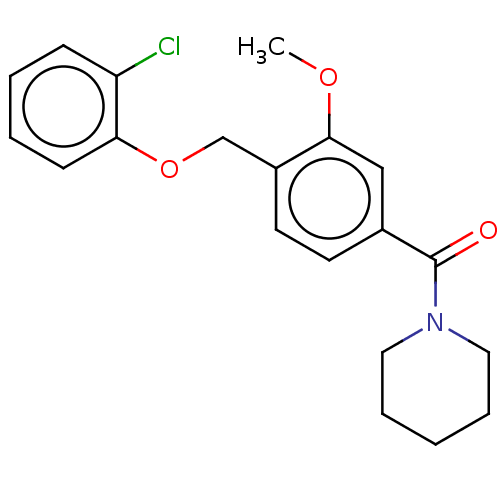
(US11345702, Table 2.3 | US20230390274, Compound A-...) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM489605
(US10961249, Entry 16 | US11220505, Entry 7)Show SMILES CCC1(CC)CC(CCN2CC3CN(CC3C2)c2ccccc2C#N)OC1=O Show InChI InChI=1S/C23H31N3O2/c1-3-23(4-2)11-20(28-22(23)27)9-10-25-13-18-15-26(16-19(18)14-25)21-8-6-5-7-17(21)12-24/h5-8,18-20H,3-4,9-11,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
A stock concentration of 5 nM [3H]-5-Hydroxytryptamine ([3H]-5HT) is prepared in 50 mM Tris-HCl, 10 mM MgCl2, 1 mM EDTA, pH 7.4 (Assay Buffer). Aliqu... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2SQ93K8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM427038
(US10544117, Entry 230071 | US11192871, Example 230...)Show InChI InChI=1S/C19H26FNO2/c1-3-19(4-2)12-17(23-18(19)22)8-10-21-9-7-14-5-6-16(20)11-15(14)13-21/h5-6,11,17H,3-4,7-10,12-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University—Of The Commonwealth System of Higher Education
US Patent
| Assay Description
A solution of the compound of the disclosure to be tested is prepared as a 1-mg/ml stock in Assay Buffer or DMSO according to its solubility. A simil... |
US Patent US10544117 (2020)
BindingDB Entry DOI: 10.7270/Q2W66P43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM352200
(US10239868, Entry 4 | US10287274, Entry 4 | US1067...)Show InChI InChI=1S/C21H32N2O2/c1-4-21(5-2)16-19(25-20(21)24)10-11-22-12-14-23(15-13-22)18-8-6-17(3)7-9-18/h6-9,19H,4-5,10-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TEMPLE UNIVERSITY OF THE COMMONWEALTH SYSTEM OF HIGHER EDUCATION
US Patent
| Assay Description
A stock concentration of 5 nM [3H]LSD (lysergic acid diethyl amide) is prepared in 50 mM Tris-HCl, 10 mM MgCl2, 1 mM EDTA, pH 7.4 (Assay Buffer). Ali... |
US Patent US9802924 (2017)
BindingDB Entry DOI: 10.7270/Q25Q4Z7N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556309
(US11345702, Table 1.27 | US20230390274, Compound A...)Show SMILES Clc1cn(-c2cccc(Cl)c2)c2ccc(cc12)C(=O)N1CCCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human 5HT7 receptor expressed in CHO-K1 cells assessed as inhibition of 5CT-induced calcium flux incubated for 60 mins at 37 d... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112709
BindingDB Entry DOI: 10.7270/Q2XK8K6M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50352724
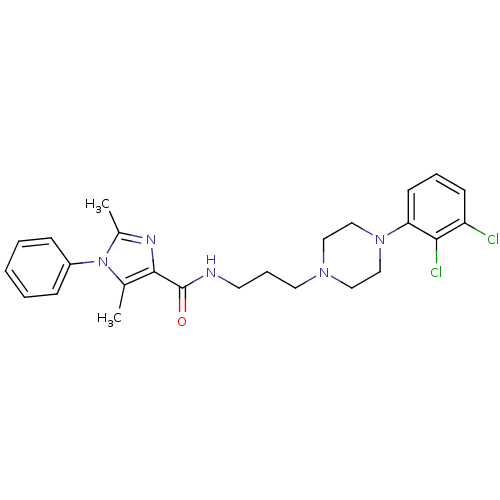
(CHEMBL1822896 | US8835436, Example 6)Show SMILES Cc1nc(C(=O)NCCCN2CCN(CC2)c2cccc(Cl)c2Cl)c(C)n1-c1ccccc1 Show InChI InChI=1S/C25H29Cl2N5O/c1-18-24(29-19(2)32(18)20-8-4-3-5-9-20)25(33)28-12-7-13-30-14-16-31(17-15-30)22-11-6-10-21(26)23(22)27/h3-6,8-11H,7,12-17H2,1-2H3,(H,28,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of 5HT7 receptor by competition binding assay |
J Med Chem 54: 6305-18 (2011)
Article DOI: 10.1021/jm200682b
BindingDB Entry DOI: 10.7270/Q2SX6DMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50375936
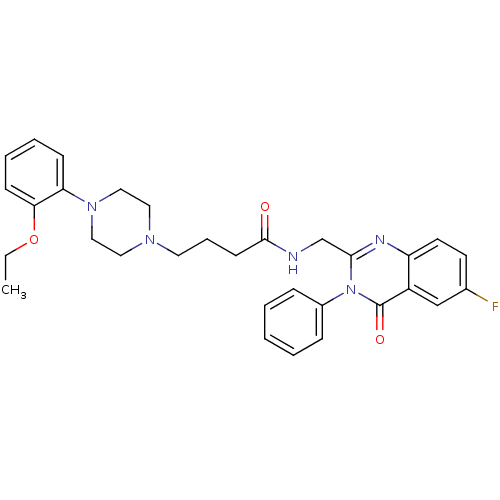
(CHEMBL260907)Show SMILES CCOc1ccccc1N1CCN(CCCC(=O)NCc2nc3ccc(F)cc3c(=O)n2-c2ccccc2)CC1 Show InChI InChI=1S/C31H34FN5O3/c1-2-40-28-12-7-6-11-27(28)36-19-17-35(18-20-36)16-8-13-30(38)33-22-29-34-26-15-14-23(32)21-25(26)31(39)37(29)24-9-4-3-5-10-24/h3-7,9-12,14-15,21H,2,8,13,16-20,22H2,1H3,(H,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Sharif University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of 5HT7 receptor by radioligand binding assay |
Eur J Med Chem 44: 1463-70 (2009)
Article DOI: 10.1016/j.ejmech.2008.09.050
BindingDB Entry DOI: 10.7270/Q2QV3NRM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50510969
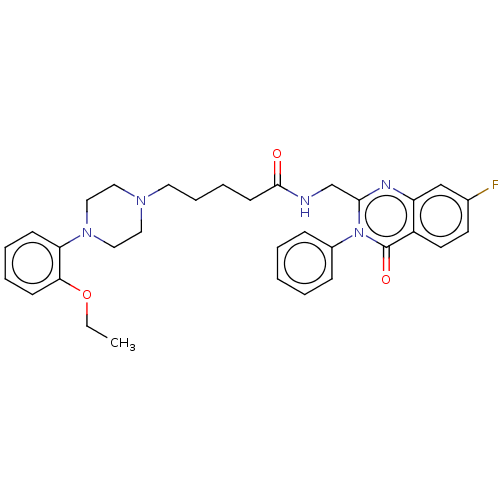
(CHEMBL4582321)Show SMILES CCOc1ccccc1N1CCN(CCCCC(=O)NCc2nc3cc(F)ccc3c(=O)n2-c2ccccc2)CC1 Show InChI InChI=1S/C32H36FN5O3/c1-2-41-29-13-7-6-12-28(29)37-20-18-36(19-21-37)17-9-8-14-31(39)34-23-30-35-27-22-24(33)15-16-26(27)32(40)38(30)25-10-4-3-5-11-25/h3-7,10-13,15-16,22H,2,8-9,14,17-21,23H2,1H3,(H,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Antagonist activity at 5-HT7 receptor (unknown origin) |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111705
BindingDB Entry DOI: 10.7270/Q2377D11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50261124
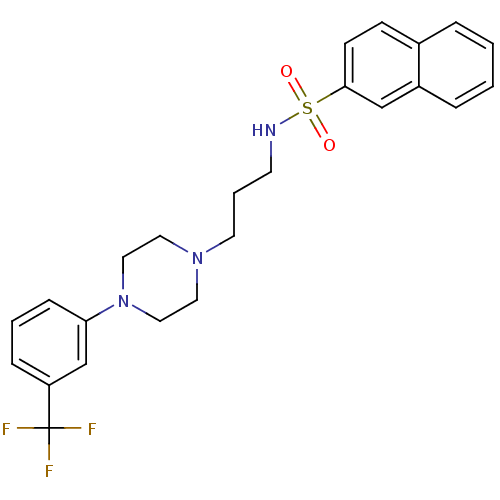
(CHEMBL495678 | Naphthalene-2-sulfonic acid {3-[4-(...)Show SMILES FC(F)(F)c1cccc(c1)N1CCN(CCCNS(=O)(=O)c2ccc3ccccc3c2)CC1 Show InChI InChI=1S/C24H26F3N3O2S/c25-24(26,27)21-7-3-8-22(18-21)30-15-13-29(14-16-30)12-4-11-28-33(31,32)23-10-9-19-5-1-2-6-20(19)17-23/h1-3,5-10,17-18,28H,4,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in CHO cell membrane |
Bioorg Med Chem 16: 5405-12 (2008)
Article DOI: 10.1016/j.bmc.2008.04.023
BindingDB Entry DOI: 10.7270/Q2RX9BVH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50375936

(CHEMBL260907)Show SMILES CCOc1ccccc1N1CCN(CCCC(=O)NCc2nc3ccc(F)cc3c(=O)n2-c2ccccc2)CC1 Show InChI InChI=1S/C31H34FN5O3/c1-2-40-28-12-7-6-11-27(28)36-19-17-35(18-20-36)16-8-13-30(38)33-22-29-34-26-15-14-23(32)21-25(26)31(39)37(29)24-9-4-3-5-10-24/h3-7,9-12,14-15,21H,2,8,13,16-20,22H2,1H3,(H,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT7 receptor |
Bioorg Med Chem 16: 2570-8 (2008)
Article DOI: 10.1016/j.bmc.2007.11.049
BindingDB Entry DOI: 10.7270/Q2JW8FRT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM427033
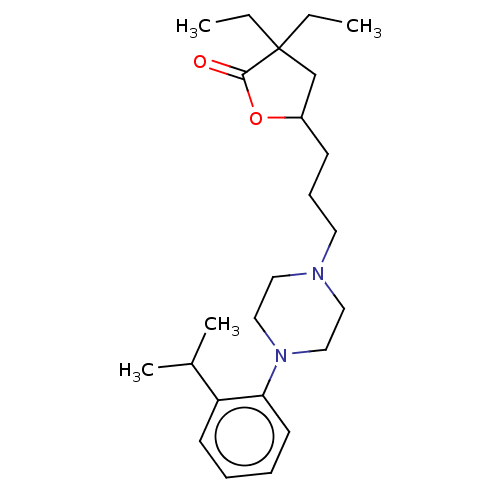
(US10544117, Entry 230062 | US11192871, Example 230...)Show SMILES CCC1(CC)CC(CCCN2CCN(CC2)c2ccccc2C(C)C)OC1=O Show InChI InChI=1S/C24H38N2O2/c1-5-24(6-2)18-20(28-23(24)27)10-9-13-25-14-16-26(17-15-25)22-12-8-7-11-21(22)19(3)4/h7-8,11-12,19-20H,5-6,9-10,13-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University—Of The Commonwealth System of Higher Education
US Patent
| Assay Description
A solution of the compound of the disclosure to be tested is prepared as a 1-mg/ml stock in Assay Buffer or DMSO according to its solubility. A simil... |
US Patent US10544117 (2020)
BindingDB Entry DOI: 10.7270/Q2W66P43 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM556301
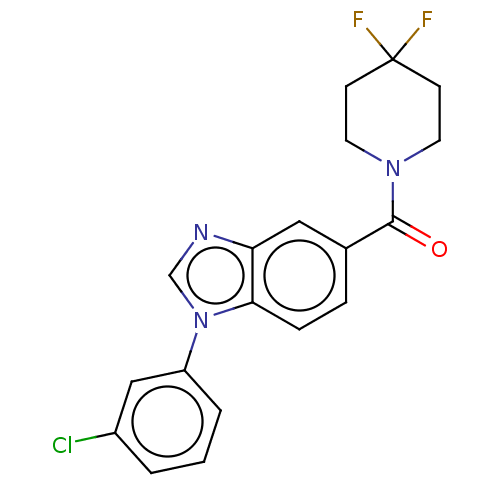
(US11345702, Table 1.19 | US20230390274, Compound A...)Show SMILES FC1(F)CCN(CC1)C(=O)c1ccc2n(cnc2c1)-c1cccc(Cl)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50306819
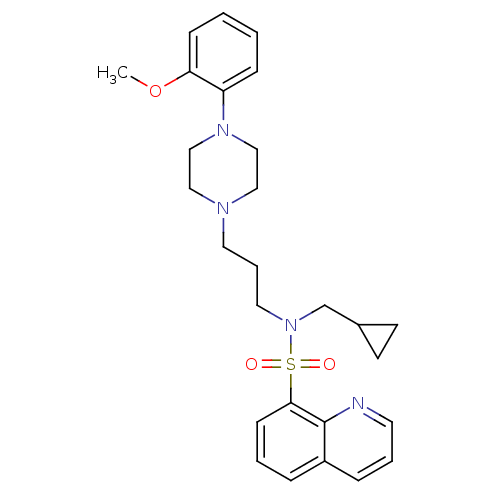
(CHEMBL602677 | N-(Cyclopropylmethyl)-N-(3-(4-(2-me...)Show SMILES COc1ccccc1N1CCN(CCCN(CC2CC2)S(=O)(=O)c2cccc3cccnc23)CC1 Show InChI InChI=1S/C27H34N4O3S/c1-34-25-10-3-2-9-24(25)30-19-17-29(18-20-30)15-6-16-31(21-22-12-13-22)35(32,33)26-11-4-7-23-8-5-14-28-27(23)26/h2-5,7-11,14,22H,6,12-13,15-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in CHO cells |
Bioorg Med Chem 18: 1665-75 (2010)
Article DOI: 10.1016/j.bmc.2009.12.067
BindingDB Entry DOI: 10.7270/Q2R78F99 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data