 Found 392 hits of ic50 data for polymerid = 2173
Found 392 hits of ic50 data for polymerid = 2173 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50385150
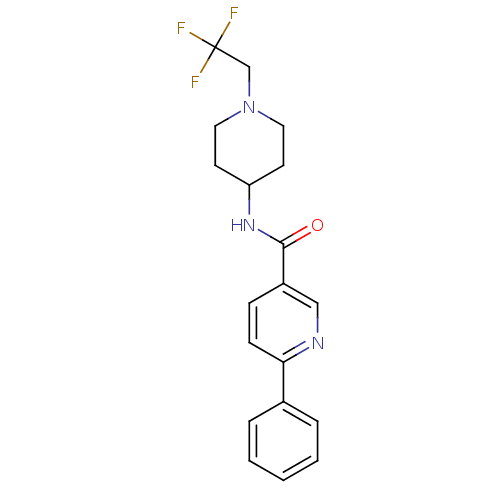
(CHEMBL2035650)Show SMILES FC(F)(F)CN1CCC(CC1)NC(=O)c1ccc(nc1)-c1ccccc1 Show InChI InChI=1S/C19H20F3N3O/c20-19(21,22)13-25-10-8-16(9-11-25)24-18(26)15-6-7-17(23-12-15)14-4-2-1-3-5-14/h1-7,12,16H,8-11,13H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HPGDS |
Bioorg Med Chem Lett 22: 3795-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.004
BindingDB Entry DOI: 10.7270/Q28C9X82 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50385142
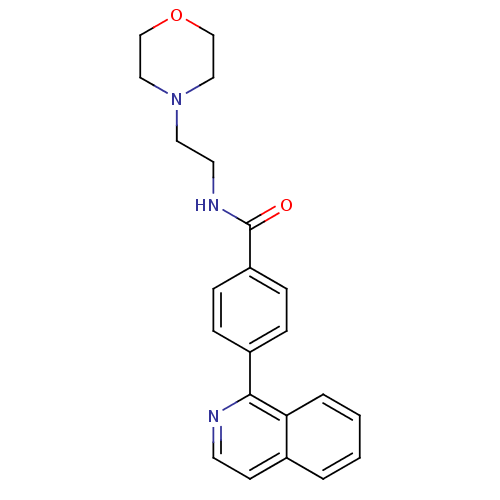
(CHEMBL2035651)Show InChI InChI=1S/C22H23N3O2/c26-22(24-11-12-25-13-15-27-16-14-25)19-7-5-18(6-8-19)21-20-4-2-1-3-17(20)9-10-23-21/h1-10H,11-16H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HPGDS using PGH2 as substrate assessed as production of PGD2 preincubated for 10 mins prior substrate addition measur... |
Bioorg Med Chem Lett 22: 3795-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.004
BindingDB Entry DOI: 10.7270/Q28C9X82 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463698
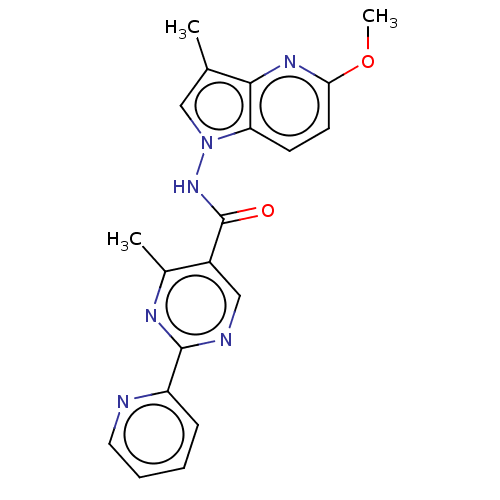
(CHEMBL4247050)Show SMILES COc1ccc2n(NC(=O)c3cnc(nc3C)-c3ccccn3)cc(C)c2n1 Show InChI InChI=1S/C20H18N6O2/c1-12-11-26(16-7-8-17(28-3)24-18(12)16)25-20(27)14-10-22-19(23-13(14)2)15-6-4-5-9-21-15/h4-11H,1-3H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Inhibition of HPGDS (unknown origin) |
Bioorg Med Chem Lett 28: 3046-3049 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.049
BindingDB Entry DOI: 10.7270/Q27P921C |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM179403

(US9126973, 8)Show SMILES Fc1cccc(c1)-c1ncc([nH]1)-c1cnc(nc1)-c1ccccc1 Show InChI InChI=1S/C19H13FN4/c20-16-8-4-7-14(9-16)19-23-12-17(24-19)15-10-21-18(22-11-15)13-5-2-1-3-6-13/h1-12H,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CAYMAN CHEMICAL COMPANY, INCORPORATED
US Patent
| Assay Description
The assay is carried out by the following steps: 1. Inhibitor screening is performed in 100 mM Tris-HCl, pH 8.0 containing 1 mM GSH, 1 mM MgCl.sub.2,... |
US Patent US9126973 (2015)
BindingDB Entry DOI: 10.7270/Q2930RZ3 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50559393

(CHEMBL4752221)Show SMILES CC(C)(O)c1nc(no1)-c1cccc(c1)-c1ncc([nH]1)-c1cnc(nc1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576323
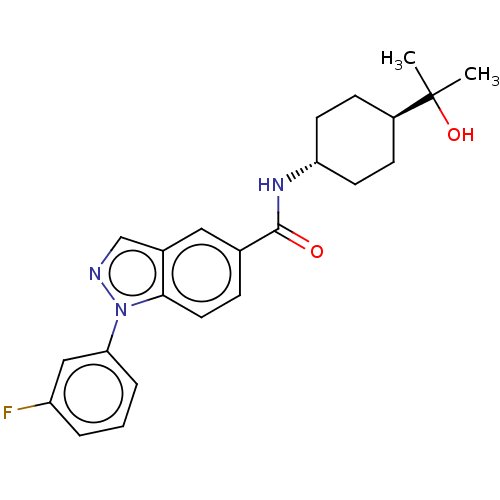
(CHEMBL4866146)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2n(ncc2c1)-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(17.72,-11.38,;16.96,-12.72,;18.5,-12.71,;16.96,-14.26,;15.63,-11.95,;15.63,-10.41,;14.29,-9.63,;12.97,-10.41,;12.97,-11.95,;14.29,-12.71,;11.64,-9.64,;10.3,-10.41,;8.97,-9.65,;10.31,-11.95,;11.65,-12.72,;11.65,-14.27,;10.31,-15.04,;10,-16.55,;8.46,-16.72,;7.83,-15.31,;8.97,-14.27,;8.98,-12.72,;11.03,-17.69,;10.55,-19.15,;11.59,-20.29,;13.09,-19.97,;13.56,-18.5,;15.07,-18.17,;12.53,-17.36,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463701
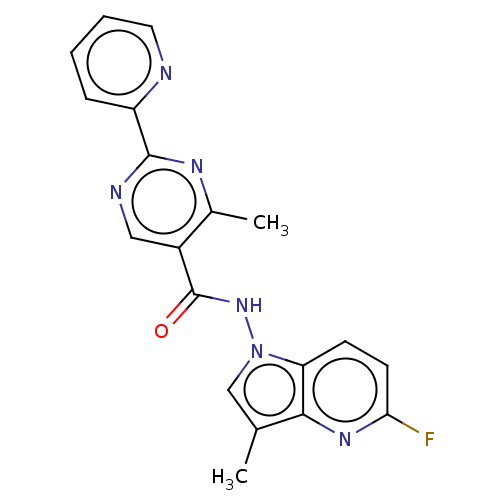
(CHEMBL4241885)Show SMILES Cc1cn(NC(=O)c2cnc(nc2C)-c2ccccn2)c2ccc(F)nc12 Show InChI InChI=1S/C19H15FN6O/c1-11-10-26(15-6-7-16(20)24-17(11)15)25-19(27)13-9-22-18(23-12(13)2)14-5-3-4-8-21-14/h3-10H,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Inhibition of HPGDS (unknown origin) |
Bioorg Med Chem Lett 28: 3046-3049 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.049
BindingDB Entry DOI: 10.7270/Q27P921C |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM179422

(US9126973, 19)Show SMILES C(N1CCC(CC1)c1ncc([nH]1)-c1cnc(nc1)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H25N5/c1-3-7-19(8-4-1)18-30-13-11-21(12-14-30)25-28-17-23(29-25)22-15-26-24(27-16-22)20-9-5-2-6-10-20/h1-10,15-17,21H,11-14,18H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CAYMAN CHEMICAL COMPANY, INCORPORATED
US Patent
| Assay Description
The assay is carried out by the following steps: 1. Inhibitor screening is performed in 100 mM Tris-HCl, pH 8.0 containing 1 mM GSH, 1 mM MgCl.sub.2,... |
US Patent US9126973 (2015)
BindingDB Entry DOI: 10.7270/Q2930RZ3 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463702
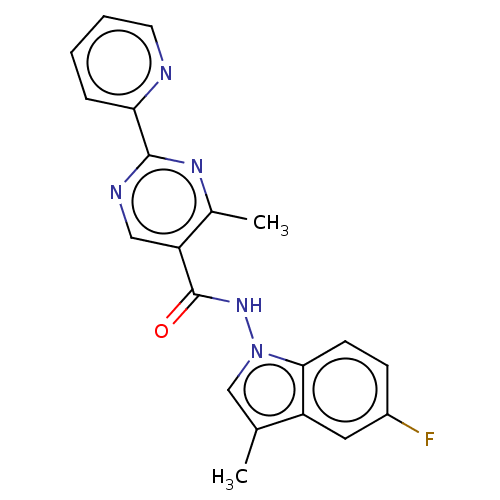
(CHEMBL3181890)Show SMILES Cc1cn(NC(=O)c2cnc(nc2C)-c2ccccn2)c2ccc(F)cc12 Show InChI InChI=1S/C20H16FN5O/c1-12-11-26(18-7-6-14(21)9-15(12)18)25-20(27)16-10-23-19(24-13(16)2)17-5-3-4-8-22-17/h3-11H,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Inhibition of HPGDS (unknown origin) |
Bioorg Med Chem Lett 28: 3046-3049 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.049
BindingDB Entry DOI: 10.7270/Q27P921C |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50559403
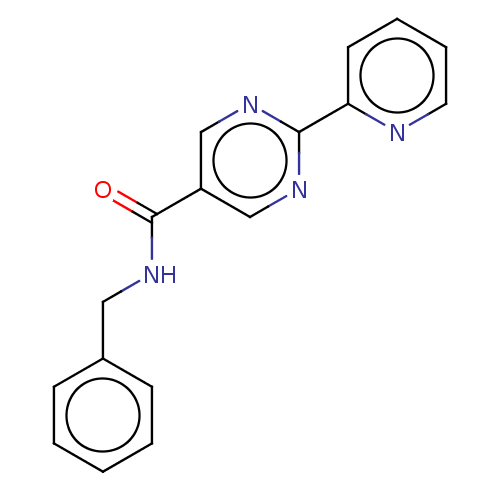
(CHEMBL4740750) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50559399
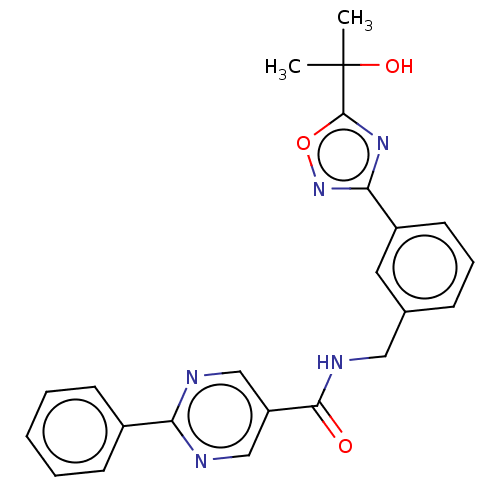
(CHEMBL4753053)Show SMILES CC(C)(O)c1nc(no1)-c1cccc(CNC(=O)c2cnc(nc2)-c2ccccc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM250506
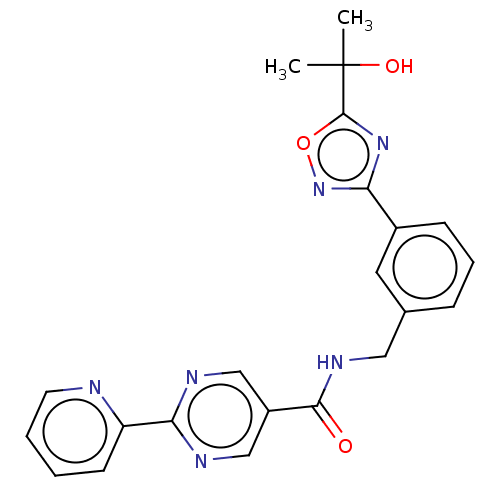
(US9469627, 1)Show SMILES CC(C)(O)c1nc(no1)-c1cccc(CNC(=O)c2cnc(nc2)-c2ccccn2)c1 Show InChI InChI=1S/C22H20N6O3/c1-22(2,30)21-27-18(28-31-21)15-7-5-6-14(10-15)11-26-20(29)16-12-24-19(25-13-16)17-8-3-4-9-23-17/h3-10,12-13,30H,11H2,1-2H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463704
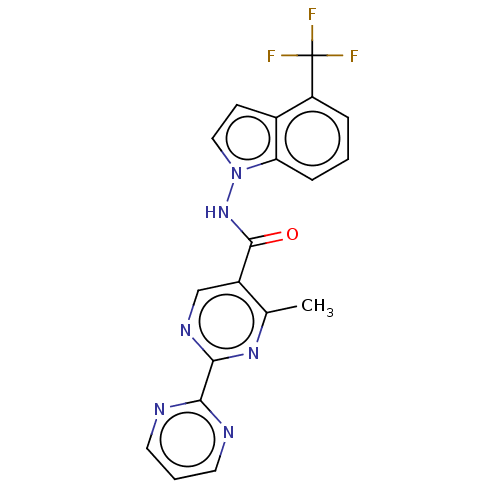
(CHEMBL4250602)Show SMILES Cc1nc(ncc1C(=O)Nn1ccc2c(cccc12)C(F)(F)F)-c1ncccn1 Show InChI InChI=1S/C19H13F3N6O/c1-11-13(10-25-17(26-11)16-23-7-3-8-24-16)18(29)27-28-9-6-12-14(19(20,21)22)4-2-5-15(12)28/h2-10H,1H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Inhibition of HPGDS (unknown origin) |
Bioorg Med Chem Lett 28: 3046-3049 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.049
BindingDB Entry DOI: 10.7270/Q27P921C |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576316
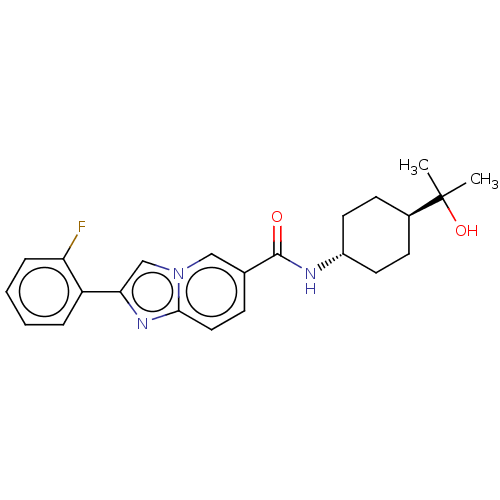
(CHEMBL4870703)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1F |r,wU:7.10,wD:4.3,(2.16,-11.57,;2.98,-12.88,;3.7,-11.51,;1.68,-13.7,;4.35,-13.61,;4.4,-15.15,;5.77,-15.87,;7.07,-15.05,;7.01,-13.51,;5.65,-12.79,;8.44,-15.77,;9.74,-14.95,;9.68,-13.41,;11.1,-15.66,;11.16,-17.2,;12.51,-17.92,;13.81,-17.1,;15.29,-17.52,;16.15,-16.25,;15.2,-15.04,;13.76,-15.57,;12.4,-14.84,;17.68,-16.19,;18.5,-17.5,;20.03,-17.44,;20.76,-16.08,;19.93,-14.77,;18.39,-14.83,;17.57,-13.53,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526494
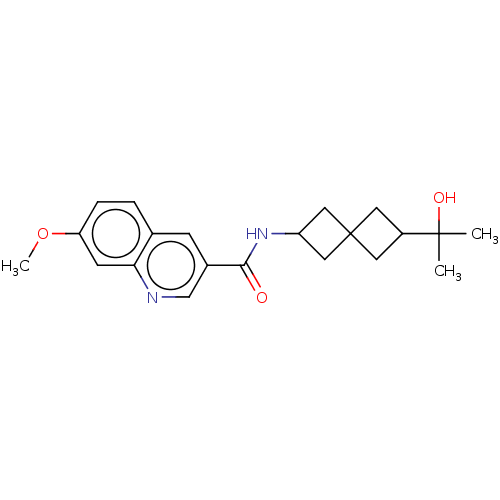
(CHEMBL4439454)Show SMILES COc1ccc2cc(cnc2c1)C(=O)NC1CC2(C1)CC(C2)C(C)(C)O |(1.86,-17.99,;3.19,-18.77,;4.53,-18,;4.53,-16.45,;5.86,-15.68,;7.2,-16.44,;8.52,-15.67,;9.86,-16.43,;9.87,-17.98,;8.53,-18.76,;7.2,-17.99,;5.86,-18.77,;11.19,-15.65,;11.17,-14.11,;12.53,-16.41,;13.86,-15.63,;15.34,-16.02,;15.74,-14.52,;14.24,-14.14,;17.22,-14.92,;17.62,-13.43,;16.13,-13.03,;18.95,-12.66,;18.17,-11.32,;19.72,-11.32,;20.29,-13.43,)| Show InChI InChI=1S/C21H26N2O3/c1-20(2,25)15-8-21(9-15)10-16(11-21)23-19(24)14-6-13-4-5-17(26-3)7-18(13)22-12-14/h4-7,12,15-16,25H,8-11H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50385144
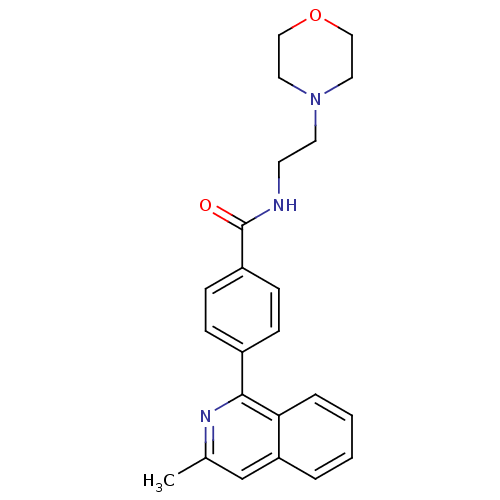
(CHEMBL2035653)Show SMILES Cc1cc2ccccc2c(n1)-c1ccc(cc1)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C23H25N3O2/c1-17-16-20-4-2-3-5-21(20)22(25-17)18-6-8-19(9-7-18)23(27)24-10-11-26-12-14-28-15-13-26/h2-9,16H,10-15H2,1H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HPGDS using PGH2 as substrate assessed as production of PGD2 preincubated for 10 mins prior substrate addition measur... |
Bioorg Med Chem Lett 22: 3795-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.004
BindingDB Entry DOI: 10.7270/Q28C9X82 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM179375

(US9126973, 1)Show InChI InChI=1S/C19H14N4/c1-3-7-14(8-4-1)18-20-11-16(12-21-18)17-13-22-19(23-17)15-9-5-2-6-10-15/h1-13H,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576314
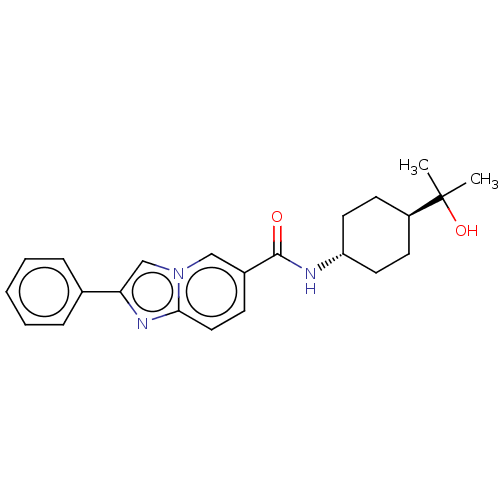
(CHEMBL4864805)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1 |r,wU:7.10,wD:4.3,(2.48,-1.86,;3.3,-3.17,;4.02,-1.8,;2,-3.99,;4.67,-3.9,;4.72,-5.44,;6.09,-6.16,;7.39,-5.34,;7.33,-3.8,;5.97,-3.08,;8.76,-6.06,;10.06,-5.24,;10,-3.7,;11.42,-5.96,;11.48,-7.5,;12.83,-8.21,;14.13,-7.39,;15.61,-7.81,;16.46,-6.54,;15.52,-5.33,;14.08,-5.86,;12.72,-5.13,;18,-6.48,;18.81,-7.79,;20.35,-7.73,;21.08,-6.37,;20.25,-5.06,;18.71,-5.12,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463699
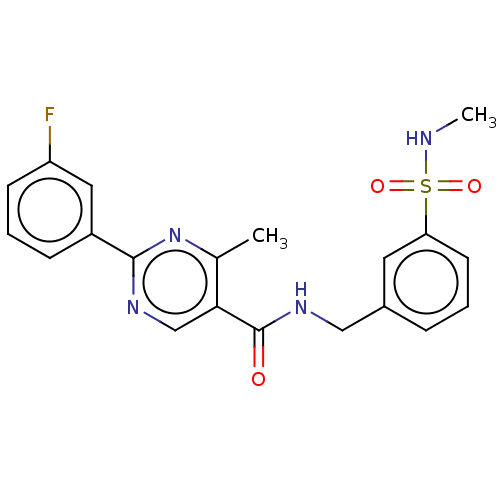
(CHEMBL4242281)Show SMILES CNS(=O)(=O)c1cccc(CNC(=O)c2cnc(nc2C)-c2cccc(F)c2)c1 Show InChI InChI=1S/C20H19FN4O3S/c1-13-18(12-23-19(25-13)15-6-4-7-16(21)10-15)20(26)24-11-14-5-3-8-17(9-14)29(27,28)22-2/h3-10,12,22H,11H2,1-2H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Inhibition of HPGDS (unknown origin) |
Bioorg Med Chem Lett 28: 3046-3049 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.049
BindingDB Entry DOI: 10.7270/Q27P921C |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526495
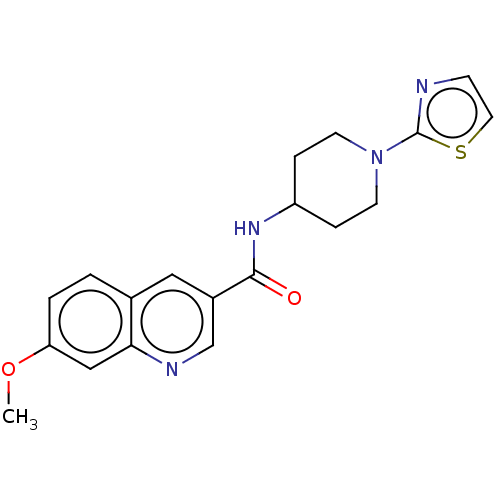
(CHEMBL4452633)Show InChI InChI=1S/C19H20N4O2S/c1-25-16-3-2-13-10-14(12-21-17(13)11-16)18(24)22-15-4-7-23(8-5-15)19-20-6-9-26-19/h2-3,6,9-12,15H,4-5,7-8H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50559399

(CHEMBL4753053)Show SMILES CC(C)(O)c1nc(no1)-c1cccc(CNC(=O)c2cnc(nc2)-c2ccccc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) assessed as reduction in reduction in LPS-induced PGD2 level by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526485
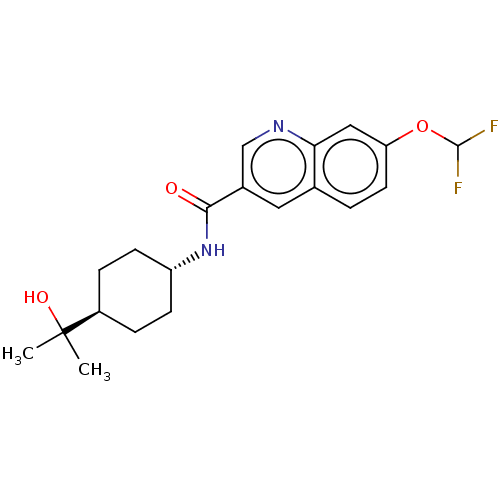
(CHEMBL4473072)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2cc(OC(F)F)ccc2c1 |r,wU:7.10,wD:4.3,(19.66,-11.66,;20.44,-13,;21.21,-11.66,;21.78,-13.76,;19.12,-13.78,;17.78,-13.01,;16.44,-13.79,;16.46,-15.32,;17.8,-16.09,;19.12,-15.32,;15.13,-16.1,;13.79,-15.34,;13.78,-13.8,;12.47,-16.12,;12.48,-17.67,;11.14,-18.44,;9.81,-17.68,;8.47,-18.45,;7.14,-17.68,;5.81,-18.45,;4.47,-17.68,;3.14,-18.45,;4.48,-16.14,;7.14,-16.14,;8.47,-15.37,;9.81,-16.13,;11.13,-15.36,)| Show InChI InChI=1S/C20H24F2N2O3/c1-20(2,26)14-4-6-15(7-5-14)24-18(25)13-9-12-3-8-16(27-19(21)22)10-17(12)23-11-13/h3,8-11,14-15,19,26H,4-7H2,1-2H3,(H,24,25)/t14-,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463700
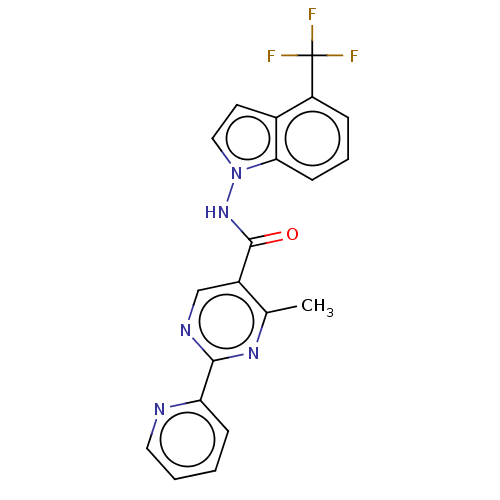
(CHEMBL4239352)Show SMILES Cc1nc(ncc1C(=O)Nn1ccc2c(cccc12)C(F)(F)F)-c1ccccn1 Show InChI InChI=1S/C20H14F3N5O/c1-12-14(11-25-18(26-12)16-6-2-3-9-24-16)19(29)27-28-10-8-13-15(20(21,22)23)5-4-7-17(13)28/h2-11H,1H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Inhibition of HPGDS (unknown origin) |
Bioorg Med Chem Lett 28: 3046-3049 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.049
BindingDB Entry DOI: 10.7270/Q27P921C |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576324
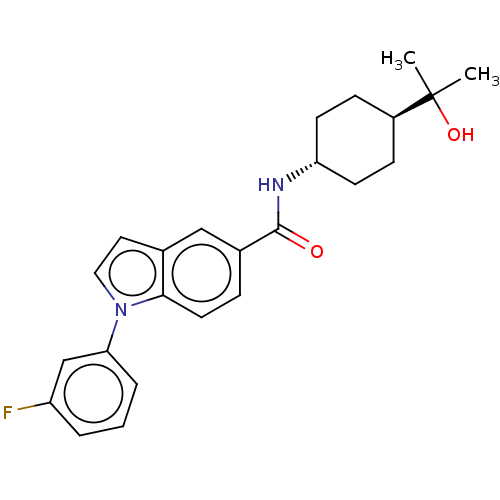
(CHEMBL4868339)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2n(ccc2c1)-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(32.28,-11.78,;31.52,-13.11,;33.06,-13.1,;31.53,-14.65,;30.2,-12.34,;30.2,-10.8,;28.86,-10.03,;27.54,-10.8,;27.53,-12.34,;28.86,-13.11,;26.2,-10.04,;24.87,-10.81,;23.54,-10.04,;24.88,-12.35,;26.21,-13.11,;26.22,-14.66,;24.88,-15.44,;24.56,-16.95,;23.03,-17.12,;22.39,-15.7,;23.54,-14.67,;23.55,-13.12,;25.6,-18.09,;25.12,-19.55,;26.15,-20.69,;27.66,-20.37,;28.13,-18.89,;29.63,-18.56,;27.09,-17.76,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM250507

(US9469627, 2)Show SMILES C[C@H](NC(=O)c1cnc(nc1)-c1ccccn1)c1cccc(c1)-c1noc(n1)C(C)(C)O |r| Show InChI InChI=1S/C23H22N6O3/c1-14(15-7-6-8-16(11-15)19-28-22(32-29-19)23(2,3)31)27-21(30)17-12-25-20(26-13-17)18-9-4-5-10-24-18/h4-14,31H,1-3H3,(H,27,30)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Sanofi
US Patent
| Assay Description
I. Assay Solutions a. Preparation of 0.1M K2HPO4/KH2PO4 buffer (pH 7.4) Prepare 0.1 M KH2PO4 from 1M KH2PO4 (Sigma, Cat# P-8709) ... |
US Patent US9469627 (2016)
BindingDB Entry DOI: 10.7270/Q2D799CT |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50559402

(CHEMBL4786374) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM250506

(US9469627, 1)Show SMILES CC(C)(O)c1nc(no1)-c1cccc(CNC(=O)c2cnc(nc2)-c2ccccn2)c1 Show InChI InChI=1S/C22H20N6O3/c1-22(2,30)21-27-18(28-31-21)15-7-5-6-14(10-15)11-26-20(29)16-12-24-19(25-13-16)17-8-3-4-9-23-17/h3-10,12-13,30H,11H2,1-2H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) assessed as reduction in reduction in LPS-induced PGD2 level by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM250506

(US9469627, 1)Show SMILES CC(C)(O)c1nc(no1)-c1cccc(CNC(=O)c2cnc(nc2)-c2ccccn2)c1 Show InChI InChI=1S/C22H20N6O3/c1-22(2,30)21-27-18(28-31-21)15-7-5-6-14(10-15)11-26-20(29)16-12-24-19(25-13-16)17-8-3-4-9-23-17/h3-10,12-13,30H,11H2,1-2H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Sanofi
US Patent
| Assay Description
I. Assay Solutions a. Preparation of 0.1M K2HPO4/KH2PO4 buffer (pH 7.4) Prepare 0.1 M KH2PO4 from 1M KH2PO4 (Sigma, Cat# P-8709) ... |
US Patent US9469627 (2016)
BindingDB Entry DOI: 10.7270/Q2D799CT |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM179375

(US9126973, 1)Show InChI InChI=1S/C19H14N4/c1-3-7-14(8-4-1)18-20-11-16(12-21-18)17-13-22-19(23-17)15-9-5-2-6-10-15/h1-13H,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CAYMAN CHEMICAL COMPANY, INCORPORATED
US Patent
| Assay Description
The assay is carried out by the following steps: 1. Inhibitor screening is performed in 100 mM Tris-HCl, pH 8.0 containing 1 mM GSH, 1 mM MgCl.sub.2,... |
US Patent US9126973 (2015)
BindingDB Entry DOI: 10.7270/Q2930RZ3 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576322
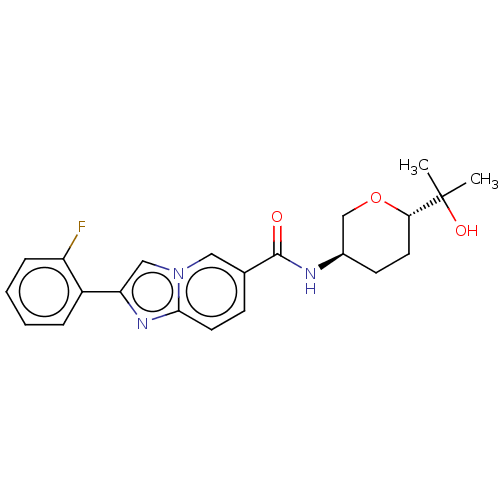
(CHEMBL4859922)Show SMILES CC(C)(O)[C@@H]1CC[C@H](CO1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM179408

(US9126973, 10)Show InChI InChI=1S/C18H13N5/c1-2-5-13(6-3-1)17-20-10-15(11-21-17)16-12-22-18(23-16)14-7-4-8-19-9-14/h1-12H,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) assessed as reduction in reduction in LPS-induced PGD2 level by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM179408

(US9126973, 10)Show InChI InChI=1S/C18H13N5/c1-2-5-13(6-3-1)17-20-10-15(11-21-17)16-12-22-18(23-16)14-7-4-8-19-9-14/h1-12H,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526492
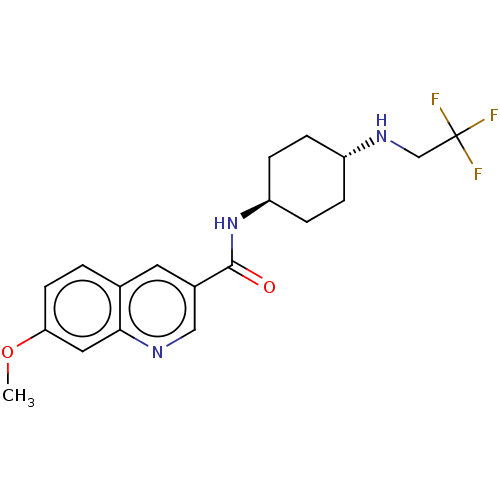
(CHEMBL4454901)Show SMILES COc1ccc2cc(cnc2c1)C(=O)N[C@H]1CC[C@@H](CC1)NCC(F)(F)F |r,wU:15.16,wD:18.23,(2.46,-40.3,;3.79,-41.07,;5.13,-40.3,;5.13,-38.76,;6.46,-37.99,;7.8,-38.75,;9.12,-37.98,;10.46,-38.74,;10.47,-40.29,;9.13,-41.06,;7.8,-40.3,;6.46,-41.07,;11.78,-37.96,;11.77,-36.42,;13.12,-38.72,;14.45,-37.94,;14.43,-36.4,;15.76,-35.63,;17.1,-36.39,;17.11,-37.93,;15.78,-38.7,;18.43,-35.61,;18.43,-34.07,;19.75,-33.29,;20.95,-32.58,;21.26,-33.85,;19.99,-31.7,)| Show InChI InChI=1S/C19H22F3N3O2/c1-27-16-7-2-12-8-13(10-23-17(12)9-16)18(26)25-15-5-3-14(4-6-15)24-11-19(20,21)22/h2,7-10,14-15,24H,3-6,11H2,1H3,(H,25,26)/t14-,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM124922
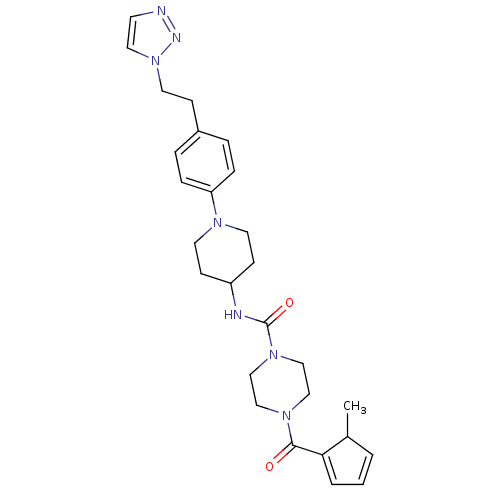
(US8765750, 7)Show SMILES CC1C=CC=C1C(=O)N1CCN(CC1)C(=O)NC1CCN(CC1)c1ccc(CCn2ccnn2)cc1 |c:2,4| Show InChI InChI=1S/C27H35N7O2/c1-21-3-2-4-25(21)26(35)32-17-19-33(20-18-32)27(36)29-23-10-13-31(14-11-23)24-7-5-22(6-8-24)9-15-34-16-12-28-30-34/h2-8,12,16,21,23H,9-11,13-15,17-20H2,1H3,(H,29,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Taiho Pharmaceutical Co., Ltd.
US Patent
| Assay Description
The test was carried out according to the method of Urade, Y. et al. (J. Biol. Chem., 262, 3820-3825, (1987)). More specifically, the reaction mixtur... |
US Patent US8765750 (2014)
BindingDB Entry DOI: 10.7270/Q2862F43 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526486
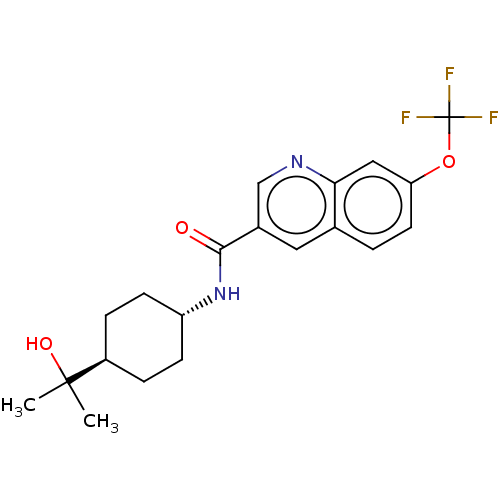
(CHEMBL4526974)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2cc(OC(F)(F)F)ccc2c1 |r,wU:7.10,wD:4.3,(60.01,-18.28,;60.78,-19.61,;61.55,-18.27,;62.13,-20.38,;59.46,-20.39,;58.12,-19.63,;56.79,-20.41,;56.81,-21.94,;58.14,-22.71,;59.47,-21.93,;55.48,-22.72,;54.14,-21.96,;54.13,-20.42,;52.82,-22.74,;52.83,-24.29,;51.49,-25.06,;50.16,-24.3,;48.83,-25.08,;47.49,-24.31,;46.16,-25.07,;44.83,-24.3,;43.61,-23.6,;44.57,-22.72,;43.32,-24.88,;47.5,-22.76,;48.82,-21.99,;50.16,-22.75,;51.48,-21.98,)| Show InChI InChI=1S/C20H23F3N2O3/c1-19(2,27)14-4-6-15(7-5-14)25-18(26)13-9-12-3-8-16(28-20(21,22)23)10-17(12)24-11-13/h3,8-11,14-15,27H,4-7H2,1-2H3,(H,25,26)/t14-,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526498

(CHEMBL4530713)Show InChI InChI=1S/C20H18N2O2/c1-24-16-8-6-14-10-15(12-21-19(14)11-16)20(23)22-18-9-7-13-4-2-3-5-17(13)18/h2-6,8,10-12,18H,7,9H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526514
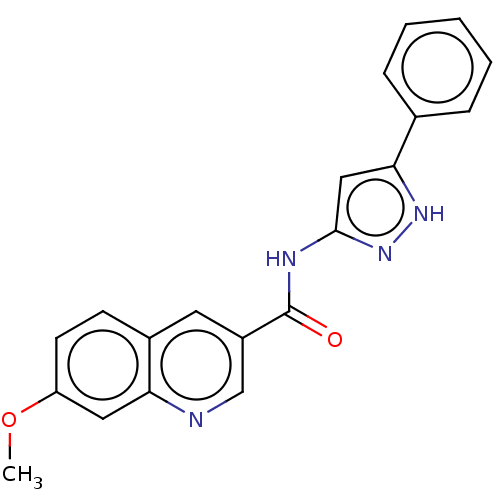
(CHEMBL4443093)Show SMILES COc1ccc2cc(cnc2c1)C(=O)Nc1cc([nH]n1)-c1ccccc1 Show InChI InChI=1S/C20H16N4O2/c1-26-16-8-7-14-9-15(12-21-17(14)10-16)20(25)22-19-11-18(23-24-19)13-5-3-2-4-6-13/h2-12H,1H3,(H2,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576315

(CHEMBL4873385)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(23.28,-2.13,;24.11,-3.43,;24.83,-2.06,;22.81,-4.26,;25.48,-4.17,;25.53,-5.7,;26.9,-6.43,;28.2,-5.61,;28.14,-4.06,;26.78,-3.35,;29.56,-6.33,;30.87,-5.5,;30.81,-3.96,;32.23,-6.22,;32.29,-7.76,;33.64,-8.47,;34.94,-7.66,;36.42,-8.08,;37.27,-6.8,;36.33,-5.6,;34.89,-6.12,;33.53,-5.4,;38.81,-6.75,;39.62,-8.05,;41.16,-8,;41.88,-6.64,;41.06,-5.33,;41.77,-3.96,;39.52,-5.39,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526487
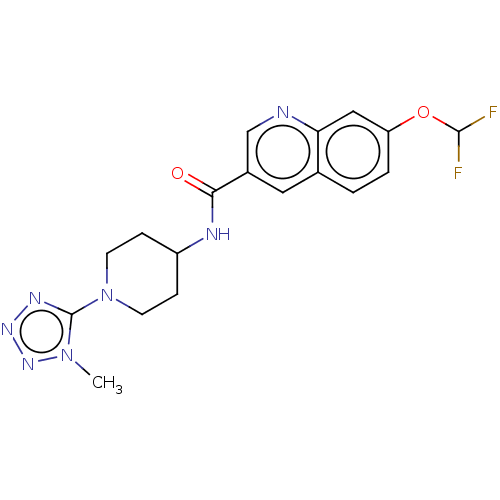
(CHEMBL4458862)Show SMILES Cn1nnnc1N1CCC(CC1)NC(=O)c1cnc2cc(OC(F)F)ccc2c1 Show InChI InChI=1S/C18H19F2N7O2/c1-26-18(23-24-25-26)27-6-4-13(5-7-27)22-16(28)12-8-11-2-3-14(29-17(19)20)9-15(11)21-10-12/h2-3,8-10,13,17H,4-7H2,1H3,(H,22,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576330
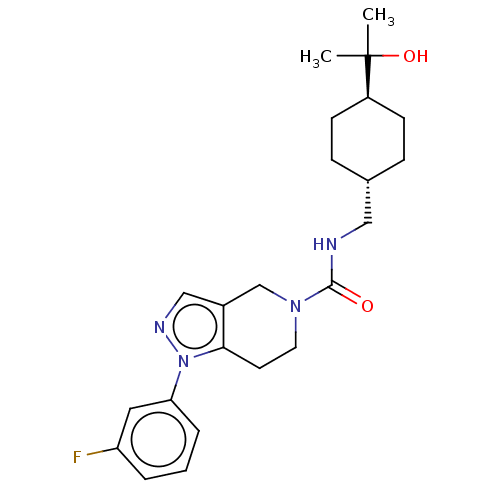
(CHEMBL4874001)Show SMILES CC(C)(O)[C@H]1CC[C@H](CNC(=O)N2CCc3c(C2)cnn3-c2cccc(F)c2)CC1 |r,wU:7.7,wD:4.3,(23.24,-24.76,;24.57,-25.53,;25.37,-24.22,;23.23,-26.3,;25.32,-26.89,;24.52,-28.21,;25.27,-29.56,;26.8,-29.58,;27.55,-30.92,;29.09,-30.95,;29.84,-32.3,;29.04,-33.62,;31.38,-32.32,;32.12,-33.66,;33.66,-33.69,;34.46,-32.37,;33.7,-31.03,;32.16,-31.01,;34.74,-29.9,;36.14,-30.54,;35.97,-32.07,;37.1,-33.11,;36.76,-34.61,;37.9,-35.65,;39.37,-35.19,;39.7,-33.67,;41.19,-33.28,;38.56,-32.64,;27.6,-28.26,;26.86,-26.92,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50559393

(CHEMBL4752221)Show SMILES CC(C)(O)c1nc(no1)-c1cccc(c1)-c1ncc([nH]1)-c1cnc(nc1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) assessed as reduction in reduction in LPS-induced PGD2 level by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM179408

(US9126973, 10)Show InChI InChI=1S/C18H13N5/c1-2-5-13(6-3-1)17-20-10-15(11-21-17)16-12-22-18(23-16)14-7-4-8-19-9-14/h1-12H,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CAYMAN CHEMICAL COMPANY, INCORPORATED
US Patent
| Assay Description
The assay is carried out by the following steps: 1. Inhibitor screening is performed in 100 mM Tris-HCl, pH 8.0 containing 1 mM GSH, 1 mM MgCl.sub.2,... |
US Patent US9126973 (2015)
BindingDB Entry DOI: 10.7270/Q2930RZ3 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM21625
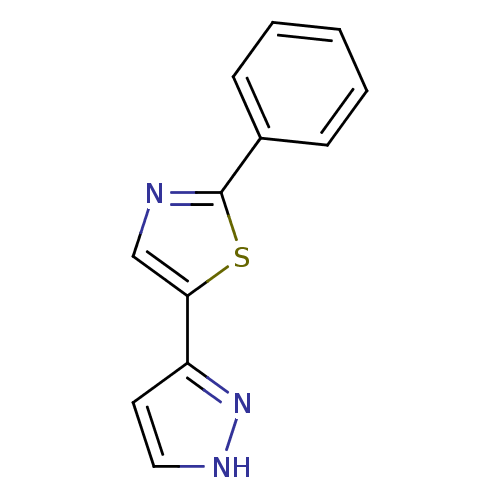
(2-phenyl-5-(1H-pyrazol-3-yl)-1,3-thiazole | 2-phen...)Show InChI InChI=1S/C12H9N3S/c1-2-4-9(5-3-1)12-13-8-11(16-12)10-6-7-14-15-10/h1-8H,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.2 | 22 |
AstraZeneca
| Assay Description
The PGDS glutathione-S-transferase (GST) activity was measured by using MonoChloroBimane (MCB) as a chromogenic substrate. The assay was run at 384-w... |
J Med Chem 51: 2178-86 (2008)
Article DOI: 10.1021/jm701509k
BindingDB Entry DOI: 10.7270/Q2Z036FR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526528
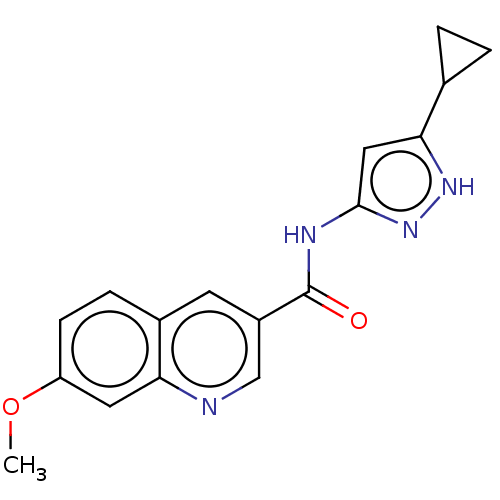
(CHEMBL4467696)Show InChI InChI=1S/C17H16N4O2/c1-23-13-5-4-11-6-12(9-18-14(11)7-13)17(22)19-16-8-15(20-21-16)10-2-3-10/h4-10H,2-3H2,1H3,(H2,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50559395
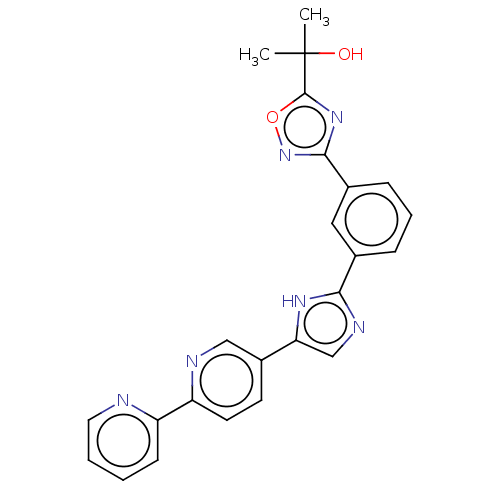
(CHEMBL4783535)Show SMILES CC(C)(O)c1nc(no1)-c1cccc(c1)-c1ncc([nH]1)-c1ccc(nc1)-c1ccccn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) assessed as reduction in reduction in LPS-induced PGD2 level by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
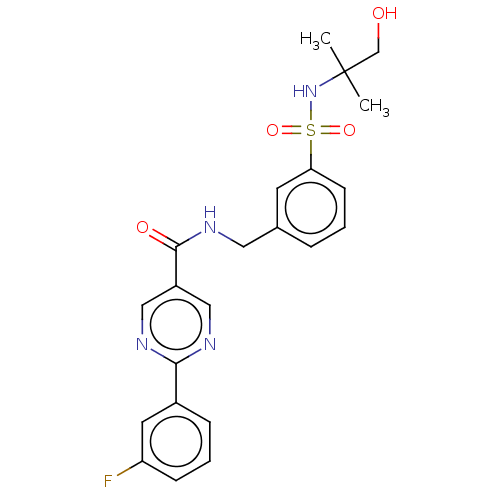
(Homo sapiens (Human)) | BDBM50463703
(CHEMBL4244590)Show SMILES CC(C)(CO)NS(=O)(=O)c1cccc(CNC(=O)c2cnc(nc2)-c2cccc(F)c2)c1 Show InChI InChI=1S/C22H23FN4O4S/c1-22(2,14-28)27-32(30,31)19-8-3-5-15(9-19)11-26-21(29)17-12-24-20(25-13-17)16-6-4-7-18(23)10-16/h3-10,12-13,27-28H,11,14H2,1-2H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Inhibition of HPGDS (unknown origin) |
Bioorg Med Chem Lett 28: 3046-3049 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.049
BindingDB Entry DOI: 10.7270/Q27P921C |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM179418

(US9126973, 15)Show SMILES C(=C/c1cccnc1)\c1ncc([nH]1)-c1cnc(nc1)-c1ccccc1 Show InChI InChI=1S/C20H15N5/c1-2-6-16(7-3-1)20-23-12-17(13-24-20)18-14-22-19(25-18)9-8-15-5-4-10-21-11-15/h1-14H,(H,22,25)/b9-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CAYMAN CHEMICAL COMPANY, INCORPORATED
US Patent
| Assay Description
The assay is carried out by the following steps: 1. Inhibitor screening is performed in 100 mM Tris-HCl, pH 8.0 containing 1 mM GSH, 1 mM MgCl.sub.2,... |
US Patent US9126973 (2015)
BindingDB Entry DOI: 10.7270/Q2930RZ3 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM21625

(2-phenyl-5-(1H-pyrazol-3-yl)-1,3-thiazole | 2-phen...)Show InChI InChI=1S/C12H9N3S/c1-2-4-9(5-3-1)12-13-8-11(16-12)10-6-7-14-15-10/h1-8H,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition human HPGDS expressed in Escherichia coli assessed as reduction in GST enzymatic activity using MCBL and glutathione incubated for 30 mins... |
Bioorg Med Chem Lett 25: 2496-500 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.065
BindingDB Entry DOI: 10.7270/Q2J9683M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50550011
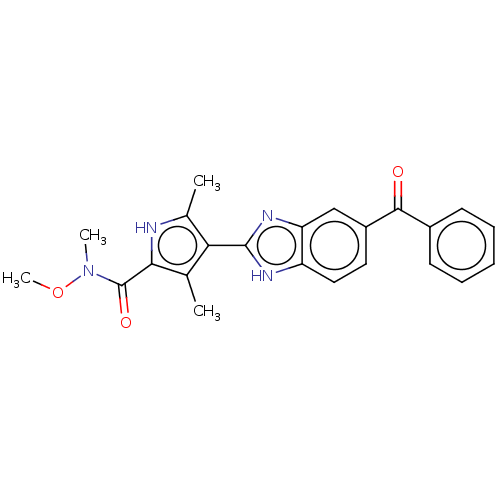
(CHEMBL4747168)Show SMILES Cl.CON(C)C(=O)c1[nH]c(C)c(c1C)-c1nc2cc(ccc2[nH]1)C(=O)c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HPGDS |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50559402

(CHEMBL4786374) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS (unknown origin) assessed as reduction in reduction in LPS-induced PGD2 level by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data