

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510487 (CHEMBL4569266) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510491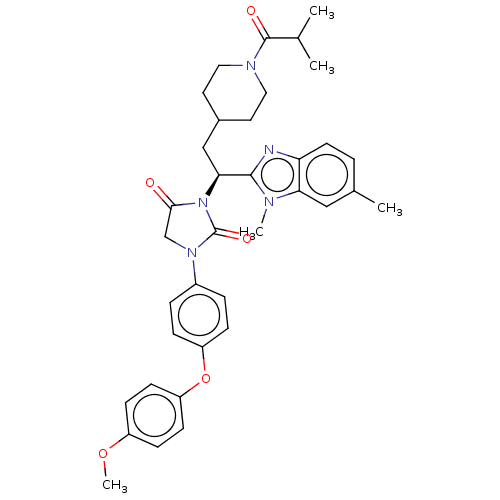 (CHEMBL4552760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286734 (CHEMBL4172988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286760 (CHEMBL4170032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50552997 (CHEMBL4797745) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510491 (CHEMBL4552760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286760 (CHEMBL4170032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286761 (CHEMBL4169187) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286736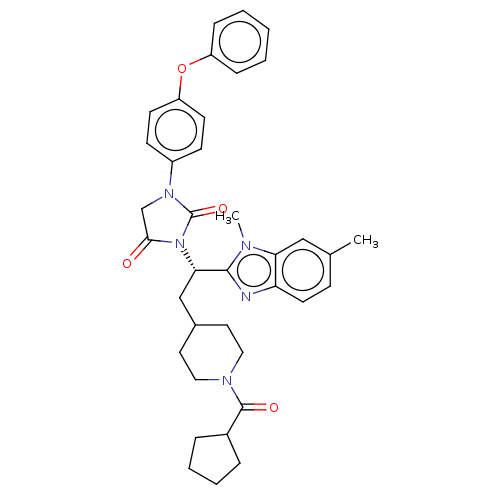 (CHEMBL4161262) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50553000 (CHEMBL4783205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286763 (CHEMBL4169596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510495 (CHEMBL4453417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510495 (CHEMBL4453417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286733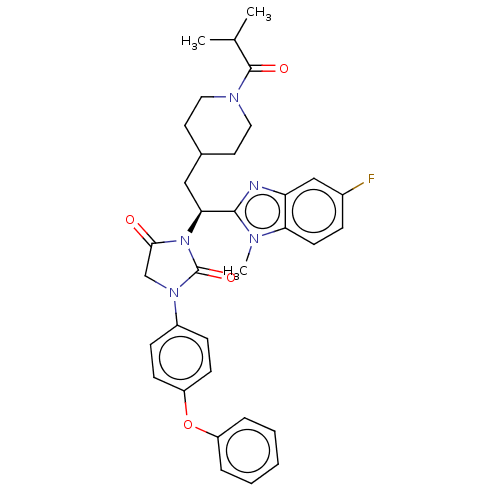 (CHEMBL4162312) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50552999 (CHEMBL4749439) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50552998 (CHEMBL4783777) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50552996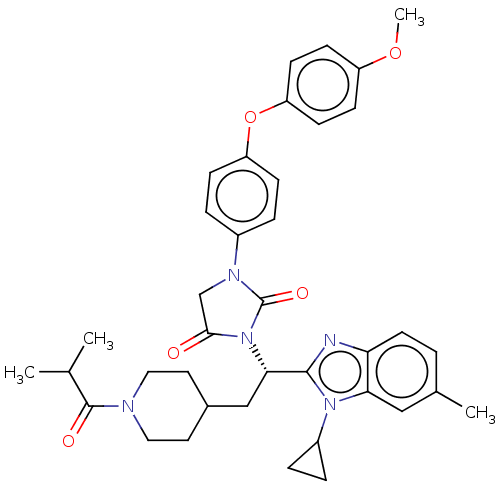 (CHEMBL4785930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM77055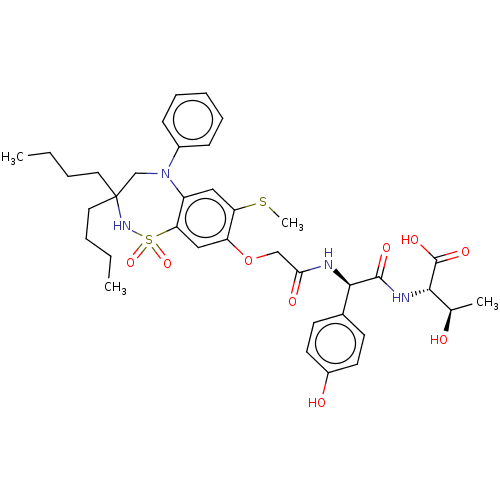 (US10093697, 8. | US10487111, Example 8. | US969401...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM77086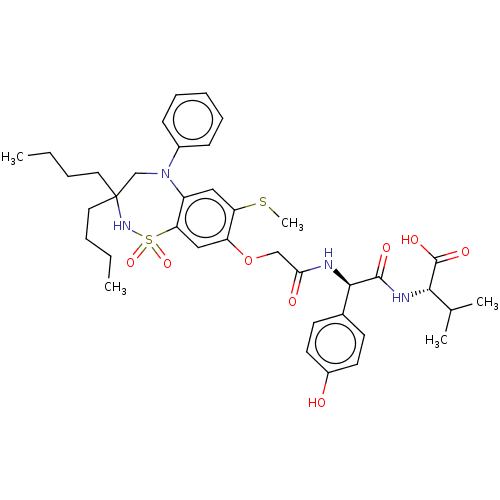 (US10093697, 13. | US10487111, Example 13. | US9694...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM77080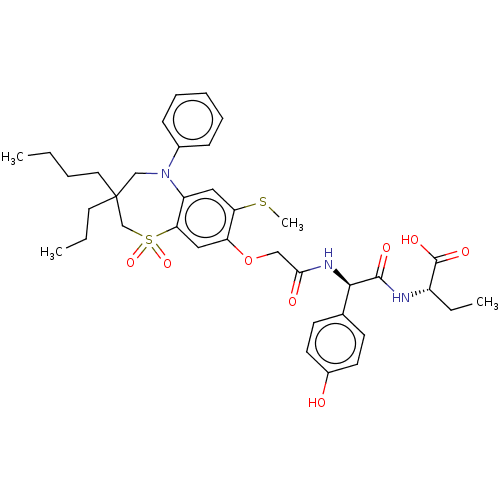 (US10093697, 11. | US9694018, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM77040 (US10093697, 5. | US10487111, Example 5. | US969401...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM77029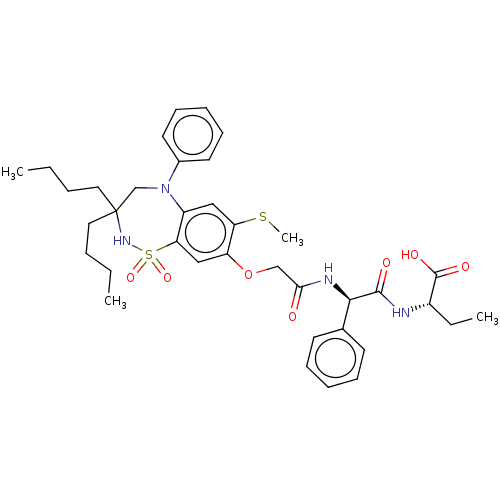 (US10093697, 3. | US10487111, Example 3. | US969401...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286762 (CHEMBL4159402) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM77074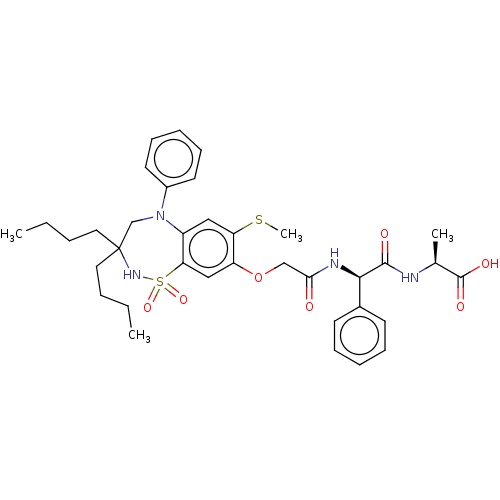 (US10093697, 10. | US10487111, Example 10. | US9694...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM225964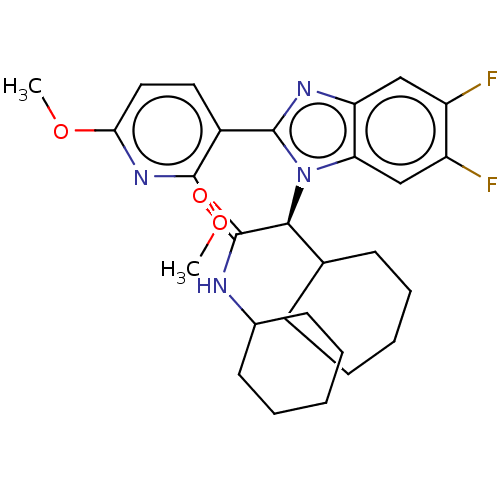 (FXR_55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | D3R | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
D3R | Assay Description The assay buffer contained 50 mM HEPES (pH 7.4), 10 mM NaCl, 5 mM MgCl2 and 0.01% CHAPS. The reactions were incubated for 30 min in the presence of [... | D3R 882: (2017) BindingDB Entry DOI: 10.7270/Q2MC8XWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM77083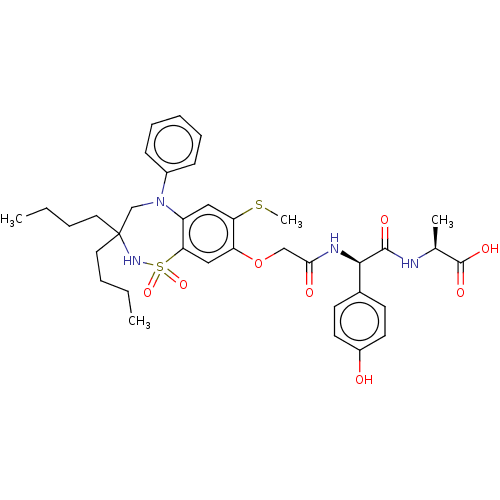 (US10093697, 12. | US10487111, Example 12. | US9694...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM77033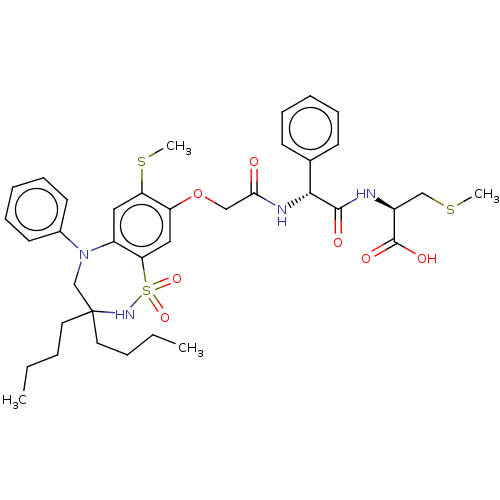 (US10093697, 4. | US10487111, Example 4. | US969401...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM77051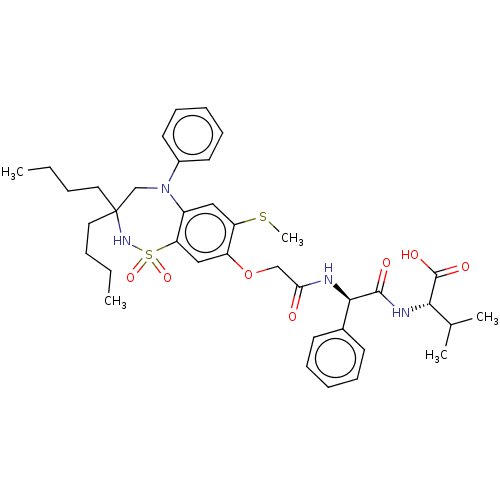 (US10093697, 7. | US10487111, Example 7. | US969401...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM76996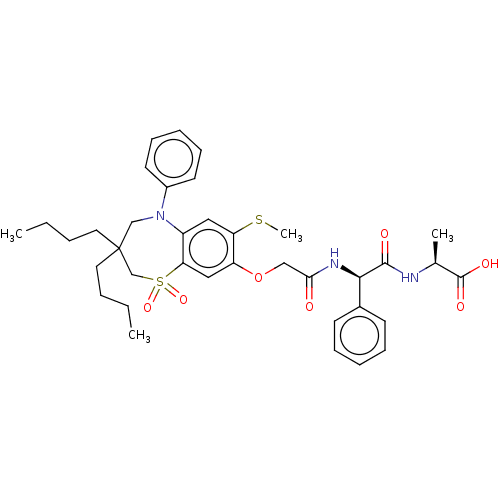 (US10487111, Example 2. | US9694018, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | CHEMBL5270123 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM76994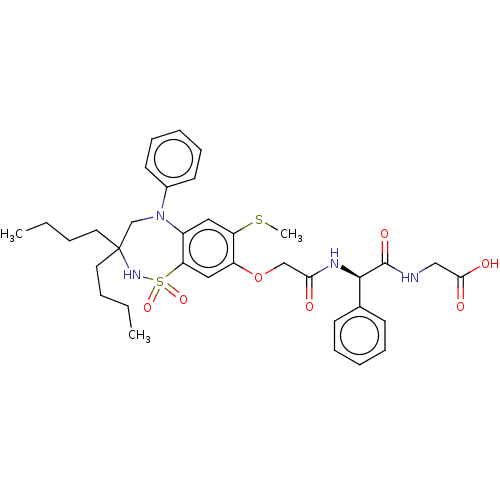 (US10093697, 1. | US10487111, Example 1. | US969401...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50236558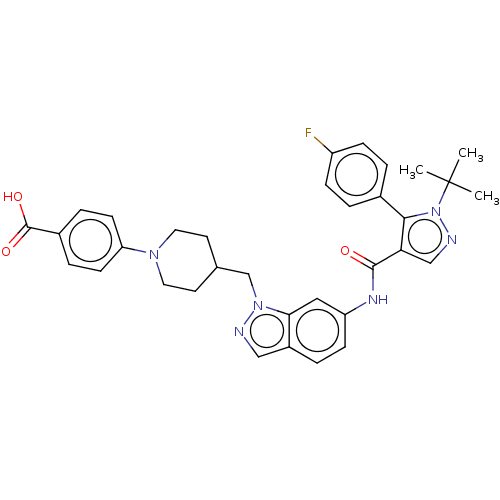 (CHEMBL4101903) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in COS1 cells assessed as inhibition of CDCA-induced receptor activation after 2 days by luciferase report... | Eur J Med Chem 129: 303-309 (2017) Article DOI: 10.1016/j.ejmech.2017.02.037 BindingDB Entry DOI: 10.7270/Q2P271DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286764 (CHEMBL4176369) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336375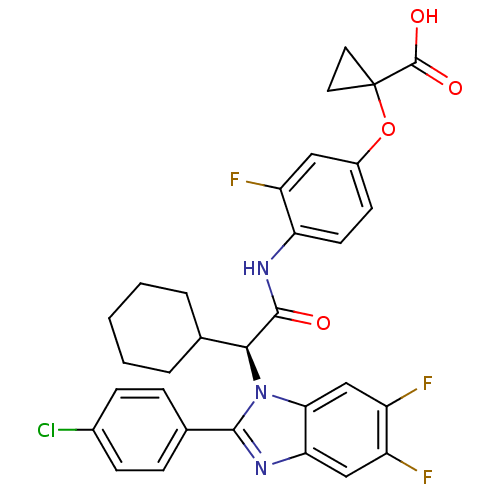 ((S)-1-(4-(2-(2-(4-chlorophenyl)-5,6-difluoro-1H-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM77088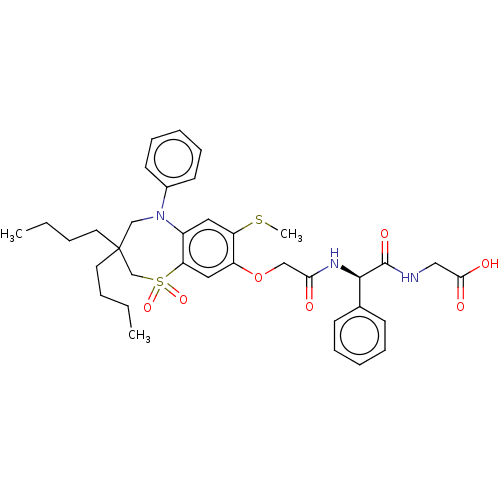 (US10093697, 14. | US10487111, Example 14. | US9694...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Albireo AB US Patent | Assay Description ISBT Hu HEK Uptake SPA 13203 IBAT HUM Ileal Bile Acid Transporter Human HEK Glycocholic acid Uptake Radiometric┐SPA Inhibitor IC50 Mean IC50 (nM) was... | US Patent US9694018 (2017) BindingDB Entry DOI: 10.7270/Q2GM85GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50463011 (CHEMBL4241985) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Antagonist activity at full length human FXR expressed in HeLa cells co-expressing BSEP-pGL3/pSG5-hRXR after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem 26: 4240-4253 (2018) Article DOI: 10.1016/j.bmc.2018.07.017 BindingDB Entry DOI: 10.7270/Q2BR8VTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50553001 (CHEMBL4778665) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510488 (CHEMBL4519419) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336376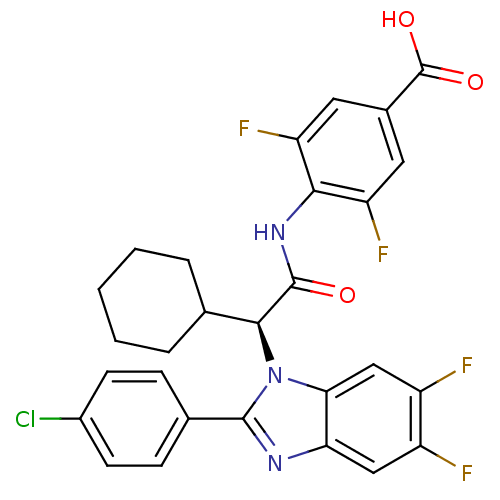 ((S)-4-(2-(2-(4-chlorophenyl)-5,6-difluoro-1H-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510491 (CHEMBL4552760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510491 (CHEMBL4552760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at recombinant GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced Fluorecein-SRC2-2 coactivator peptide... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50015421 (CHEMBL3264644) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Beckman Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human GST-tagged FXR after 20 mins by TR-FRET assay | Bioorg Med Chem 22: 2919-38 (2014) Article DOI: 10.1016/j.bmc.2014.04.014 BindingDB Entry DOI: 10.7270/Q2FT8NMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50552997 (CHEMBL4797745) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at recombinant GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced Fluorecein-SRC2-2 coactivator peptide... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00640 BindingDB Entry DOI: 10.7270/Q2QZ2FKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM225944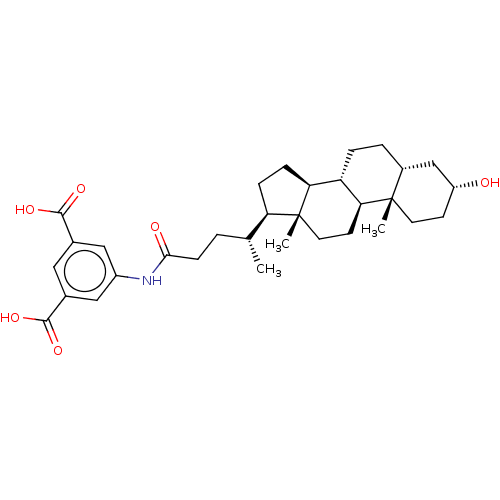 (FXR_34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB D3R | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
D3R | Assay Description The assay buffer contained 50 mM HEPES (pH 7.4), 10 mM NaCl, 5 mM MgCl2 and 0.01% CHAPS. The reactions were incubated for 30 min in the presence of [... | D3R 882: (2017) BindingDB Entry DOI: 10.7270/Q2MC8XWV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336377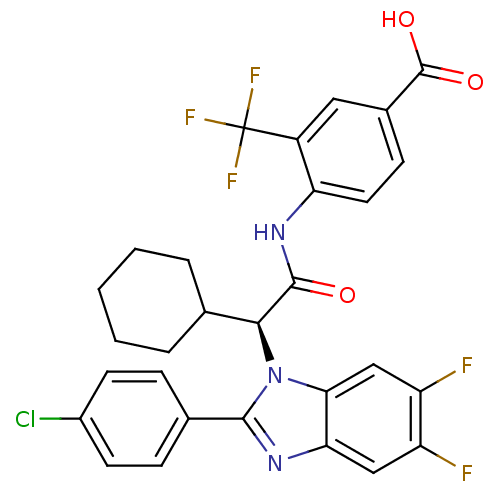 ((S)-4-(2-(2-(4-chlorophenyl)-5,6-difluoro-1H-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336378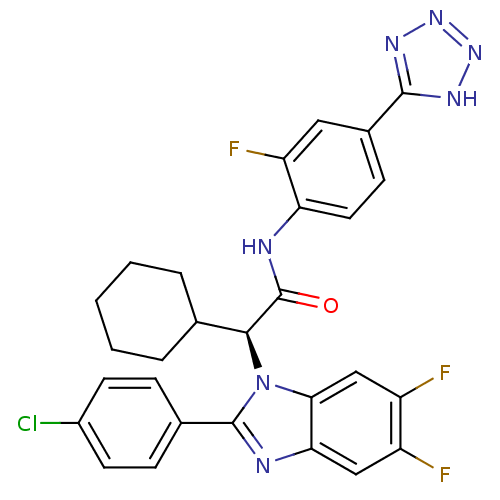 ((S)-2-(2-(4-chlorophenyl)-5,6-difluoro-1H-benzo[d]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM225941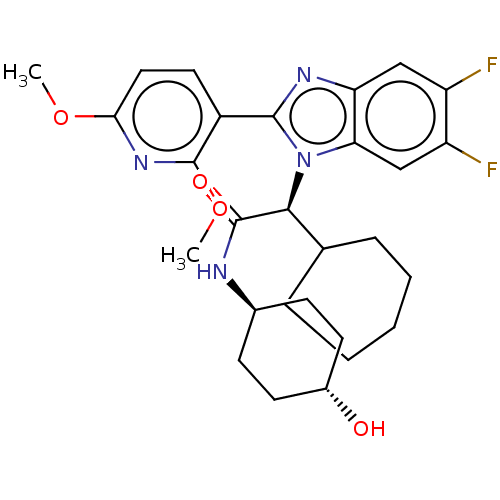 (FXR_31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB D3R | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
D3R | Assay Description The assay buffer contained 50 mM HEPES (pH 7.4), 10 mM NaCl, 5 mM MgCl2 and 0.01% CHAPS. The reactions were incubated for 30 min in the presence of [... | D3R 882: (2017) BindingDB Entry DOI: 10.7270/Q2MC8XWV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336379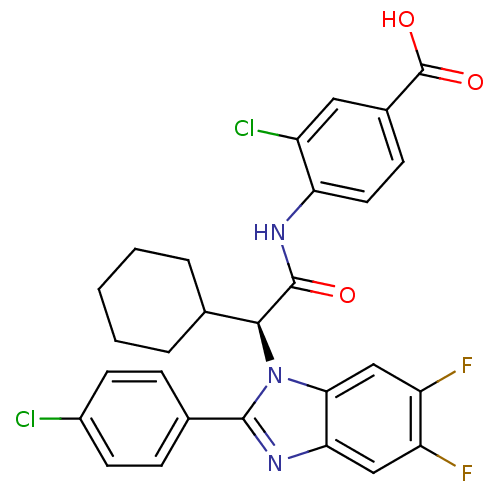 ((S)-3-chloro-4-(2-(2-(4-chlorophenyl)-5,6-difluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB D3R | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
D3R | Assay Description The assay buffer contained 50 mM HEPES (pH 7.4), 10 mM NaCl, 5 mM MgCl2 and 0.01% CHAPS. The reactions were incubated for 30 min in the presence of [... | D3R 882: (2017) BindingDB Entry DOI: 10.7270/Q2MC8XWV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM225933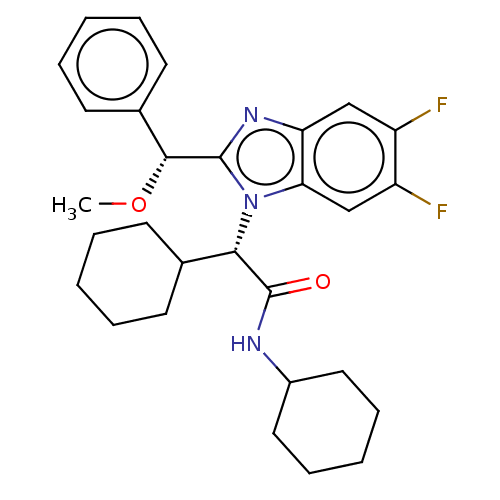 (FXR_22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB D3R | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
D3R | Assay Description The assay buffer contained 50 mM HEPES (pH 7.4), 10 mM NaCl, 5 mM MgCl2 and 0.01% CHAPS. The reactions were incubated for 30 min in the presence of [... | D3R 882: (2017) BindingDB Entry DOI: 10.7270/Q2MC8XWV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50336379 ((S)-3-chloro-4-(2-(2-(4-chlorophenyl)-5,6-difluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of radioligand from human FXR by scintillation proximity assay | Bioorg Med Chem Lett 21: 1134-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.123 BindingDB Entry DOI: 10.7270/Q2MS3T1N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 575 total ) | Next | Last >> |