 Found 89 hits of kd for UniProtKB: P37231
Found 89 hits of kd for UniProtKB: P37231 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 14.8 | 3.80 | n/a | n/a | 7.5 | 37 |
Consiglio Nazionale delle Ricerche
| Assay Description
Kd values were obtained by incubating His-PPARgamma-LBD with biotinylated peptide, europium-labeled anti-histidine antibody, and allophycocyanin-labe... |
J Biol Chem 282: 17314-24 (2007)
Article DOI: 10.1074/jbc.M702316200
BindingDB Entry DOI: 10.7270/Q27M0682 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28762
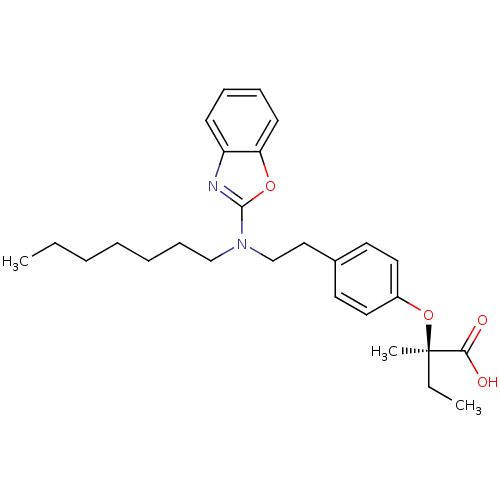
((2R)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...)Show SMILES CCCCCCCN(CCc1ccc(O[C@](C)(CC)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C27H36N2O4/c1-4-6-7-8-11-19-29(26-28-23-12-9-10-13-24(23)32-26)20-18-21-14-16-22(17-15-21)33-27(3,5-2)25(30)31/h9-10,12-17H,4-8,11,18-20H2,1-3H3,(H,30,31)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 685 | 73.3 | n/a | n/a | 7.5 | 37 |
Consiglio Nazionale delle Ricerche
| Assay Description
Kd values were obtained by incubating His-PPARgamma-LBD with biotinylated peptide, europium-labeled anti-histidine antibody, and allophycocyanin-labe... |
J Biol Chem 282: 17314-24 (2007)
Article DOI: 10.1074/jbc.M702316200
BindingDB Entry DOI: 10.7270/Q27M0682 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28763
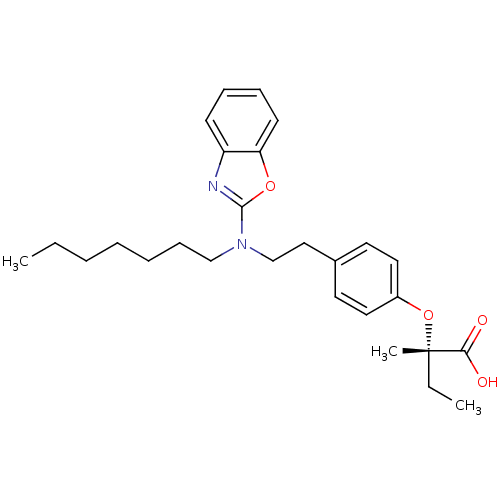
((2S)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...)Show SMILES CCCCCCCN(CCc1ccc(O[C@@](C)(CC)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C27H36N2O4/c1-4-6-7-8-11-19-29(26-28-23-12-9-10-13-24(23)32-26)20-18-21-14-16-22(17-15-21)33-27(3,5-2)25(30)31/h9-10,12-17H,4-8,11,18-20H2,1-3H3,(H,30,31)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 1.98E+3 | 593 | n/a | n/a | 7.5 | 37 |
Consiglio Nazionale delle Ricerche
| Assay Description
Kd values were obtained by incubating His-PPARgamma-LBD with biotinylated peptide, europium-labeled anti-histidine antibody, and allophycocyanin-labe... |
J Biol Chem 282: 17314-24 (2007)
Article DOI: 10.1074/jbc.M702316200
BindingDB Entry DOI: 10.7270/Q27M0682 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50075315
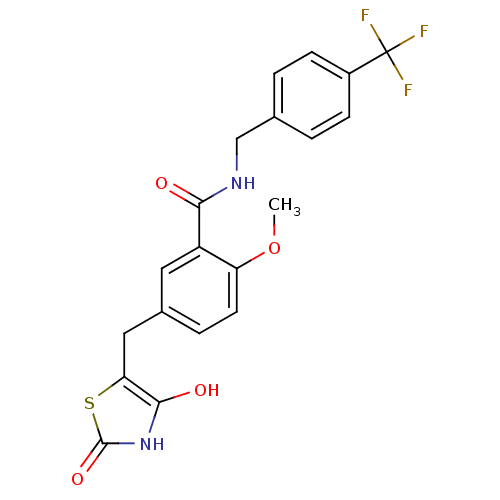
(5-(2,4-Dioxo-thiazolidin-5-ylmethyl)-2-methoxy-N-(...)Show SMILES COc1ccc(Cc2sc(=O)[nH]c2O)cc1C(=O)NCc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H17F3N2O4S/c1-29-15-7-4-12(9-16-18(27)25-19(28)30-16)8-14(15)17(26)24-10-11-2-5-13(6-3-11)20(21,22)23/h2-8,27H,9-10H2,1H3,(H,24,26)(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity for Peroxisome proliferator activated receptor gamma (PPAR gamma) |
Bioorg Med Chem Lett 9: 533-8 (1999)
BindingDB Entry DOI: 10.7270/Q25X283W |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50050550
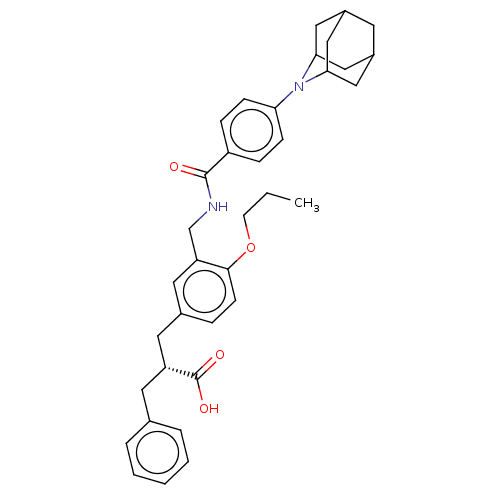
(CHEMBL3317867)Show SMILES CCCOc1ccc(C[C@@H](Cc2ccccc2)C(O)=O)cc1CNC(=O)c1ccc(cc1)N1C2CC3CC(C2)CC1C3 |r,TLB:38:37:41:34.33.32,38:33:36.37.39:41,THB:32:33:36:39.40.41,32:40:36:34.38.33,29:32:36.37.39:41| Show InChI InChI=1S/C36H42N2O4/c1-2-14-42-34-13-8-25(17-29(36(40)41)16-24-6-4-3-5-7-24)18-30(34)23-37-35(39)28-9-11-31(12-10-28)38-32-19-26-15-27(21-32)22-33(38)20-26/h3-13,18,26-27,29,32-33H,2,14-17,19-23H2,1H3,(H,37,39)(H,40,41)/t26?,27?,29-,32?,33?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged PPARgamma LBD by SPR method |
Bioorg Med Chem Lett 24: 4001-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.023
BindingDB Entry DOI: 10.7270/Q20G3MTS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50050551
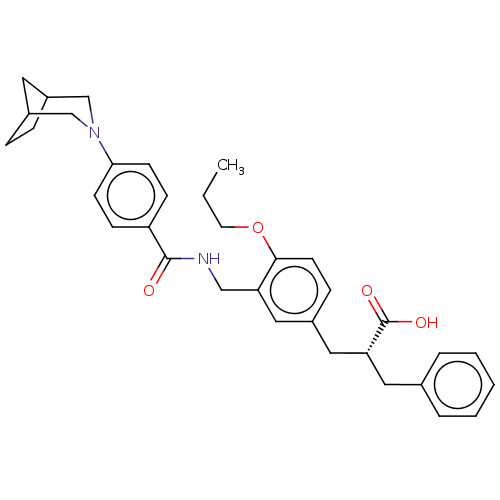
(CHEMBL3317866)Show SMILES CCCOc1ccc(C[C@@H](Cc2ccccc2)C(O)=O)cc1CNC(=O)c1ccc(cc1)N1CC2CCC(C2)C1 |r,TLB:29:32:38:35.36| Show InChI InChI=1S/C34H40N2O4/c1-2-16-40-32-15-10-25(19-29(34(38)39)18-24-6-4-3-5-7-24)20-30(32)21-35-33(37)28-11-13-31(14-12-28)36-22-26-8-9-27(17-26)23-36/h3-7,10-15,20,26-27,29H,2,8-9,16-19,21-23H2,1H3,(H,35,37)(H,38,39)/t26?,27?,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged PPARgamma LBD by SPR method |
Bioorg Med Chem Lett 24: 4001-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.023
BindingDB Entry DOI: 10.7270/Q20G3MTS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50050552

(CHEMBL3317865)Show SMILES CCCOc1ccc(C[C@@H](Cc2ccccc2)C(O)=O)cc1CNC(=O)c1ccc(cc1)N1C2CCCC1CCC2 |r,TLB:29:32:34.35.36:38.39.40| Show InChI InChI=1S/C35H42N2O4/c1-2-20-41-33-19-14-26(22-28(35(39)40)21-25-8-4-3-5-9-25)23-29(33)24-36-34(38)27-15-17-32(18-16-27)37-30-10-6-11-31(37)13-7-12-30/h3-5,8-9,14-19,23,28,30-31H,2,6-7,10-13,20-22,24H2,1H3,(H,36,38)(H,39,40)/t28-,30?,31?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged PPARgamma LBD by SPR method |
Bioorg Med Chem Lett 24: 4001-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.023
BindingDB Entry DOI: 10.7270/Q20G3MTS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50050553
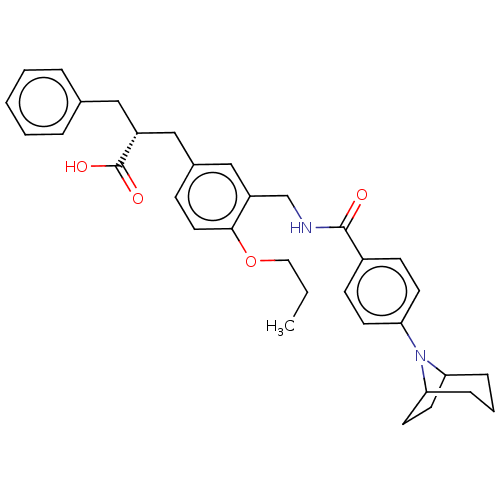
(CHEMBL3317864)Show SMILES CCCOc1ccc(C[C@@H](Cc2ccccc2)C(O)=O)cc1CNC(=O)c1ccc(cc1)N1C2CCC1CCC2 |r,TLB:29:32:38.39.37:34.35| Show InChI InChI=1S/C34H40N2O4/c1-2-19-40-32-18-11-25(21-27(34(38)39)20-24-7-4-3-5-8-24)22-28(32)23-35-33(37)26-12-14-31(15-13-26)36-29-9-6-10-30(36)17-16-29/h3-5,7-8,11-15,18,22,27,29-30H,2,6,9-10,16-17,19-21,23H2,1H3,(H,35,37)(H,38,39)/t27-,29?,30?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged PPARgamma LBD by SPR method |
Bioorg Med Chem Lett 24: 4001-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.023
BindingDB Entry DOI: 10.7270/Q20G3MTS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50050554
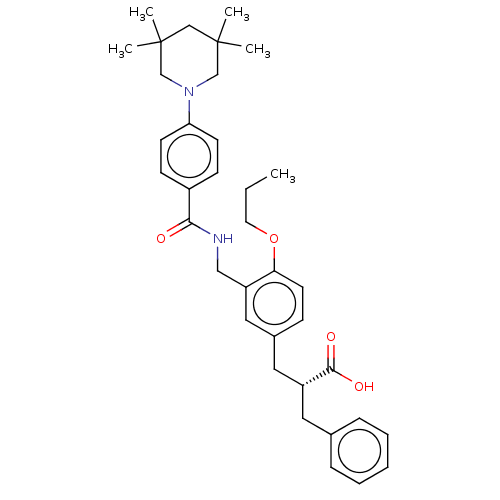
(CHEMBL3317863)Show SMILES CCCOc1ccc(C[C@@H](Cc2ccccc2)C(O)=O)cc1CNC(=O)c1ccc(cc1)N1CC(C)(C)CC(C)(C)C1 |r| Show InChI InChI=1S/C36H46N2O4/c1-6-18-42-32-17-12-27(20-29(34(40)41)19-26-10-8-7-9-11-26)21-30(32)22-37-33(39)28-13-15-31(16-14-28)38-24-35(2,3)23-36(4,5)25-38/h7-17,21,29H,6,18-20,22-25H2,1-5H3,(H,37,39)(H,40,41)/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged PPARgamma LBD by SPR method |
Bioorg Med Chem Lett 24: 4001-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.023
BindingDB Entry DOI: 10.7270/Q20G3MTS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50050555

(CHEMBL3317455)Show SMILES CCCOc1ccc(C[C@@H](Cc2ccccc2)C(O)=O)cc1CNC(=O)c1ccc(cc1)N1CCCCCCCC1 |r| Show InChI InChI=1S/C35H44N2O4/c1-2-22-41-33-19-14-28(24-30(35(39)40)23-27-12-8-7-9-13-27)25-31(33)26-36-34(38)29-15-17-32(18-16-29)37-20-10-5-3-4-6-11-21-37/h7-9,12-19,25,30H,2-6,10-11,20-24,26H2,1H3,(H,36,38)(H,39,40)/t30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged PPARgamma LBD by SPR method |
Bioorg Med Chem Lett 24: 4001-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.023
BindingDB Entry DOI: 10.7270/Q20G3MTS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50050556
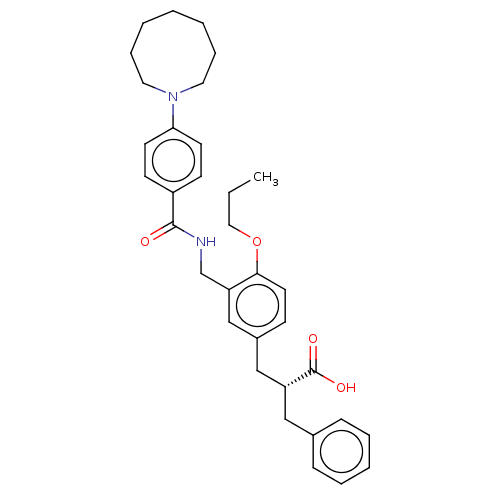
(CHEMBL3317860)Show SMILES CCCOc1ccc(C[C@@H](Cc2ccccc2)C(O)=O)cc1CNC(=O)c1ccc(cc1)N1CCCCCCC1 |r| Show InChI InChI=1S/C34H42N2O4/c1-2-21-40-32-18-13-27(23-29(34(38)39)22-26-11-7-6-8-12-26)24-30(32)25-35-33(37)28-14-16-31(17-15-28)36-19-9-4-3-5-10-20-36/h6-8,11-18,24,29H,2-5,9-10,19-23,25H2,1H3,(H,35,37)(H,38,39)/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged PPARgamma LBD by SPR method |
Bioorg Med Chem Lett 24: 4001-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.023
BindingDB Entry DOI: 10.7270/Q20G3MTS |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50050557
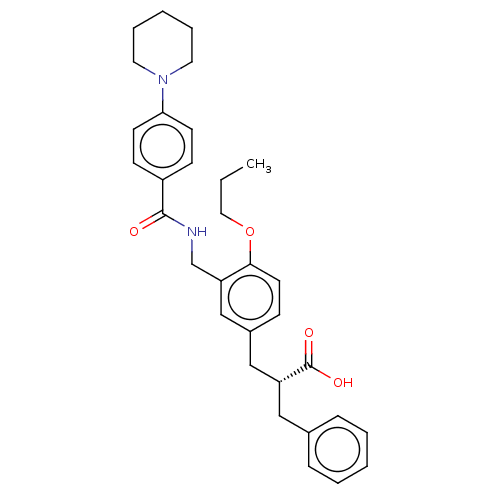
(CHEMBL3317858)Show SMILES CCCOc1ccc(C[C@@H](Cc2ccccc2)C(O)=O)cc1CNC(=O)c1ccc(cc1)N1CCCCC1 |r| Show InChI InChI=1S/C32H38N2O4/c1-2-19-38-30-16-11-25(21-27(32(36)37)20-24-9-5-3-6-10-24)22-28(30)23-33-31(35)26-12-14-29(15-13-26)34-17-7-4-8-18-34/h3,5-6,9-16,22,27H,2,4,7-8,17-21,23H2,1H3,(H,33,35)(H,36,37)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a |
Okayama University
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged PPARgamma LBD by SPR method |
Bioorg Med Chem Lett 24: 4001-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.023
BindingDB Entry DOI: 10.7270/Q20G3MTS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM19264

((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...)Show InChI InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma |
Bioorg Med Chem Lett 17: 4767-70 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.059
BindingDB Entry DOI: 10.7270/Q21R6Q65 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50122340
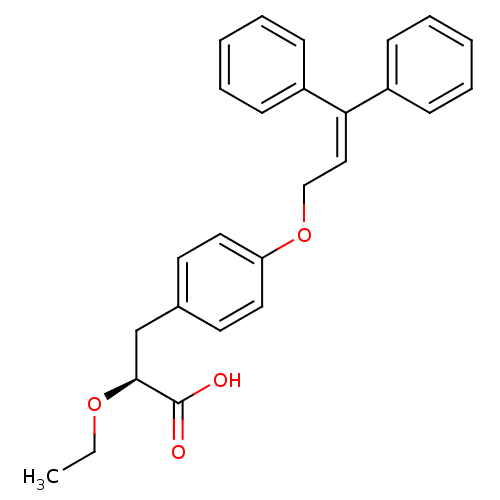
((S)-3-[4-(3,3-Diphenyl-allyloxy)-phenyl]-2-ethoxy-...)Show SMILES [#6]-[#6]-[#8]-[#6@@H](-[#6]-c1ccc(-[#8]-[#6]\[#6]=[#6](/c2ccccc2)-c2ccccc2)cc1)-[#6](-[#8])=O Show InChI InChI=1S/C26H26O4/c1-2-29-25(26(27)28)19-20-13-15-23(16-14-20)30-18-17-24(21-9-5-3-6-10-21)22-11-7-4-8-12-22/h3-17,25H,2,18-19H2,1H3,(H,27,28)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor gamma |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50075315

(5-(2,4-Dioxo-thiazolidin-5-ylmethyl)-2-methoxy-N-(...)Show SMILES COc1ccc(Cc2sc(=O)[nH]c2O)cc1C(=O)NCc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H17F3N2O4S/c1-29-15-7-4-12(9-16-18(27)25-19(28)30-16)8-14(15)17(26)24-10-11-2-5-13(6-3-11)20(21,22)23/h2-8,27H,9-10H2,1H3,(H,24,26)(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor gamma |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50122338
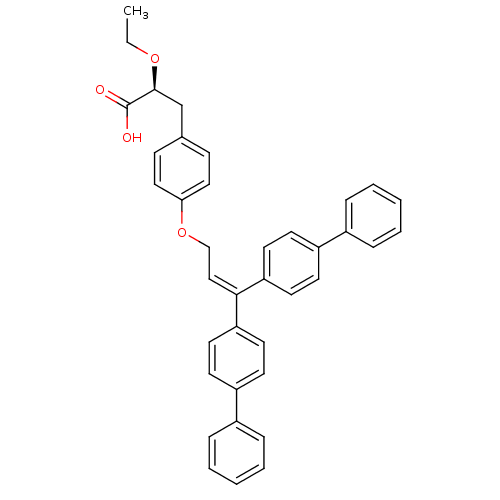
((S)-3-[4-(3,3-Bis-biphenyl-4-yl-allyloxy)-phenyl]-...)Show SMILES [#6]-[#6]-[#8]-[#6@@H](-[#6]-c1ccc(-[#8]-[#6]\[#6]=[#6](/c2ccc(cc2)-c2ccccc2)-c2ccc(cc2)-c2ccccc2)cc1)-[#6](-[#8])=O Show InChI InChI=1S/C38H34O4/c1-2-41-37(38(39)40)27-28-13-23-35(24-14-28)42-26-25-36(33-19-15-31(16-20-33)29-9-5-3-6-10-29)34-21-17-32(18-22-34)30-11-7-4-8-12-30/h3-25,37H,2,26-27H2,1H3,(H,39,40)/t37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor gamma |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50175504
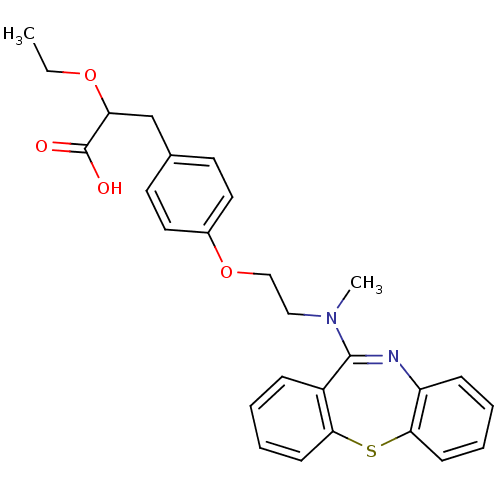
(3-{4-[2-(Dibenzo[b,f][1,4]thiazepin-11-yl-methyl-a...)Show SMILES CCOC(Cc1ccc(OCCN(C)C2=Nc3ccccc3Sc3ccccc23)cc1)C(O)=O |t:14| Show InChI InChI=1S/C27H28N2O4S/c1-3-32-23(27(30)31)18-19-12-14-20(15-13-19)33-17-16-29(2)26-21-8-4-6-10-24(21)34-25-11-7-5-9-22(25)28-26/h4-15,23H,3,16-18H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor gamma |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50109547
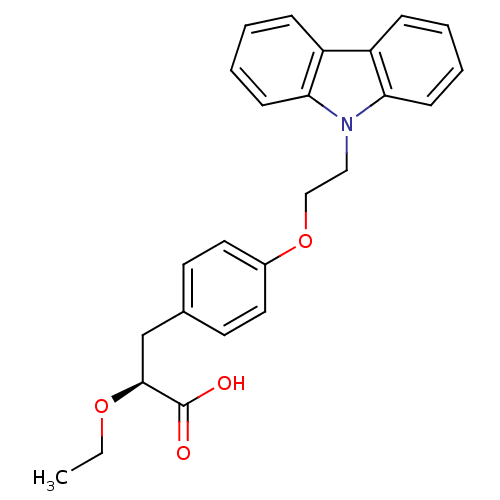
((S)-3-(4-(2-CARBAZOL-9-YL-ETHOXY)-PHENYL)-2-ETHOXY...)Show SMILES CCO[C@@H](Cc1ccc(OCCn2c3ccccc3c3ccccc23)cc1)C(O)=O Show InChI InChI=1S/C25H25NO4/c1-2-29-24(25(27)28)17-18-11-13-19(14-12-18)30-16-15-26-22-9-5-3-7-20(22)21-8-4-6-10-23(21)26/h3-14,24H,2,15-17H2,1H3,(H,27,28)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards peroxisome proliferator activated receptor gamma |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 4.98E+3 | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma |
Bioorg Med Chem Lett 16: 5913-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.093
BindingDB Entry DOI: 10.7270/Q20002W3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50370846
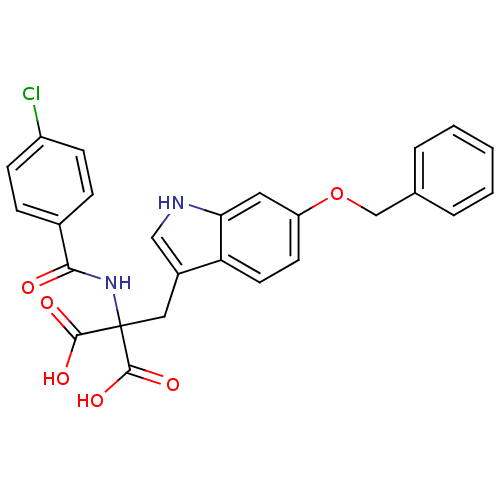
(CHEMBL375353)Show SMILES OC(=O)C(Cc1c[nH]c2cc(OCc3ccccc3)ccc12)(NC(=O)c1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C26H21ClN2O6/c27-19-8-6-17(7-9-19)23(30)29-26(24(31)32,25(33)34)13-18-14-28-22-12-20(10-11-21(18)22)35-15-16-4-2-1-3-5-16/h1-12,14,28H,13,15H2,(H,29,30)(H,31,32)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.69E+3 | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma |
Bioorg Med Chem Lett 16: 5913-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.093
BindingDB Entry DOI: 10.7270/Q20002W3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50370847

(CHEMBL223249)Show SMILES OC(=O)C(Cc1c[nH]c2cc(OCc3ccccc3)ccc12)NC(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C25H21ClN2O4/c26-19-8-6-17(7-9-19)24(29)28-23(25(30)31)12-18-14-27-22-13-20(10-11-21(18)22)32-15-16-4-2-1-3-5-16/h1-11,13-14,23,27H,12,15H2,(H,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.86E+3 | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma |
Bioorg Med Chem Lett 16: 5913-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.093
BindingDB Entry DOI: 10.7270/Q20002W3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50203462

(CHEMBL3931161)Show SMILES CN(C)C(=O)c1ccc2n(nc(Oc3c(Cl)cccc3C(F)(F)F)c2c1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H17ClF3N3O4/c1-30(2)22(32)14-8-11-19-16(12-14)21(29-31(19)15-9-6-13(7-10-15)23(33)34)35-20-17(24(26,27)28)4-3-5-18(20)25/h3-12H,1-2H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a |
Galderma R&D
Curated by ChEMBL
| Assay Description
Activity at GAL4 DBD-fused PPARgamma LBD (unknown origin) expressed in HG5LN cells after 18 hrs by luciferase reporter gene assay |
Bioorg Med Chem Lett 26: 5802-5808 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.023
BindingDB Entry DOI: 10.7270/Q2D79DDH |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Helmholtz Centre for Infection Research
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma (unknown origin) by competitive TR-FRET assay |
J Med Chem 56: 1535-43 (2013)
Article DOI: 10.1021/jm3013272
BindingDB Entry DOI: 10.7270/Q2R49S4J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428826
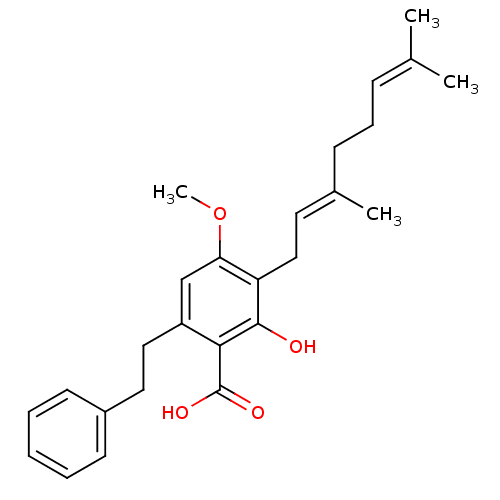
(CHEMBL2337127)Show SMILES [#6]-[#8]-c1cc(-[#6]-[#6]-c2ccccc2)c(-[#6](-[#8])=O)c(-[#8])c1-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C26H32O4/c1-18(2)9-8-10-19(3)13-16-22-23(30-4)17-21(24(25(22)27)26(28)29)15-14-20-11-6-5-7-12-20/h5-7,9,11-13,17,27H,8,10,14-16H2,1-4H3,(H,28,29)/b19-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a |
Helmholtz Centre for Infection Research
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma (unknown origin) by competitive TR-FRET assay |
J Med Chem 56: 1535-43 (2013)
Article DOI: 10.1021/jm3013272
BindingDB Entry DOI: 10.7270/Q2R49S4J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428827
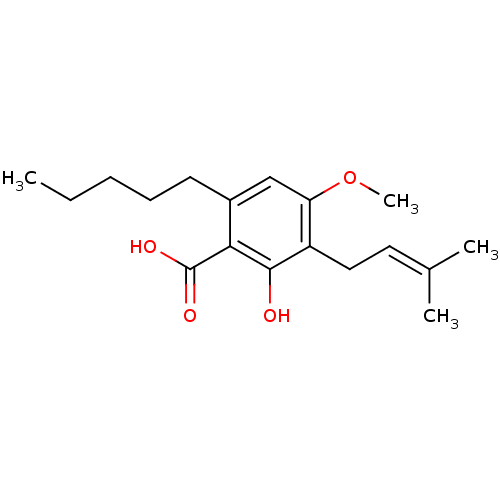
(CHEMBL2337126)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-c1cc(-[#8]-[#6])c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c1-[#6](-[#8])=O Show InChI InChI=1S/C18H26O4/c1-5-6-7-8-13-11-15(22-4)14(10-9-12(2)3)17(19)16(13)18(20)21/h9,11,19H,5-8,10H2,1-4H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 287 | n/a | n/a | n/a | n/a | n/a |
Helmholtz Centre for Infection Research
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma (unknown origin) by competitive TR-FRET assay |
J Med Chem 56: 1535-43 (2013)
Article DOI: 10.1021/jm3013272
BindingDB Entry DOI: 10.7270/Q2R49S4J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM91427
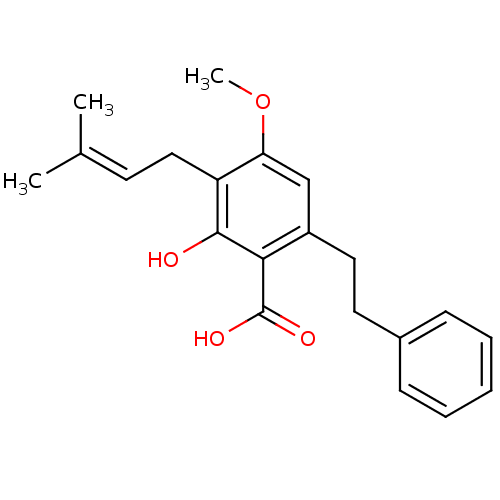
(2-hydroxy-4-methoxy-3-(3-methylbut-2-enyl)-6-(2-ph...)Show SMILES [#6]-[#8]-c1cc(-[#6]-[#6]-c2ccccc2)c(-[#6](-[#8])=O)c(-[#8])c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C21H24O4/c1-14(2)9-12-17-18(25-3)13-16(19(20(17)22)21(23)24)11-10-15-7-5-4-6-8-15/h4-9,13,22H,10-12H2,1-3H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 236 | n/a | n/a | n/a | n/a | n/a |
Helmholtz Centre for Infection Research
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma (unknown origin) by competitive TR-FRET assay |
J Med Chem 56: 1535-43 (2013)
Article DOI: 10.1021/jm3013272
BindingDB Entry DOI: 10.7270/Q2R49S4J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50099491
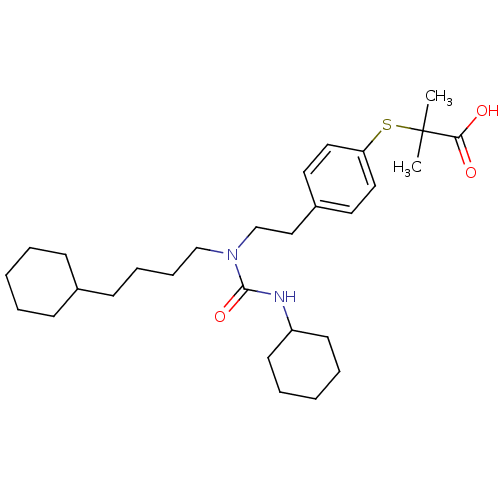
(2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...)Show SMILES CC(C)(Sc1ccc(CCN(CCCCC2CCCCC2)C(=O)NC2CCCCC2)cc1)C(O)=O Show InChI InChI=1S/C29H46N2O3S/c1-29(2,27(32)33)35-26-18-16-24(17-19-26)20-22-31(28(34)30-25-14-7-4-8-15-25)21-10-9-13-23-11-5-3-6-12-23/h16-19,23,25H,3-15,20-22H2,1-2H3,(H,30,34)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a |
Helmholtz Centre for Infection Research
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma (unknown origin) by competitive TR-FRET assay |
J Med Chem 56: 1535-43 (2013)
Article DOI: 10.1021/jm3013272
BindingDB Entry DOI: 10.7270/Q2R49S4J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50173365
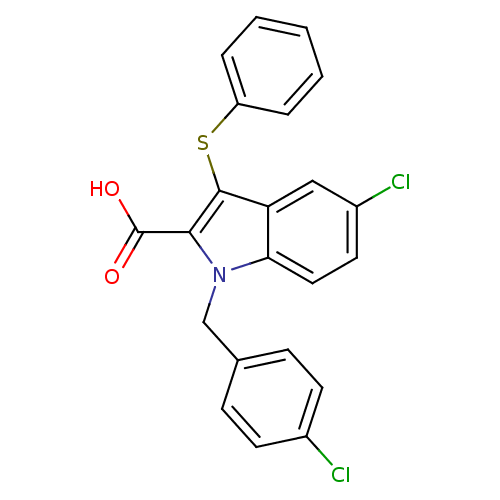
(5-CHLORO-1-(4-CHLOROBENZYL)-3-(PHENYLTHIO)-1H-INDO...)Show SMILES OC(=O)c1c(Sc2ccccc2)c2cc(Cl)ccc2n1Cc1ccc(Cl)cc1 Show InChI InChI=1S/C22H15Cl2NO2S/c23-15-8-6-14(7-9-15)13-25-19-11-10-16(24)12-18(19)21(20(25)22(26)27)28-17-4-2-1-3-5-17/h1-12H,13H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a |
Helmholtz Centre for Infection Research
Curated by ChEMBL
| Assay Description
Binding affinity to human PPARgamma (unknown origin) by competitive TR-FRET assay |
J Med Chem 56: 1535-43 (2013)
Article DOI: 10.1021/jm3013272
BindingDB Entry DOI: 10.7270/Q2R49S4J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma ligand binding domain by isothermal titration calorimetry |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50401010
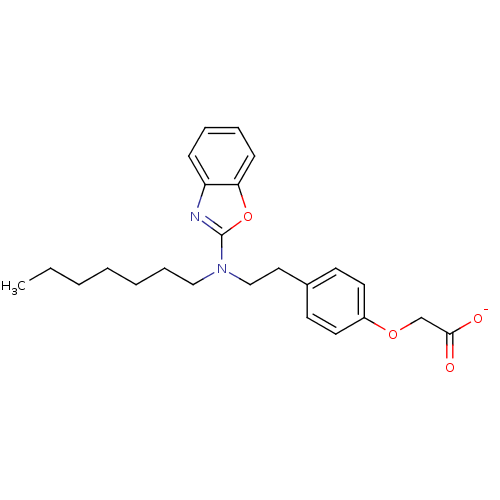
(CHEMBL2206304)Show SMILES CCCCCCCN(CCc1ccc(OCC([O-])=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C24H30N2O4/c1-2-3-4-5-8-16-26(24-25-21-9-6-7-10-22(21)30-24)17-15-19-11-13-20(14-12-19)29-18-23(27)28/h6-7,9-14H,2-5,8,15-18H2,1H3,(H,27,28)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma ligand binding domain by isothermal titration calorimetry |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50401011
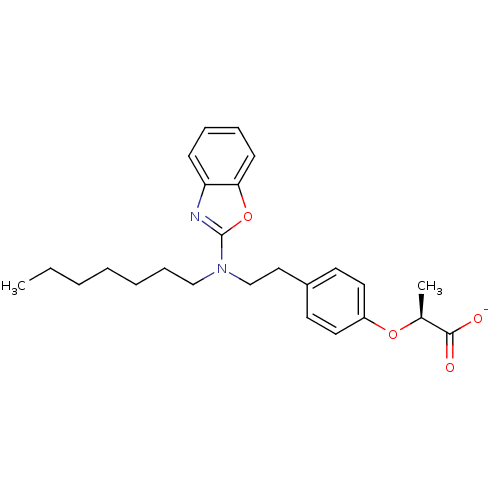
(CHEMBL2206303)Show SMILES CCCCCCCN(CCc1ccc(O[C@@H](C)C([O-])=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C25H32N2O4/c1-3-4-5-6-9-17-27(25-26-22-10-7-8-11-23(22)31-25)18-16-20-12-14-21(15-13-20)30-19(2)24(28)29/h7-8,10-15,19H,3-6,9,16-18H2,1-2H3,(H,28,29)/p-1/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma ligand binding domain by isothermal titration calorimetry |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
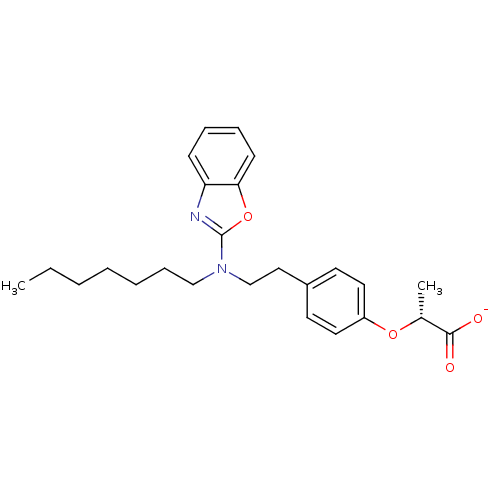
(Homo sapiens (Human)) | BDBM50401012
(CHEMBL2206302)Show SMILES CCCCCCCN(CCc1ccc(O[C@H](C)C([O-])=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C25H32N2O4/c1-3-4-5-6-9-17-27(25-26-22-10-7-8-11-23(22)31-25)18-16-20-12-14-21(15-13-20)30-19(2)24(28)29/h7-8,10-15,19H,3-6,9,16-18H2,1-2H3,(H,28,29)/p-1/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma ligand binding domain by isothermal titration calorimetry |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
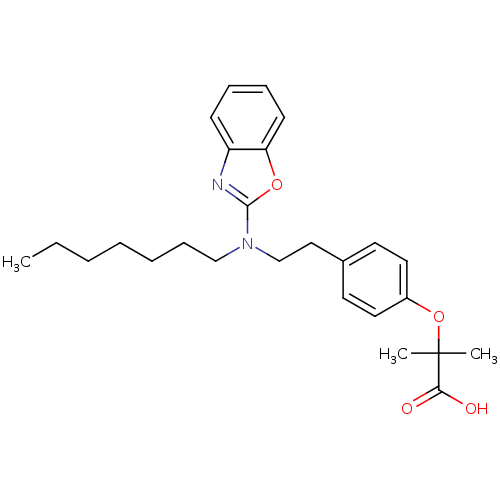
(Homo sapiens (Human)) | BDBM50401013
(CHEMBL477312)Show SMILES CCCCCCCN(CCc1ccc(OC(C)(C)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C26H34N2O4/c1-4-5-6-7-10-18-28(25-27-22-11-8-9-12-23(22)31-25)19-17-20-13-15-21(16-14-20)32-26(2,3)24(29)30/h8-9,11-16H,4-7,10,17-19H2,1-3H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma ligand binding domain by isothermal titration calorimetry |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28763

((2S)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...)Show SMILES CCCCCCCN(CCc1ccc(O[C@@](C)(CC)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C27H36N2O4/c1-4-6-7-8-11-19-29(26-28-23-12-9-10-13-24(23)32-26)20-18-21-14-16-22(17-15-21)33-27(3,5-2)25(30)31/h9-10,12-17H,4-8,11,18-20H2,1-3H3,(H,30,31)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma ligand binding domain by isothermal titration calorimetry |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28762

((2R)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...)Show SMILES CCCCCCCN(CCc1ccc(O[C@](C)(CC)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C27H36N2O4/c1-4-6-7-8-11-19-29(26-28-23-12-9-10-13-24(23)32-26)20-18-21-14-16-22(17-15-21)33-27(3,5-2)25(30)31/h9-10,12-17H,4-8,11,18-20H2,1-3H3,(H,30,31)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma ligand binding domain by isothermal titration calorimetry |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
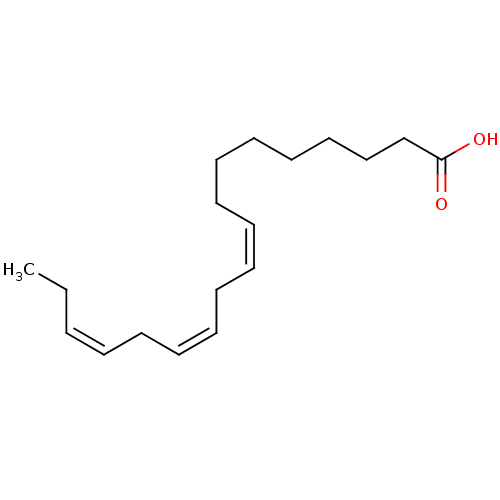
(Homo sapiens (Human)) | BDBM50240347
((9,12,15)-linolenic acid | (9Z,12Z,15Z)-octadeca-9...)Show InChI InChI=1S/C18H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h3-4,6-7,9-10H,2,5,8,11-17H2,1H3,(H,19,20)/b4-3-,7-6-,10-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma |
J Med Chem 55: 4027-61 (2012)
Article DOI: 10.1021/jm101360s
BindingDB Entry DOI: 10.7270/Q2ST7QW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50270612
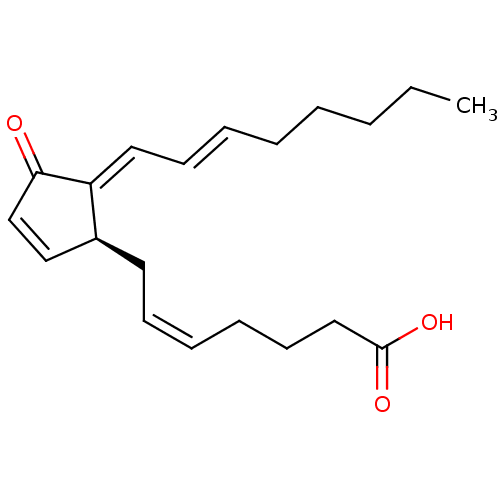
(15-deoxy-d12,14-prostaglandin J2 | 15-deoxy-delta1...)Show SMILES CCCCC\C=C\C=C1/[C@@H](C\C=C/CCCC(O)=O)C=CC1=O |r,c:19| Show InChI InChI=1S/C20H28O3/c1-2-3-4-5-6-10-13-18-17(15-16-19(18)21)12-9-7-8-11-14-20(22)23/h6-7,9-10,13,15-17H,2-5,8,11-12,14H2,1H3,(H,22,23)/b9-7-,10-6+,18-13+/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma |
J Med Chem 55: 4027-61 (2012)
Article DOI: 10.1021/jm101360s
BindingDB Entry DOI: 10.7270/Q2ST7QW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50389674
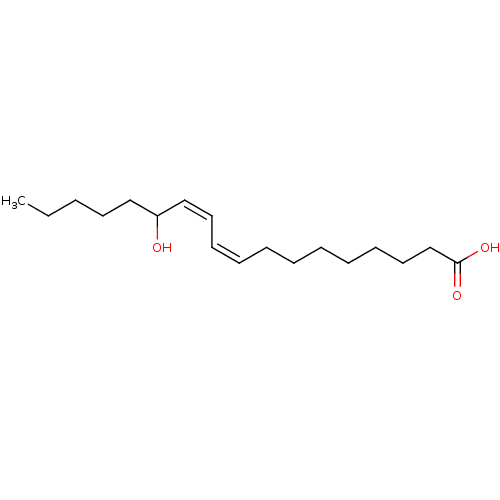
(CHEMBL2069934)Show InChI InChI=1S/C18H32O3/c1-2-3-11-14-17(19)15-12-9-7-5-4-6-8-10-13-16-18(20)21/h7,9,12,15,17,19H,2-6,8,10-11,13-14,16H2,1H3,(H,20,21)/b9-7-,15-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma |
J Med Chem 55: 4027-61 (2012)
Article DOI: 10.1021/jm101360s
BindingDB Entry DOI: 10.7270/Q2ST7QW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50389675
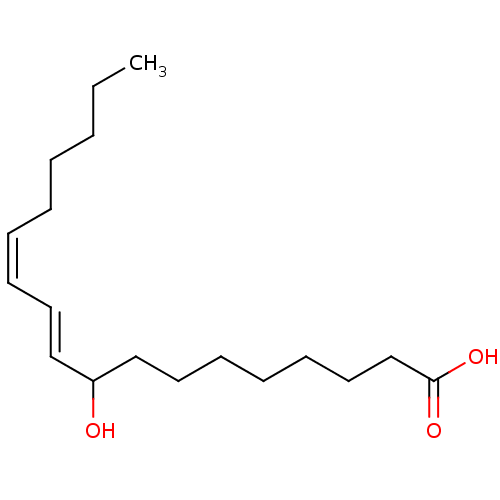
(CHEMBL1077284)Show InChI InChI=1S/C18H32O3/c1-2-3-4-5-6-8-11-14-17(19)15-12-9-7-10-13-16-18(20)21/h6,8,11,14,17,19H,2-5,7,9-10,12-13,15-16H2,1H3,(H,20,21)/b8-6-,14-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma |
J Med Chem 55: 4027-61 (2012)
Article DOI: 10.1021/jm101360s
BindingDB Entry DOI: 10.7270/Q2ST7QW1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50242349

((5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-eicosapentaenoic ...)Show InChI InChI=1S/C20H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h3-4,6-7,9-10,12-13,15-16H,2,5,8,11,14,17-19H2,1H3,(H,21,22)/b4-3-,7-6-,10-9-,13-12-,16-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ Lille-Nord de France
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma |
J Med Chem 55: 4027-61 (2012)
Article DOI: 10.1021/jm101360s
BindingDB Entry DOI: 10.7270/Q2ST7QW1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50235985
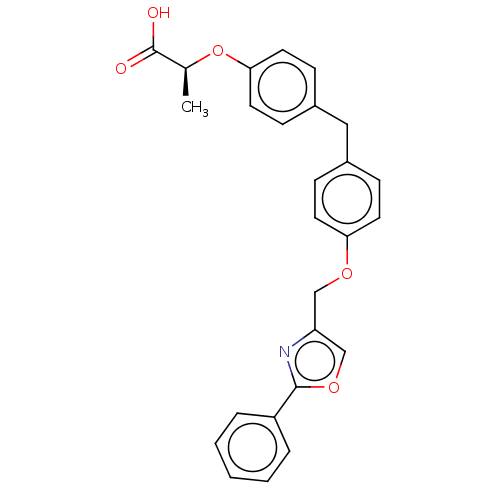
(CHEMBL4092600)Show SMILES C[C@H](Oc1ccc(Cc2ccc(OCc3coc(n3)-c3ccccc3)cc2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H23NO5/c1-18(26(28)29)32-24-13-9-20(10-14-24)15-19-7-11-23(12-8-19)30-16-22-17-31-25(27-22)21-5-3-2-4-6-21/h2-14,17-18H,15-16H2,1H3,(H,28,29)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro"
Curated by ChEMBL
| Assay Description
Inhibition of FTase-catalyzed incorporation of [3H]- FPP radioligand into recombinant Ha-Ras by 50% at an enzyme concentration of 1 nM. |
Eur J Med Chem 127: 379-397 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.047
BindingDB Entry DOI: 10.7270/Q2474D4J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50235986
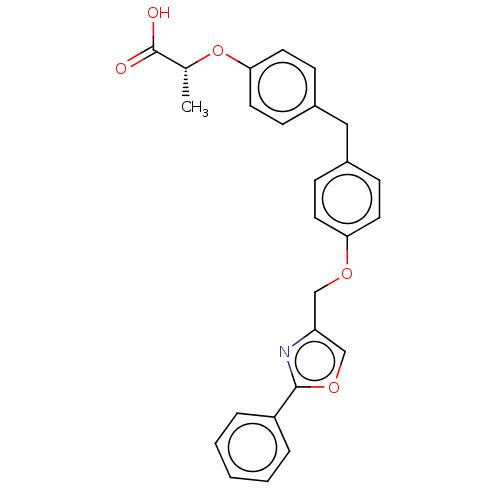
(CHEMBL4073499)Show SMILES C[C@@H](Oc1ccc(Cc2ccc(OCc3coc(n3)-c3ccccc3)cc2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H23NO5/c1-18(26(28)29)32-24-13-9-20(10-14-24)15-19-7-11-23(12-8-19)30-16-22-17-31-25(27-22)21-5-3-2-4-6-21/h2-14,17-18H,15-16H2,1H3,(H,28,29)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro"
Curated by ChEMBL
| Assay Description
Inhibition of FTase-catalyzed incorporation of [3H]- FPP radioligand into recombinant Ha-Ras by 50% at an enzyme concentration of 1 nM. |
Eur J Med Chem 127: 379-397 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.047
BindingDB Entry DOI: 10.7270/Q2474D4J |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM24566
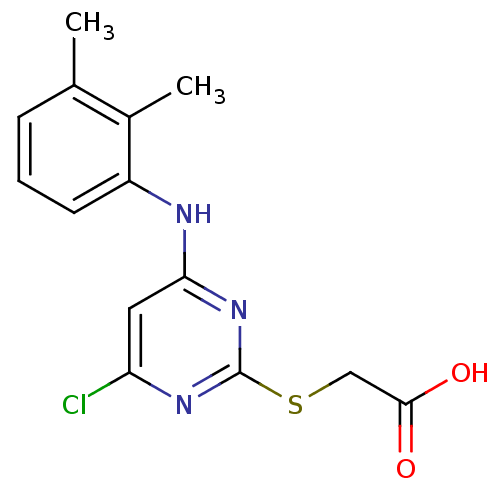
(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show InChI InChI=1S/C14H14ClN3O2S/c1-8-4-3-5-10(9(8)2)16-12-6-11(15)17-14(18-12)21-7-13(19)20/h3-6H,7H2,1-2H3,(H,19,20)(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro"
Curated by ChEMBL
| Assay Description
Inhibition of FTase-catalyzed incorporation of [3H]- FPP radioligand into recombinant Ha-Ras by 50% at an enzyme concentration of 1 nM. |
Eur J Med Chem 127: 379-397 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.047
BindingDB Entry DOI: 10.7270/Q2474D4J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50030474
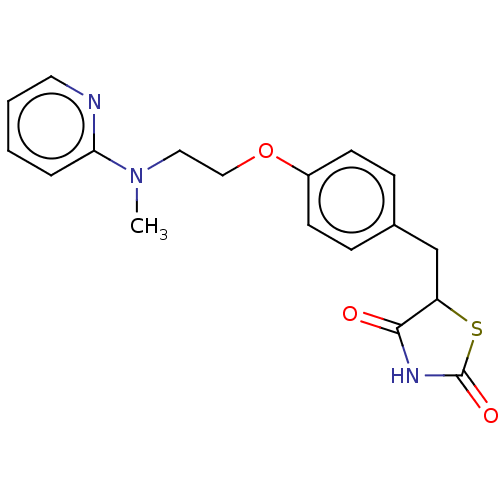
(Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,15H,10-12H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro"
Curated by ChEMBL
| Assay Description
Inhibition of FTase-catalyzed incorporation of [3H]- FPP radioligand into recombinant Ha-Ras by 50% at an enzyme concentration of 1 nM. |
Eur J Med Chem 127: 379-397 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.047
BindingDB Entry DOI: 10.7270/Q2474D4J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50461063
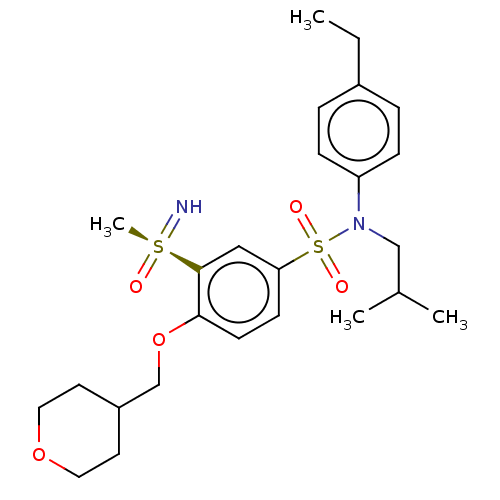
(CHEMBL4226803 | US10457637, Compound 8)Show SMILES CCc1ccc(cc1)N(CC(C)C)S(=O)(=O)c1ccc(OCC2CCOCC2)c(c1)[S@@](C)(=N)=O |r| Show InChI InChI=1S/C25H36N2O5S2/c1-5-20-6-8-22(9-7-20)27(17-19(2)3)34(29,30)23-10-11-24(25(16-23)33(4,26)28)32-18-21-12-14-31-15-13-21/h6-11,16,19,21,26H,5,12-15,17-18H2,1-4H3/t33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health
Curated by ChEMBL
| Assay Description
Activity at PPARgamma (unknown origin) expressed in human HeLa-derived HG5LN cells transfected with the GAL4 DNA-binding domain fused to the PPARgamm... |
Bioorg Med Chem Lett 28: 1269-1273 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.041
BindingDB Entry DOI: 10.7270/Q2WD437Q |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50536780
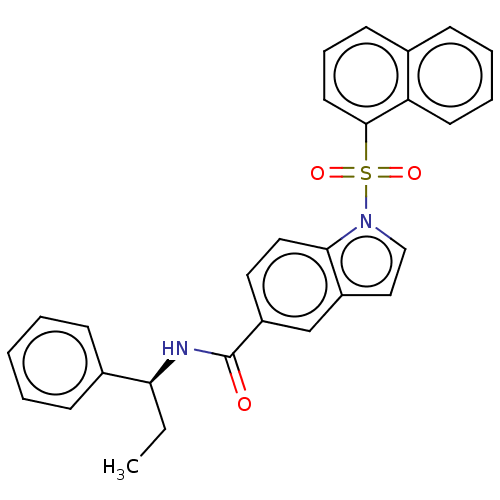
(CHEMBL4466393)Show SMILES CC[C@H](NC(=O)c1ccc2n(ccc2c1)S(=O)(=O)c1cccc2ccccc12)c1ccccc1 |r| Show InChI InChI=1S/C28H24N2O3S/c1-2-25(21-10-4-3-5-11-21)29-28(31)23-15-16-26-22(19-23)17-18-30(26)34(32,33)27-14-8-12-20-9-6-7-13-24(20)27/h3-19,25H,2H2,1H3,(H,29,31)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to N-terminal His6-tagged human PPARgamma (205 to 477 residues) expressed in Escherichia coli BL21(DE3) by surface plasmon resonance... |
Bioorg Med Chem Lett 26: 4157-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.067
BindingDB Entry DOI: 10.7270/Q2W66Q9R |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50536784
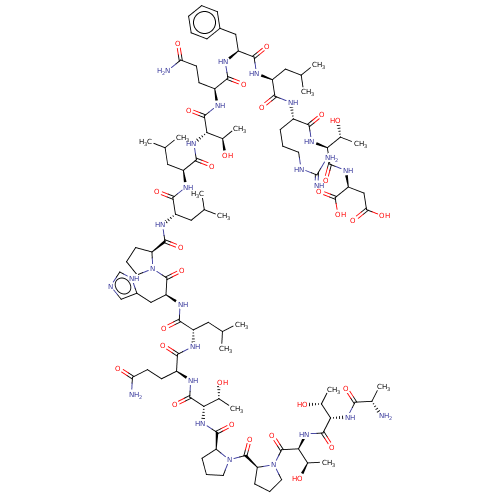
(CHEMBL4552580)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](C)N)[C@@H](C)O)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O |r| Show InChI InChI=1S/C97H156N26O29/c1-45(2)36-60(82(137)106-57(24-18-32-104-97(101)102)81(136)117-74(51(11)125)91(146)115-66(96(151)152)42-72(131)132)110-85(140)64(40-55-22-16-15-17-23-55)112-80(135)59(29-31-71(100)130)107-89(144)73(50(10)124)118-86(141)63(39-48(7)8)111-83(138)62(38-47(5)6)113-87(142)67-25-19-33-121(67)93(148)65(41-56-43-103-44-105-56)114-84(139)61(37-46(3)4)109-79(134)58(28-30-70(99)129)108-90(145)75(52(12)126)119-88(143)68-26-20-34-122(68)94(149)69-27-21-35-123(69)95(150)77(54(14)128)120-92(147)76(53(13)127)116-78(133)49(9)98/h15-17,22-23,43-54,57-69,73-77,124-128H,18-21,24-42,98H2,1-14H3,(H2,99,129)(H2,100,130)(H,103,105)(H,106,137)(H,107,144)(H,108,145)(H,109,134)(H,110,140)(H,111,138)(H,112,135)(H,113,142)(H,114,139)(H,115,146)(H,116,133)(H,117,136)(H,118,141)(H,119,143)(H,120,147)(H,131,132)(H,151,152)(H4,101,102,104)/t49-,50+,51+,52+,53+,54+,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,73-,74-,75-,76-,77-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of 5-FAM-labeled NBM131 from human PPARgamma ligand binding domain expressed in human HepG2 cells by fluorescence polarization assay |
Bioorg Med Chem Lett 26: 4157-64 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.067
BindingDB Entry DOI: 10.7270/Q2W66Q9R |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50103521
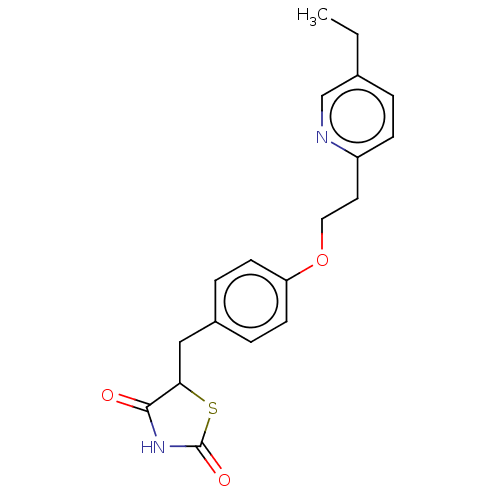
(Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 871 | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human 6His-tagged PPARgamma LBD expressed in Escherichia coli BL21(DE3) using FITC-NTKNHPMLMNLLKDNPAQD peptide by isothermal titr... |
J Med Chem 62: 2008-2023 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01573
BindingDB Entry DOI: 10.7270/Q2TH8R5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50530214

(CHEBI:82937 | Leriglitazone | Min-102)Show InChI InChI=1S/C19H20N2O4S/c1-12(22)14-4-5-15(20-11-14)8-9-25-16-6-2-13(3-7-16)10-17-18(23)21-19(24)26-17/h2-7,11-12,17,22H,8-10H2,1H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human 6His-tagged PPARgamma LBD expressed in Escherichia coli BL21(DE3) using FITC-NTKNHPMLMNLLKDNPAQD peptide by isothermal titr... |
J Med Chem 62: 2008-2023 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01573
BindingDB Entry DOI: 10.7270/Q2TH8R5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50530214

(CHEBI:82937 | Leriglitazone | Min-102)Show InChI InChI=1S/C19H20N2O4S/c1-12(22)14-4-5-15(20-11-14)8-9-25-16-6-2-13(3-7-16)10-17-18(23)21-19(24)26-17/h2-7,11-12,17,22H,8-10H2,1H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human 6His-tagged PPARgamma LBD expressed in Escherichia coli BL21(DE3) using FITC-NTKNHPMLMNLLKDNPAQD peptide by isothermal titr... |
J Med Chem 62: 2008-2023 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01573
BindingDB Entry DOI: 10.7270/Q2TH8R5M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data