Found 518 hits of ec50 data for polymerid = 2210,49000820
Found 518 hits of ec50 data for polymerid = 2210,49000820 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50255899

(1-[1-(1-Methylcyclooctyl)-4-piperidinyl]-2-[(3S)-3...)Show SMILES CC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)[C@H]1CCCNC1 |r| Show InChI InChI=1S/C26H40N4/c1-26(15-7-3-2-4-8-16-26)29-18-13-22(14-19-29)30-24-12-6-5-11-23(24)28-25(30)21-10-9-17-27-20-21/h5-6,11-12,21-22,27H,2-4,7-10,13-20H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a |
ShanghaiTech University
Curated by ChEMBL
| Assay Description
Agonist activity at human NOPR expressed in human BHK cells assessed as inhibition of forskolin-mediated cAMP accumulation after 10 mins by radioimmu... |
J Med Chem 61: 9841-9878 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00435
BindingDB Entry DOI: 10.7270/Q2F76GX7 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50333098
(CHEMBL1631926)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@H]-1-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6]-1=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@H]-1-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6]-1=O)-[#6](-[#7])=O |r| Show InChI InChI=1S/C90H145FN32O24/c1-48(125)73(123-86(145)62(40-50-26-28-51(91)29-27-50)111-71(131)45-107-70(130)44-108-75(134)52(95)39-49-17-3-2-4-18-49)88(147)109-46-72(132)110-53-22-8-13-36-104-69(129)43-64(121-87(146)65(47-124)122-82(141)56(21-7-12-34-94)114-79(138)58(116-76(53)135)24-15-37-105-89(99)100)85(144)117-59(25-16-38-106-90(101)102)80(139)112-54(19-5-10-32-92)77(136)115-57-23-9-14-35-103-68(128)42-61(74(98)133)119-83(142)60(30-31-66(96)126)118-84(143)63(41-67(97)127)120-81(140)55(113-78(57)137)20-6-11-33-93/h2-4,17-18,26-29,48,52-65,73,124-125H,5-16,19-25,30-47,92-95H2,1H3,(H2,96,126)(H2,97,127)(H2,98,133)(H,103,128)(H,104,129)(H,107,130)(H,108,134)(H,109,147)(H,110,132)(H,111,131)(H,112,139)(H,113,137)(H,114,138)(H,115,136)(H,116,135)(H,117,144)(H,118,143)(H,119,142)(H,120,140)(H,121,146)(H,122,141)(H,123,145)(H4,99,100,105)(H4,101,102,106)/t48-,52+,53+,54+,55+,56+,57+,58+,59+,60+,61+,62+,63+,64+,65+,73+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at NOR in mouse Neuro-2a cells assessed as stimulation of ERK phopshorylation after 30 mins post dose by Alphascreen Surefire assay |
J Med Chem 53: 8400-8408 (2010)
Article DOI: 10.1021/jm101139f
BindingDB Entry DOI: 10.7270/Q24Q7V77 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121247
(CHEMBL414542 | F-G-G-F-T-G-Aib-R-K-S-A-R-K-L-A-N-Q...)Show SMILES CC[C@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C80H132N28O21/c1-7-49(98-63(115)40-95-78(129)64(45(6)110)108-76(127)56(36-47-22-12-9-13-23-47)99-62(114)39-93-61(113)38-94-68(119)48(83)35-46-20-10-8-11-21-46)69(120)102-54(27-19-33-92-80(89)90)71(122)104-52(25-15-17-31-82)73(124)107-58(41-109)77(128)97-43(4)66(117)101-53(26-18-32-91-79(87)88)70(121)103-51(24-14-16-30-81)72(123)106-55(34-42(2)3)74(125)96-44(5)67(118)105-57(37-60(85)112)75(126)100-50(65(86)116)28-29-59(84)111/h8-13,20-23,42-45,48-58,64,109-110H,7,14-19,24-41,81-83H2,1-6H3,(H2,84,111)(H2,85,112)(H2,86,116)(H,93,113)(H,94,119)(H,95,129)(H,96,125)(H,97,128)(H,98,115)(H,99,114)(H,100,126)(H,101,117)(H,102,120)(H,103,121)(H,104,122)(H,105,118)(H,106,123)(H,107,124)(H,108,127)(H4,87,88,91)(H4,89,90,92)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475333
(CHEMBL264846)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H129FN28O21/c1-40(2)32-54(73(125)97-43(5)67(119)105-56(35-59(85)112)74(126)100-49(64(86)116)26-27-58(84)111)106-71(123)50(18-10-12-28-81)103-70(122)53(21-15-31-92-79(89)90)102-66(118)42(4)98-76(128)57(39-109)107-72(124)51(19-11-13-29-82)104-69(121)52(20-14-30-91-78(87)88)101-65(117)41(3)96-61(114)38-95-77(129)63(44(6)110)108-75(127)55(34-46-22-24-47(80)25-23-46)99-62(115)37-93-60(113)36-94-68(120)48(83)33-45-16-8-7-9-17-45/h7-9,16-17,22-25,40-44,48-57,63,109-110H,10-15,18-21,26-39,81-83H2,1-6H3,(H2,84,111)(H2,85,112)(H2,86,116)(H,93,113)(H,94,120)(H,95,129)(H,96,114)(H,97,125)(H,98,128)(H,99,115)(H,100,126)(H,101,117)(H,102,118)(H,103,122)(H,104,121)(H,105,119)(H,106,123)(H,107,124)(H,108,127)(H4,87,88,91)(H4,89,90,92)/t41-,42-,43-,44+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0661 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP levels in CHO cell membranes expressing the human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475328

(CHEMBL414782)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H140FN35O21/c1-43(104-62(123)41-103-79(139)65(45(3)120)116-76(136)57(37-47-24-26-48(83)27-25-47)106-63(124)40-101-61(122)39-102-68(128)49(87)36-46-16-5-4-6-17-46)66(126)107-53(21-13-33-98-80(89)90)70(130)111-52(20-9-12-32-86)74(134)115-59(42-119)77(137)105-44(2)67(127)108-54(22-14-34-99-81(91)92)71(131)109-50(18-7-10-30-84)69(129)112-55(23-15-35-100-82(93)94)72(132)110-51(19-8-11-31-85)73(133)114-58(38-60(88)121)75(135)113-56(78(138)117-95)28-29-64(125)118(96)97/h4-6,16-17,24-27,43-45,49-59,65,119-120H,7-15,18-23,28-42,84-87,95-97H2,1-3H3,(H2,88,121)(H,101,122)(H,102,128)(H,103,139)(H,104,123)(H,105,137)(H,106,124)(H,107,126)(H,108,127)(H,109,131)(H,110,132)(H,111,130)(H,112,129)(H,113,135)(H,114,133)(H,115,134)(H,116,136)(H,117,138)(H4,89,90,98)(H4,91,92,99)(H4,93,94,100)/t43-,44-,45+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0676 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP levels in CHO cell membranes expressing the human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475328

(CHEMBL414782)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H140FN35O21/c1-43(104-62(123)41-103-79(139)65(45(3)120)116-76(136)57(37-47-24-26-48(83)27-25-47)106-63(124)40-101-61(122)39-102-68(128)49(87)36-46-16-5-4-6-17-46)66(126)107-53(21-13-33-98-80(89)90)70(130)111-52(20-9-12-32-86)74(134)115-59(42-119)77(137)105-44(2)67(127)108-54(22-14-34-99-81(91)92)71(131)109-50(18-7-10-30-84)69(129)112-55(23-15-35-100-82(93)94)72(132)110-51(19-8-11-31-85)73(133)114-58(38-60(88)121)75(135)113-56(78(138)117-95)28-29-64(125)118(96)97/h4-6,16-17,24-27,43-45,49-59,65,119-120H,7-15,18-23,28-42,84-87,95-97H2,1-3H3,(H2,88,121)(H,101,122)(H,102,128)(H,103,139)(H,104,123)(H,105,137)(H,106,124)(H,107,126)(H,108,127)(H,109,131)(H,110,132)(H,111,130)(H,112,129)(H,113,135)(H,114,133)(H,115,134)(H,116,136)(H,117,138)(H4,89,90,98)(H4,91,92,99)(H4,93,94,100)/t43-,44-,45+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0759 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Agonist potency against GTPgammaS binding in CHO cell membranes expressing the human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM21842
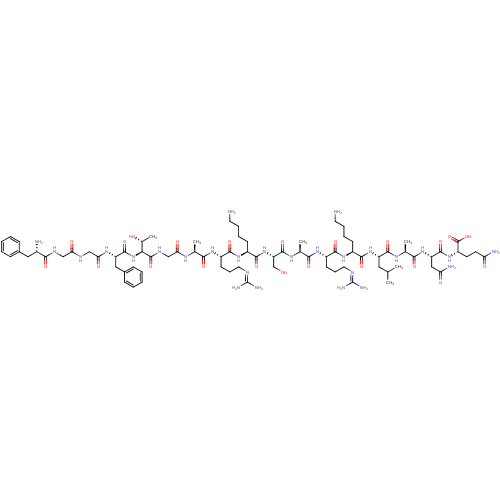
((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated for increase in [35S]GTP-gamma-S, binding for human ORL1 receptor carried out in CHO cell membranes ; Nd: no data |
Bioorg Med Chem Lett 12: 3157-60 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9C9R |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121245
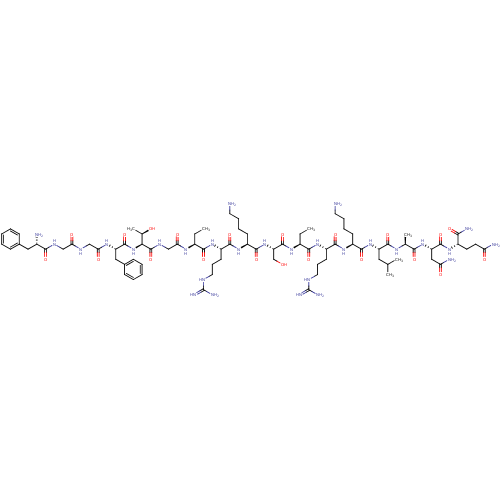
(CHEMBL266191 | F-G-G-F-T-G-Aib-R-K-S-Aib-R-K-L-A-N...)Show SMILES CC[C@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C81H134N28O21/c1-7-49(98-64(116)41-96-79(130)65(45(6)111)109-77(128)57(37-47-23-13-10-14-24-47)99-63(115)40-94-62(114)39-95-68(119)48(84)36-46-21-11-9-12-22-46)69(120)102-54(27-19-33-92-80(88)89)71(122)105-53(26-16-18-32-83)74(125)108-59(42-110)78(129)100-50(8-2)70(121)103-55(28-20-34-93-81(90)91)72(123)104-52(25-15-17-31-82)73(124)107-56(35-43(3)4)75(126)97-44(5)67(118)106-58(38-61(86)113)76(127)101-51(66(87)117)29-30-60(85)112/h9-14,21-24,43-45,48-59,65,110-111H,7-8,15-20,25-42,82-84H2,1-6H3,(H2,85,112)(H2,86,113)(H2,87,117)(H,94,114)(H,95,119)(H,96,130)(H,97,126)(H,98,116)(H,99,115)(H,100,129)(H,101,127)(H,102,120)(H,103,121)(H,104,123)(H,105,122)(H,106,118)(H,107,124)(H,108,125)(H,109,128)(H4,88,89,92)(H4,90,91,93)/t44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50546920
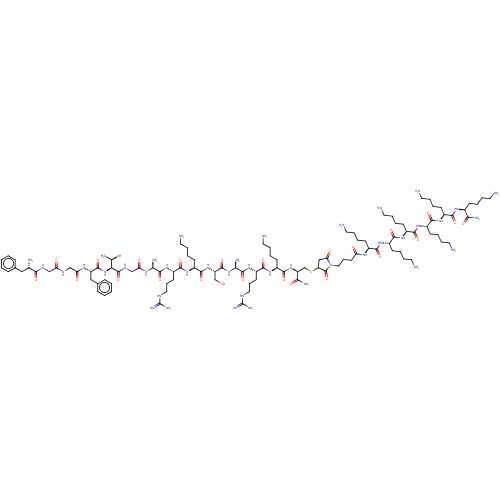
(CHEMBL4781235)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CSC1CC(=O)N(CCCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(N)=O)C1=O)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0871 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at NOP (unknown origin) expressed in HEK293 cell membranes co-expressing Gbeta1-RGFP protein assessed as induction of G protein acti... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02057
BindingDB Entry DOI: 10.7270/Q23X8B75 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Agonist activity as stimulation of [35S]-GTP-gamma binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475327
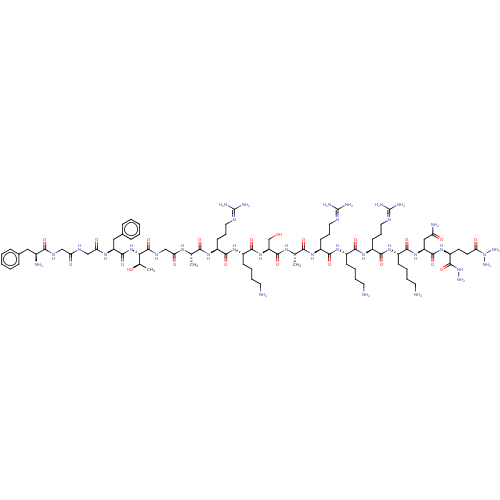
(CHEMBL410653)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H141N35O21/c1-44(103-62(122)42-102-79(138)65(46(3)119)115-76(135)57(38-48-21-8-5-9-22-48)105-63(123)41-100-61(121)40-101-68(127)49(86)37-47-19-6-4-7-20-47)66(125)106-53(26-16-34-97-80(88)89)70(129)110-52(25-12-15-33-85)74(133)114-59(43-118)77(136)104-45(2)67(126)107-54(27-17-35-98-81(90)91)71(130)108-50(23-10-13-31-83)69(128)111-55(28-18-36-99-82(92)93)72(131)109-51(24-11-14-32-84)73(132)113-58(39-60(87)120)75(134)112-56(78(137)116-94)29-30-64(124)117(95)96/h4-9,19-22,44-46,49-59,65,118-119H,10-18,23-43,83-86,94-96H2,1-3H3,(H2,87,120)(H,100,121)(H,101,127)(H,102,138)(H,103,122)(H,104,136)(H,105,123)(H,106,125)(H,107,126)(H,108,130)(H,109,131)(H,110,129)(H,111,128)(H,112,134)(H,113,132)(H,114,133)(H,115,135)(H,116,137)(H4,88,89,97)(H4,90,91,98)(H4,92,93,99)/t44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP levels in CHO cell membranes expressing the human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50304173
(3,4-dichloro-N-[(1S,5R,13R,14R,17S)-4-(cyclopropyl...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)[C@@H](CC[C@@]35O)NC(=O)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C27H28Cl2N2O4/c28-17-5-3-16(11-18(17)29)25(33)30-19-7-8-27(34)21-12-15-4-6-20(32)23-22(15)26(27,24(19)35-23)9-10-31(21)13-14-1-2-14/h3-6,11,14,19,21,24,32,34H,1-2,7-10,12-13H2,(H,30,33)/t19-,21-,24+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at NOP expressed in HEK293 cells assessed as inhibition of [35S]GTPgammaS binding |
Bioorg Med Chem 17: 6671-81 (2009)
Article DOI: 10.1016/j.bmc.2009.07.069
BindingDB Entry DOI: 10.7270/Q2PK0H37 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.115 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP levels in CHO cell membranes expressing the human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50473478

(CHEMBL409204)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(cc1)-[#7+](-[#8-])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H99N23O17/c1-33(75-47(88)31-74-59(99)49(35(3)86)83-57(97)44(28-37-19-21-38(22-20-37)84(100)101)77-48(89)30-72-46(87)29-73-53(93)39(64)27-36-13-5-4-6-14-36)51(91)79-43(18-12-26-71-61(68)69)55(95)81-41(16-8-10-24-63)56(96)82-45(32-85)58(98)76-34(2)52(92)80-42(17-11-25-70-60(66)67)54(94)78-40(50(65)90)15-7-9-23-62/h4-6,13-14,19-22,33-35,39-45,49,85-86H,7-12,15-18,23-32,62-64H2,1-3H3,(H2,65,90)(H,72,87)(H,73,93)(H,74,99)(H,75,88)(H,76,98)(H,77,89)(H,78,94)(H,79,91)(H,80,92)(H,81,95)(H,82,96)(H,83,97)(H4,66,67,70)(H4,68,69,71)/t33-,34-,35+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.135 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP accumulation in CHO cells stably expressing the human OP4 receptor |
J Med Chem 44: 3956-64 (2001)
Article DOI: 10.1021/jm010221v
BindingDB Entry DOI: 10.7270/Q26D5WR4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121253
(CHEMBL408356 | F-G-G-F-T-G-A-R-K-S-Aib-R-K-L-A-N-Q...)Show SMILES CC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C80H132N28O21/c1-7-49(69(120)102-54(27-19-33-92-80(89)90)71(122)103-51(24-14-16-30-81)72(123)106-55(34-42(2)3)74(125)97-44(5)67(118)105-57(37-60(85)112)75(126)100-50(65(86)116)28-29-59(84)111)99-77(128)58(41-109)107-73(124)52(25-15-17-31-82)104-70(121)53(26-18-32-91-79(87)88)101-66(117)43(4)96-62(114)40-95-78(129)64(45(6)110)108-76(127)56(36-47-22-12-9-13-23-47)98-63(115)39-93-61(113)38-94-68(119)48(83)35-46-20-10-8-11-21-46/h8-13,20-23,42-45,48-58,64,109-110H,7,14-19,24-41,81-83H2,1-6H3,(H2,84,111)(H2,85,112)(H2,86,116)(H,93,113)(H,94,119)(H,95,129)(H,96,114)(H,97,125)(H,98,115)(H,99,128)(H,100,126)(H,101,117)(H,102,120)(H,103,122)(H,104,121)(H,105,118)(H,106,123)(H,107,124)(H,108,127)(H4,87,88,91)(H4,89,90,92)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475327

(CHEMBL410653)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H141N35O21/c1-44(103-62(122)42-102-79(138)65(46(3)119)115-76(135)57(38-48-21-8-5-9-22-48)105-63(123)41-100-61(121)40-101-68(127)49(86)37-47-19-6-4-7-20-47)66(125)106-53(26-16-34-97-80(88)89)70(129)110-52(25-12-15-33-85)74(133)114-59(43-118)77(136)104-45(2)67(126)107-54(27-17-35-98-81(90)91)71(130)108-50(23-10-13-31-83)69(128)111-55(28-18-36-99-82(92)93)72(131)109-51(24-11-14-32-84)73(132)113-58(39-60(87)120)75(134)112-56(78(137)116-94)29-30-64(124)117(95)96/h4-9,19-22,44-46,49-59,65,118-119H,10-18,23-43,83-86,94-96H2,1-3H3,(H2,87,120)(H,100,121)(H,101,127)(H,102,138)(H,103,122)(H,104,136)(H,105,123)(H,106,125)(H,107,126)(H,108,130)(H,109,131)(H,110,129)(H,111,128)(H,112,134)(H,113,132)(H,114,133)(H,115,135)(H,116,137)(H4,88,89,97)(H4,90,91,98)(H4,92,93,99)/t44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.141 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Agonist potency against GTPgammaS binding in CHO cell membranes expressing the human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50473476

(CHEMBL261997)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H99FN22O15/c1-33(76-47(88)31-75-59(99)49(35(3)86)84-57(97)44(28-37-19-21-38(62)22-20-37)78-48(89)30-73-46(87)29-74-53(93)39(65)27-36-13-5-4-6-14-36)51(91)80-43(18-12-26-72-61(69)70)55(95)82-41(16-8-10-24-64)56(96)83-45(32-85)58(98)77-34(2)52(92)81-42(17-11-25-71-60(67)68)54(94)79-40(50(66)90)15-7-9-23-63/h4-6,13-14,19-22,33-35,39-45,49,85-86H,7-12,15-18,23-32,63-65H2,1-3H3,(H2,66,90)(H,73,87)(H,74,93)(H,75,99)(H,76,88)(H,77,98)(H,78,89)(H,79,94)(H,80,91)(H,81,92)(H,82,95)(H,83,96)(H,84,97)(H4,67,68,71)(H4,69,70,72)/t33-,34-,35+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.158 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP accumulation in CHO cells stably expressing the human OP4 receptor |
J Med Chem 44: 3956-64 (2001)
Article DOI: 10.1021/jm010221v
BindingDB Entry DOI: 10.7270/Q26D5WR4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121249

(CHEMBL415845 | F-G-G-F-T-G-A-R-K-S-A-R-K-L-Aib-N-Q...)Show SMILES CC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C80H132N28O21/c1-7-49(69(120)106-57(37-60(85)112)75(126)100-50(65(86)116)28-29-59(84)111)99-74(125)55(34-42(2)3)105-72(123)51(24-14-16-30-81)103-71(122)54(27-19-33-92-80(89)90)102-67(118)44(5)97-77(128)58(41-109)107-73(124)52(25-15-17-31-82)104-70(121)53(26-18-32-91-79(87)88)101-66(117)43(4)96-62(114)40-95-78(129)64(45(6)110)108-76(127)56(36-47-22-12-9-13-23-47)98-63(115)39-93-61(113)38-94-68(119)48(83)35-46-20-10-8-11-21-46/h8-13,20-23,42-45,48-58,64,109-110H,7,14-19,24-41,81-83H2,1-6H3,(H2,84,111)(H2,85,112)(H2,86,116)(H,93,113)(H,94,119)(H,95,129)(H,96,114)(H,97,128)(H,98,115)(H,99,125)(H,100,126)(H,101,117)(H,102,118)(H,103,122)(H,104,121)(H,105,123)(H,106,120)(H,107,124)(H,108,127)(H4,87,88,91)(H4,89,90,92)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475330

(CHEMBL442297)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H142FN35O20/c1-44(104-63(123)42-103-79(138)66(46(3)120)116-76(135)58(37-48-24-26-49(83)27-25-48)106-64(124)41-102-62(122)40-98-39-50(87)36-47-16-5-4-6-17-47)67(126)107-54(21-13-33-99-80(89)90)70(129)111-53(20-9-12-32-86)74(133)115-60(43-119)77(136)105-45(2)68(127)108-55(22-14-34-100-81(91)92)71(130)109-51(18-7-10-30-84)69(128)112-56(23-15-35-101-82(93)94)72(131)110-52(19-8-11-31-85)73(132)114-59(38-61(88)121)75(134)113-57(78(137)117-95)28-29-65(125)118(96)97/h4-6,16-17,24-27,44-46,50-60,66,98,119-120H,7-15,18-23,28-43,84-87,95-97H2,1-3H3,(H2,88,121)(H,102,122)(H,103,138)(H,104,123)(H,105,136)(H,106,124)(H,107,126)(H,108,127)(H,109,130)(H,110,131)(H,111,129)(H,112,128)(H,113,134)(H,114,132)(H,115,133)(H,116,135)(H,117,137)(H4,89,90,99)(H4,91,92,100)(H4,93,94,101)/t44-,45-,46+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.209 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Agonist potency against GTPgammaS binding in CHO cell membranes expressing the human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50106477
(CHEMBL265801 | FGGFTGARKSARKLADE | Phe-GGFTGARKSAR...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O Show InChI InChI=1S/C79H128N26O23/c1-41(2)33-54(73(124)94-44(5)67(118)102-56(36-62(113)114)74(125)97-49(64(83)115)27-28-61(111)112)103-71(122)50(23-13-15-29-80)100-70(121)53(26-18-32-89-79(86)87)99-66(117)43(4)95-76(127)57(40-106)104-72(123)51(24-14-16-30-81)101-69(120)52(25-17-31-88-78(84)85)98-65(116)42(3)93-59(109)39-92-77(128)63(45(6)107)105-75(126)55(35-47-21-11-8-12-22-47)96-60(110)38-90-58(108)37-91-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,106-107H,13-18,23-40,80-82H2,1-6H3,(H2,83,115)(H,90,108)(H,91,119)(H,92,128)(H,93,109)(H,94,124)(H,95,127)(H,96,110)(H,97,125)(H,98,116)(H,99,117)(H,100,121)(H,101,120)(H,102,118)(H,103,122)(H,104,123)(H,105,126)(H,111,112)(H,113,114)(H4,84,85,88)(H4,86,87,89)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.219 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP accumulation in CHO cells stably expressing the human OP4 receptor |
J Med Chem 44: 3956-64 (2001)
Article DOI: 10.1021/jm010221v
BindingDB Entry DOI: 10.7270/Q26D5WR4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Agonist activity at human nociceptin receptor expressed in HEK293 cells assessed as inhibition of forskolin-stimulated cAMP production preincubated f... |
J Med Chem 59: 3777-92 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01976
BindingDB Entry DOI: 10.7270/Q2T72KC6 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475325
(CHEMBL262544)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H143N35O20/c1-45(103-63(122)43-102-79(137)66(47(3)119)115-76(134)58(38-49-21-8-5-9-22-49)105-64(123)42-101-62(121)41-97-40-50(86)37-48-19-6-4-7-20-48)67(125)106-54(26-16-34-98-80(88)89)70(128)110-53(25-12-15-33-85)74(132)114-60(44-118)77(135)104-46(2)68(126)107-55(27-17-35-99-81(90)91)71(129)108-51(23-10-13-31-83)69(127)111-56(28-18-36-100-82(92)93)72(130)109-52(24-11-14-32-84)73(131)113-59(39-61(87)120)75(133)112-57(78(136)116-94)29-30-65(124)117(95)96/h4-9,19-22,45-47,50-60,66,97,118-119H,10-18,23-44,83-86,94-96H2,1-3H3,(H2,87,120)(H,101,121)(H,102,137)(H,103,122)(H,104,135)(H,105,123)(H,106,125)(H,107,126)(H,108,129)(H,109,130)(H,110,128)(H,111,127)(H,112,133)(H,113,131)(H,114,132)(H,115,134)(H,116,136)(H4,88,89,98)(H4,90,91,99)(H4,92,93,100)/t45-,46-,47+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP levels in CHO cell membranes expressing the human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50473472
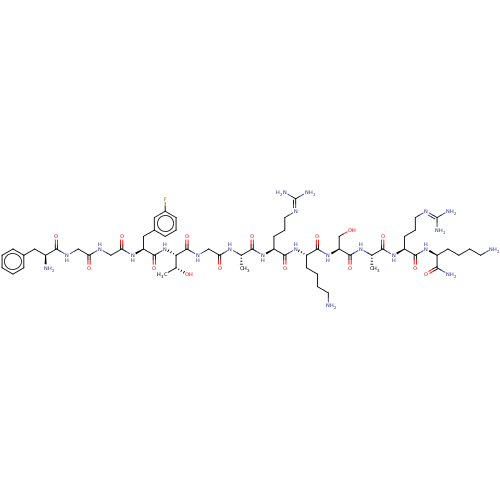
(CHEMBL412537)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1cccc(F)c1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H99FN22O15/c1-33(76-47(88)31-75-59(99)49(35(3)86)84-57(97)44(28-37-16-11-17-38(62)26-37)78-48(89)30-73-46(87)29-74-53(93)39(65)27-36-14-5-4-6-15-36)51(91)80-43(21-13-25-72-61(69)70)55(95)82-41(19-8-10-23-64)56(96)83-45(32-85)58(98)77-34(2)52(92)81-42(20-12-24-71-60(67)68)54(94)79-40(50(66)90)18-7-9-22-63/h4-6,11,14-17,26,33-35,39-45,49,85-86H,7-10,12-13,18-25,27-32,63-65H2,1-3H3,(H2,66,90)(H,73,87)(H,74,93)(H,75,99)(H,76,88)(H,77,98)(H,78,89)(H,79,94)(H,80,91)(H,81,92)(H,82,95)(H,83,96)(H,84,97)(H4,67,68,71)(H4,69,70,72)/t33-,34-,35+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP accumulation in CHO cells stably expressing the human OP4 receptor |
J Med Chem 44: 3956-64 (2001)
Article DOI: 10.1021/jm010221v
BindingDB Entry DOI: 10.7270/Q26D5WR4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50473474
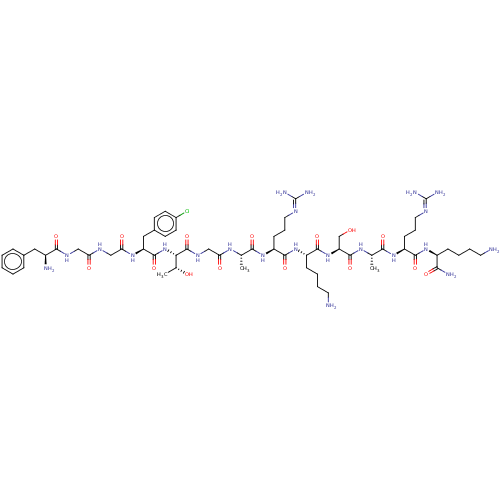
(CHEMBL405648)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H99ClN22O15/c1-33(76-47(88)31-75-59(99)49(35(3)86)84-57(97)44(28-37-19-21-38(62)22-20-37)78-48(89)30-73-46(87)29-74-53(93)39(65)27-36-13-5-4-6-14-36)51(91)80-43(18-12-26-72-61(69)70)55(95)82-41(16-8-10-24-64)56(96)83-45(32-85)58(98)77-34(2)52(92)81-42(17-11-25-71-60(67)68)54(94)79-40(50(66)90)15-7-9-23-63/h4-6,13-14,19-22,33-35,39-45,49,85-86H,7-12,15-18,23-32,63-65H2,1-3H3,(H2,66,90)(H,73,87)(H,74,93)(H,75,99)(H,76,88)(H,77,98)(H,78,89)(H,79,94)(H,80,91)(H,81,92)(H,82,95)(H,83,96)(H,84,97)(H4,67,68,71)(H4,69,70,72)/t33-,34-,35+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.269 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP accumulation in CHO cells stably expressing the human OP4 receptor |
J Med Chem 44: 3956-64 (2001)
Article DOI: 10.1021/jm010221v
BindingDB Entry DOI: 10.7270/Q26D5WR4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121244
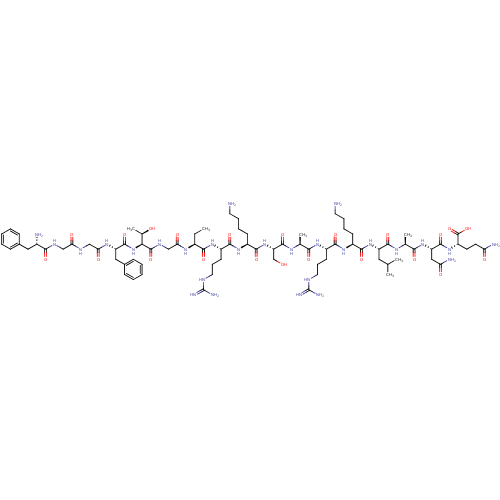
(CHEMBL264084 | F-G-G-F-T-G-Aib-R-K-S-A-R-K-L-A-N-Q...)Show SMILES CC[C@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C80H131N27O22/c1-7-49(97-63(114)40-94-77(127)64(45(6)109)107-75(125)56(36-47-22-12-9-13-23-47)98-62(113)39-92-61(112)38-93-67(117)48(83)35-46-20-10-8-11-21-46)68(118)100-53(27-19-33-91-80(88)89)70(120)102-51(25-15-17-31-82)72(122)106-58(41-108)76(126)96-43(4)65(115)99-52(26-18-32-90-79(86)87)69(119)101-50(24-14-16-30-81)71(121)105-55(34-42(2)3)73(123)95-44(5)66(116)104-57(37-60(85)111)74(124)103-54(78(128)129)28-29-59(84)110/h8-13,20-23,42-45,48-58,64,108-109H,7,14-19,24-41,81-83H2,1-6H3,(H2,84,110)(H2,85,111)(H,92,112)(H,93,117)(H,94,127)(H,95,123)(H,96,126)(H,97,114)(H,98,113)(H,99,115)(H,100,118)(H,101,119)(H,102,120)(H,103,124)(H,104,116)(H,105,121)(H,106,122)(H,107,125)(H,128,129)(H4,86,87,90)(H4,88,89,91)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50413782
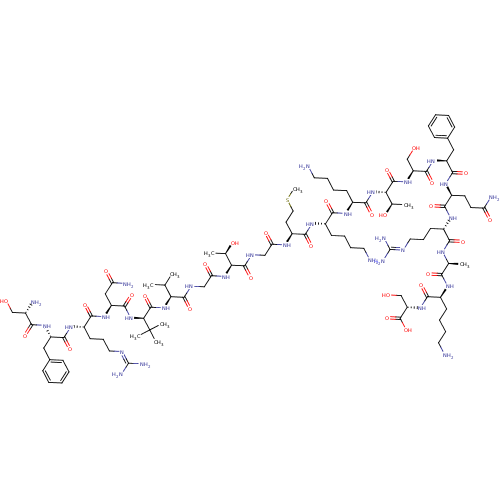
(CHEMBL1627325)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#8])C([#6])([#6])[#6])-[#6](-[#6])-[#6])-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](-[#8])=O Show InChI InChI=1S/C97H163N31O28S/c1-50(2)73(126-93(154)76(97(6,7)8)128-88(149)66(44-70(103)135)122-81(142)61(32-23-40-109-96(106)107)118-86(147)64(120-78(139)56(101)47-129)42-54-24-12-10-13-25-54)90(151)110-46-72(137)125-74(52(4)132)91(152)111-45-71(136)113-63(35-41-157-9)84(145)115-58(29-17-20-37-99)80(141)116-59(30-18-21-38-100)85(146)127-75(53(5)133)92(153)123-67(48-130)89(150)121-65(43-55-26-14-11-15-27-55)87(148)119-62(33-34-69(102)134)83(144)117-60(31-22-39-108-95(104)105)79(140)112-51(3)77(138)114-57(28-16-19-36-98)82(143)124-68(49-131)94(155)156/h10-15,24-27,50-53,56-68,73-76,129-133H,16-23,28-49,98-101H2,1-9H3,(H2,102,134)(H2,103,135)(H,110,151)(H,111,152)(H,112,140)(H,113,136)(H,114,138)(H,115,145)(H,116,141)(H,117,144)(H,118,147)(H,119,148)(H,120,139)(H,121,150)(H,122,142)(H,123,153)(H,124,143)(H,125,137)(H,126,154)(H,127,146)(H,128,149)(H,155,156)(H4,104,105,108)(H4,106,107,109)/t51-,52+,53+,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,73-,74-,75-,76-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.282 | n/a | n/a | n/a | n/a |
National Institute of Neuroscienc
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NOP receptor expressed in CHO cells coexpressing alphaqi5 assessed as inhibition of N/OFQ-induced intracellu... |
J Med Chem 52: 4068-71 (2009)
Article DOI: 10.1021/jm900604g
BindingDB Entry DOI: 10.7270/Q2445NQD |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50546925

(CHEMBL4799815)Show SMILES CCCCCCCCCCCCCC(=O)NCCCN1C(=O)CC(SC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc2ccccc2)[C@@H](C)O)C(N)=O)C1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.288 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at NOP (unknown origin) expressed in HEK293 cell membranes co-expressing Gbeta1-RGFP protein assessed as induction of G protein acti... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02057
BindingDB Entry DOI: 10.7270/Q23X8B75 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50546916

(CHEMBL4780822)Show SMILES CCCCCCCCCCCCCCCC(=O)NCCCN1C(=O)CC(SC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc2ccccc2)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(N)=O)C1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.288 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at NOP (unknown origin) expressed in HEK293 cell membranes co-expressing Gbeta1-RGFP protein assessed as induction of G protein acti... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02057
BindingDB Entry DOI: 10.7270/Q23X8B75 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50533341
(CHEMBL4544227)Show SMILES CNC(=O)Cn1c2ccccc2n(C2CCN(CC2)[C@H]2Cc3cccc4cccc2c34)c1=O |r| Show InChI InChI=1S/C27H28N4O2/c1-28-25(32)17-30-22-10-2-3-11-23(22)31(27(30)33)20-12-14-29(15-13-20)24-16-19-8-4-6-18-7-5-9-21(24)26(18)19/h2-11,20,24H,12-17H2,1H3,(H,28,32)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Astraea Therapeutics
Curated by ChEMBL
| Assay Description
Agonist activity at human nociceptin opioid receptor expressed in HEK293 cell membranes by GTPgamma(35)S binding assay |
J Med Chem 59: 7011-28 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01499
BindingDB Entry DOI: 10.7270/Q2057KDD |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50473477

(CHEMBL427791)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(cc1)C#N)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C62H99N23O15/c1-34(77-48(89)32-76-60(100)50(36(3)87)85-58(98)45(28-38-19-21-39(29-65)22-20-38)79-49(90)31-74-47(88)30-75-54(94)40(66)27-37-13-5-4-6-14-37)52(92)81-44(18-12-26-73-62(70)71)56(96)83-42(16-8-10-24-64)57(97)84-46(33-86)59(99)78-35(2)53(93)82-43(17-11-25-72-61(68)69)55(95)80-41(51(67)91)15-7-9-23-63/h4-6,13-14,19-22,34-36,40-46,50,86-87H,7-12,15-18,23-28,30-33,63-64,66H2,1-3H3,(H2,67,91)(H,74,88)(H,75,94)(H,76,100)(H,77,89)(H,78,99)(H,79,90)(H,80,95)(H,81,92)(H,82,93)(H,83,96)(H,84,97)(H,85,98)(H4,68,69,72)(H4,70,71,73)/t34-,35-,36+,40-,41-,42-,43-,44-,45-,46-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.302 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP accumulation in CHO cells stably expressing the human OP4 receptor |
J Med Chem 44: 3956-64 (2001)
Article DOI: 10.1021/jm010221v
BindingDB Entry DOI: 10.7270/Q26D5WR4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475330

(CHEMBL442297)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H142FN35O20/c1-44(104-63(123)42-103-79(138)66(46(3)120)116-76(135)58(37-48-24-26-49(83)27-25-48)106-64(124)41-102-62(122)40-98-39-50(87)36-47-16-5-4-6-17-47)67(126)107-54(21-13-33-99-80(89)90)70(129)111-53(20-9-12-32-86)74(133)115-60(43-119)77(136)105-45(2)68(127)108-55(22-14-34-100-81(91)92)71(130)109-51(18-7-10-30-84)69(128)112-56(23-15-35-101-82(93)94)72(131)110-52(19-8-11-31-85)73(132)114-59(38-61(88)121)75(134)113-57(78(137)117-95)28-29-65(125)118(96)97/h4-6,16-17,24-27,44-46,50-60,66,98,119-120H,7-15,18-23,28-43,84-87,95-97H2,1-3H3,(H2,88,121)(H,102,122)(H,103,138)(H,104,123)(H,105,136)(H,106,124)(H,107,126)(H,108,127)(H,109,130)(H,110,131)(H,111,129)(H,112,128)(H,113,134)(H,114,132)(H,115,133)(H,116,135)(H,117,137)(H4,89,90,99)(H4,91,92,100)(H4,93,94,101)/t44-,45-,46+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.302 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP levels in CHO cell membranes expressing the human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475333

(CHEMBL264846)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H129FN28O21/c1-40(2)32-54(73(125)97-43(5)67(119)105-56(35-59(85)112)74(126)100-49(64(86)116)26-27-58(84)111)106-71(123)50(18-10-12-28-81)103-70(122)53(21-15-31-92-79(89)90)102-66(118)42(4)98-76(128)57(39-109)107-72(124)51(19-11-13-29-82)104-69(121)52(20-14-30-91-78(87)88)101-65(117)41(3)96-61(114)38-95-77(129)63(44(6)110)108-75(127)55(34-46-22-24-47(80)25-23-46)99-62(115)37-93-60(113)36-94-68(120)48(83)33-45-16-8-7-9-17-45/h7-9,16-17,22-25,40-44,48-57,63,109-110H,10-15,18-21,26-39,81-83H2,1-6H3,(H2,84,111)(H2,85,112)(H2,86,116)(H,93,113)(H,94,120)(H,95,129)(H,96,114)(H,97,125)(H,98,128)(H,99,115)(H,100,126)(H,101,117)(H,102,118)(H,103,122)(H,104,121)(H,105,119)(H,106,123)(H,107,124)(H,108,127)(H4,87,88,91)(H4,89,90,92)/t41-,42-,43-,44+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.309 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Agonist potency against GTPgammaS binding in CHO cell membranes expressing the human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM29986
(arylpyrazole, 31)Show SMILES Cc1c(CN[C@H]2CC[C@@H](F)C2)nn(c1-c1cnc(C)c(F)c1)-c1ncccc1Cl |r| Show InChI InChI=1S/C21H22ClF2N5/c1-12-19(11-27-16-6-5-15(23)9-16)28-29(21-17(22)4-3-7-25-21)20(12)14-8-18(24)13(2)26-10-14/h3-4,7-8,10,15-16,27H,5-6,9,11H2,1-2H3/t15-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | 0.310 | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co.
| Assay Description
Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... |
Bioorg Med Chem Lett 19: 3627-31 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.116
BindingDB Entry DOI: 10.7270/Q289145G |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50106479
(CHEMBL384755 | FGGFTGARKSARK | H-FGGFTGARKSARK-NH2...)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.324 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP accumulation in CHO cells stably expressing the human OP4 receptor |
J Med Chem 44: 3956-64 (2001)
Article DOI: 10.1021/jm010221v
BindingDB Entry DOI: 10.7270/Q26D5WR4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50333105
(CHEMBL1631909 | H-FGGF(4-F)TGARKSARKLKNQ-NH2)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O |r| Show InChI InChI=1S/C82H136FN29O21/c1-43(2)35-57(76(129)108-53(20-10-13-31-85)74(127)110-59(38-62(89)116)77(130)103-51(67(90)120)28-29-61(88)115)109-73(126)52(19-9-12-30-84)106-72(125)56(23-16-34-96-82(93)94)105-69(122)45(4)101-79(132)60(42-113)111-75(128)54(21-11-14-32-86)107-71(124)55(22-15-33-95-81(91)92)104-68(121)44(3)100-64(118)41-99-80(133)66(46(5)114)112-78(131)58(37-48-24-26-49(83)27-25-48)102-65(119)40-97-63(117)39-98-70(123)50(87)36-47-17-7-6-8-18-47/h6-8,17-18,24-27,43-46,50-60,66,113-114H,9-16,19-23,28-42,84-87H2,1-5H3,(H2,88,115)(H2,89,116)(H2,90,120)(H,97,117)(H,98,123)(H,99,133)(H,100,118)(H,101,132)(H,102,119)(H,103,130)(H,104,121)(H,105,122)(H,106,125)(H,107,124)(H,108,129)(H,109,126)(H,110,127)(H,111,128)(H,112,131)(H4,91,92,95)(H4,93,94,96)/t44-,45-,46+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,66-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at NOR in mouse Neuro-2a cells assessed as stimulation of ERK phopshorylation after 30 mins post dose by Alphascreen Surefire assay |
J Med Chem 53: 8400-8408 (2010)
Article DOI: 10.1021/jm101139f
BindingDB Entry DOI: 10.7270/Q24Q7V77 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.389 | n/a | n/a | n/a | n/a |
National Institute of Neuroscienc
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant NOP receptor expressed in CHO cells coexpressing alphaqi5 assessed as inhibition of N/OFQ-induced intracellu... |
J Med Chem 52: 4068-71 (2009)
Article DOI: 10.1021/jm900604g
BindingDB Entry DOI: 10.7270/Q2445NQD |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50255850
(1-[1-(1-Methylcyclooctyl)-4-piperidinyl]-2-[(3R)-3...)Show SMILES CC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C26H40N4/c1-26(15-7-3-2-4-8-16-26)29-18-13-22(14-19-29)30-24-12-6-5-11-23(24)28-25(30)21-10-9-17-27-20-21/h5-6,11-12,21-22,27H,2-4,7-10,13-20H2,1H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human NOP receptor expressed in HEK293 cells by [35S]GTPgammaS binding |
J Med Chem 52: 610-25 (2009)
Article DOI: 10.1021/jm7012979
BindingDB Entry DOI: 10.7270/Q29K4C5K |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475331
(CHEMBL411649)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H140FN35O21/c1-44(104-63(124)42-103-79(139)66(46(3)120)116-76(136)57(36-47-24-26-49(83)27-25-47)106-64(125)41-102-62(123)40-101-61(122)39-97-38-48-16-5-4-6-17-48)67(127)107-53(21-13-33-98-80(88)89)70(130)111-52(20-9-12-32-86)74(134)115-59(43-119)77(137)105-45(2)68(128)108-54(22-14-34-99-81(90)91)71(131)109-50(18-7-10-30-84)69(129)112-55(23-15-35-100-82(92)93)72(132)110-51(19-8-11-31-85)73(133)114-58(37-60(87)121)75(135)113-56(78(138)117-94)28-29-65(126)118(95)96/h4-6,16-17,24-27,44-46,50-59,66,97,119-120H,7-15,18-23,28-43,84-86,94-96H2,1-3H3,(H2,87,121)(H,101,122)(H,102,123)(H,103,139)(H,104,124)(H,105,137)(H,106,125)(H,107,127)(H,108,128)(H,109,131)(H,110,132)(H,111,130)(H,112,129)(H,113,135)(H,114,133)(H,115,134)(H,116,136)(H,117,138)(H4,88,89,98)(H4,90,91,99)(H4,92,93,100)/t44-,45-,46+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,66-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.407 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Agonist potency against GTPgammaS binding in CHO cell membranes expressing human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.427 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human NOP receptor expressed in CHO cells assessed as dynamic mass redistribution by DMR assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02062
BindingDB Entry DOI: 10.7270/Q2KH0S68 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(MOUSE) | BDBM50475328

(CHEMBL414782)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](=O)-[#7](-[#7])-[#7])-[#6](=O)-[#7]-[#7] Show InChI InChI=1S/C82H140FN35O21/c1-43(104-62(123)41-103-79(139)65(45(3)120)116-76(136)57(37-47-24-26-48(83)27-25-47)106-63(124)40-101-61(122)39-102-68(128)49(87)36-46-16-5-4-6-17-46)66(126)107-53(21-13-33-98-80(89)90)70(130)111-52(20-9-12-32-86)74(134)115-59(42-119)77(137)105-44(2)67(127)108-54(22-14-34-99-81(91)92)71(131)109-50(18-7-10-30-84)69(129)112-55(23-15-35-100-82(93)94)72(132)110-51(19-8-11-31-85)73(133)114-58(38-60(88)121)75(135)113-56(78(138)117-95)28-29-64(125)118(96)97/h4-6,16-17,24-27,43-45,49-59,65,119-120H,7-15,18-23,28-42,84-87,95-97H2,1-3H3,(H2,88,121)(H,101,122)(H,102,128)(H,103,139)(H,104,123)(H,105,137)(H,106,124)(H,107,126)(H,108,127)(H,109,131)(H,110,132)(H,111,130)(H,112,129)(H,113,135)(H,114,133)(H,115,134)(H,116,136)(H,117,138)(H4,89,90,98)(H4,91,92,99)(H4,93,94,100)/t43-,44-,45+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.437 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of electrically evoked contraction of mouse vas deferens |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50546910
(CHEMBL4797388)Show SMILES CCCCCCCCCCCCCCCC(=O)NCCCN1C(=O)CC(SC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc2ccccc2)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(N)=O)C1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.437 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at NOP (unknown origin) expressed in HEK293 cell membranes co-expressing Gbeta1-RGFP protein assessed as induction of G protein acti... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02057
BindingDB Entry DOI: 10.7270/Q23X8B75 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM21842

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50546946

(CHEMBL4757730)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(F)cc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.457 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at NOP (unknown origin) expressed in HEK293 cell membranes co-expressing Gbeta1-RGFP protein assessed as induction of G protein acti... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02057
BindingDB Entry DOI: 10.7270/Q23X8B75 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121256
(CHEMBL410979 | F-G-G-F-T-G-A-R-K-S-A-R-K-L-Aib-N-Q...)Show SMILES CC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C80H131N27O22/c1-7-49(68(118)105-57(37-60(85)111)74(124)103-54(78(128)129)28-29-59(84)110)98-73(123)55(34-42(2)3)104-71(121)50(24-14-16-30-81)101-70(120)53(27-19-33-91-80(88)89)100-66(116)44(5)96-76(126)58(41-108)106-72(122)51(25-15-17-31-82)102-69(119)52(26-18-32-90-79(86)87)99-65(115)43(4)95-62(113)40-94-77(127)64(45(6)109)107-75(125)56(36-47-22-12-9-13-23-47)97-63(114)39-92-61(112)38-93-67(117)48(83)35-46-20-10-8-11-21-46/h8-13,20-23,42-45,48-58,64,108-109H,7,14-19,24-41,81-83H2,1-6H3,(H2,84,110)(H2,85,111)(H,92,112)(H,93,117)(H,94,127)(H,95,113)(H,96,126)(H,97,114)(H,98,123)(H,99,115)(H,100,116)(H,101,120)(H,102,119)(H,103,124)(H,104,121)(H,105,118)(H,106,122)(H,107,125)(H,128,129)(H4,86,87,90)(H4,88,89,91)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50475329
(CHEMBL263588)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H131FN28O20/c1-41(2)32-55(73(124)97-44(5)68(119)105-57(35-60(85)112)74(125)100-50(65(86)116)26-27-59(84)111)106-71(122)51(18-10-12-28-81)103-70(121)54(21-15-31-93-79(89)90)102-67(118)43(4)98-76(127)58(40-109)107-72(123)52(19-11-13-29-82)104-69(120)53(20-14-30-92-78(87)88)101-66(117)42(3)96-62(114)39-95-77(128)64(45(6)110)108-75(126)56(34-47-22-24-48(80)25-23-47)99-63(115)38-94-61(113)37-91-36-49(83)33-46-16-8-7-9-17-46/h7-9,16-17,22-25,41-45,49-58,64,91,109-110H,10-15,18-21,26-40,81-83H2,1-6H3,(H2,84,111)(H2,85,112)(H2,86,116)(H,94,113)(H,95,128)(H,96,114)(H,97,124)(H,98,127)(H,99,115)(H,100,125)(H,101,117)(H,102,118)(H,103,121)(H,104,120)(H,105,119)(H,106,122)(H,107,123)(H,108,126)(H4,87,88,92)(H4,89,90,93)/t42-,43-,44-,45+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.479 | n/a | n/a | n/a | n/a |
University of Ferrara
Curated by ChEMBL
| Assay Description
Inhibition of forskolin stimulated cAMP levels in CHO cell membranes expressing the human NOP receptor (CHOhNOP) |
J Med Chem 48: 1421-7 (2005)
Article DOI: 10.1021/jm040106v
BindingDB Entry DOI: 10.7270/Q2J38WBW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50255899

(1-[1-(1-Methylcyclooctyl)-4-piperidinyl]-2-[(3S)-3...)Show SMILES CC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)[C@H]1CCCNC1 |r| Show InChI InChI=1S/C26H40N4/c1-26(15-7-3-2-4-8-16-26)29-18-13-22(14-19-29)30-24-12-6-5-11-23(24)28-25(30)21-10-9-17-27-20-21/h5-6,11-12,21-22,27H,2-4,7-10,13-20H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human NOP receptor expressed in HEK293 cells by [35S]GTPgammaS binding |
J Med Chem 52: 610-25 (2009)
Article DOI: 10.1021/jm7012979
BindingDB Entry DOI: 10.7270/Q29K4C5K |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50106479

(CHEMBL384755 | FGGFTGARKSARK | H-FGGFTGARKSARK-NH2...)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.513 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human NOP receptor expressed in CHO cells assessed as stimulation of calcium mobilization by Fluo-4 AM dye based analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02062
BindingDB Entry DOI: 10.7270/Q2KH0S68 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50546911
(CHEMBL4749802)Show SMILES CCCCCCCCCCCCCCCC(=O)NCCCN1C(=O)CC(SC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc2ccccc2)[C@@H](C)O)C(=O)N[C@@H](CCCCN)C(N)=O)C1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.525 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at NOP (unknown origin) expressed in HEK293 cell membranes co-expressing Gbeta1-RGFP protein assessed as induction of G protein acti... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02057
BindingDB Entry DOI: 10.7270/Q23X8B75 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50546926
(CHEMBL4747497)Show SMILES CCCCCCCCCCCCCCCC(=O)NCCCN1C(=O)CC(SC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc2ccccc2)[C@@H](C)O)C(N)=O)C1=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.537 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at NOP (unknown origin) expressed in HEK293 cell membranes co-expressing Gbeta1-RGFP protein assessed as induction of G protein acti... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02057
BindingDB Entry DOI: 10.7270/Q23X8B75 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM30011
(benzimidazole analogue, 7j)Show SMILES CCN1CCN(CC1)c1cc2[nH]c(S[C@@]3(C)CC[C@@H](CC3)N3CCCC3=O)nc2cc1Cl |r,wU:14.15,18.22,wD:14.14,(-10.04,.77,;-8.71,,;-7.38,.77,;-7.38,2.31,;-6.04,3.08,;-4.71,2.31,;-4.71,.77,;-6.04,,;-3.38,3.08,;-2.04,2.31,;-.71,3.08,;.76,2.6,;1.66,3.85,;3.2,3.85,;3.97,2.52,;4.74,3.85,;3.48,1.06,;4.51,-.1,;6.01,.21,;6.5,1.67,;5.48,2.82,;7.04,-.94,;6.75,-2.46,;8.11,-3.19,;9.22,-2.13,;8.56,-.74,;9.3,.61,;.76,5.1,;-.71,4.62,;-2.04,5.39,;-3.38,4.62,;-4.71,5.39,)| Show InChI InChI=1S/C24H34ClN5OS/c1-3-28-11-13-29(14-12-28)21-16-20-19(15-18(21)25)26-23(27-20)32-24(2)8-6-17(7-9-24)30-10-4-5-22(30)31/h15-17H,3-14H2,1-2H3,(H,26,27)/t17-,24- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | 0.550 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co.
| Assay Description
Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... |
Bioorg Med Chem Lett 19: 3100-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.022
BindingDB Entry DOI: 10.7270/Q20R9MRX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data