 Found 836 hits of ic50 data for polymerid = 2210,49000820
Found 836 hits of ic50 data for polymerid = 2210,49000820 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50103533
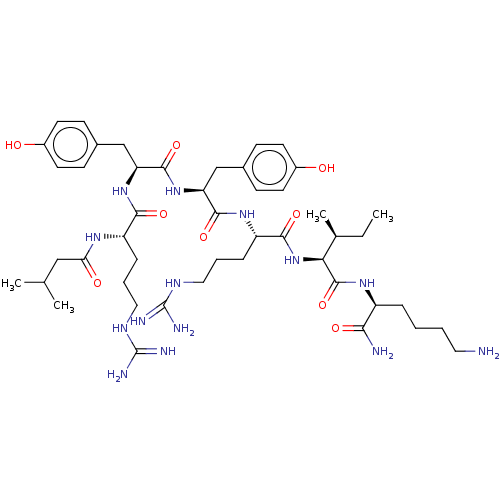
(CHEMBL3343947)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)CC(C)C)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C47H76N14O9/c1-5-28(4)39(45(70)57-33(40(49)65)10-6-7-21-48)61-42(67)35(12-9-23-55-47(52)53)58-43(68)36(25-29-13-17-31(62)18-14-29)60-44(69)37(26-30-15-19-32(63)20-16-30)59-41(66)34(11-8-22-54-46(50)51)56-38(64)24-27(2)3/h13-20,27-28,33-37,39,62-63H,5-12,21-26,48H2,1-4H3,(H2,49,65)(H,56,64)(H,57,70)(H,58,68)(H,59,66)(H,60,69)(H,61,67)(H4,50,51,54)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]]nociceptin from human ORL1 receptor high affinity binding site expressed in African green monkey COS7 cells after 90 mins by top... |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50190307
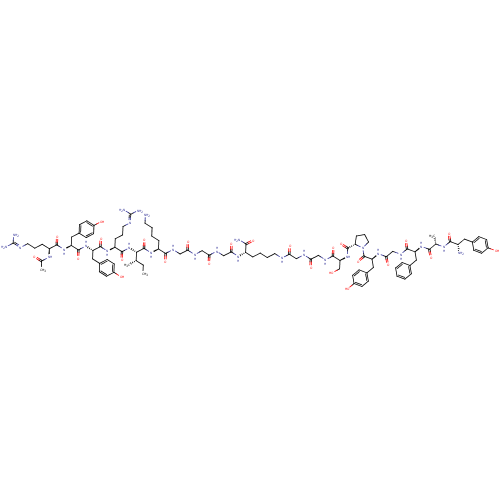
(Ac-RYYRIK-GGG-K-(NH2)-YAFGYPS-GG | CHEMBL414736)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](-[#7])=O Show InChI InChI=1S/C100H144N28O25/c1-5-56(2)85(127-93(148)72(21-14-42-110-100(106)107)121-94(149)74(46-61-25-33-65(132)34-26-61)125-95(150)75(47-62-27-35-66(133)36-28-62)124-92(147)71(118-58(4)130)20-13-41-109-99(104)105)97(152)122-70(19-9-11-39-101)89(144)114-51-81(137)112-50-80(136)113-53-83(139)119-69(86(103)141)18-10-12-40-108-79(135)49-111-82(138)52-115-91(146)77(55-129)126-96(151)78-22-15-43-128(78)98(153)76(48-63-29-37-67(134)38-30-63)120-84(140)54-116-90(145)73(45-59-16-7-6-8-17-59)123-87(142)57(3)117-88(143)68(102)44-60-23-31-64(131)32-24-60/h6-8,16-17,23-38,56-57,68-78,85,129,131-134H,5,9-15,18-22,39-55,101-102H2,1-4H3,(H2,103,141)(H,108,135)(H,111,138)(H,112,137)(H,113,136)(H,114,144)(H,115,146)(H,116,145)(H,117,143)(H,118,130)(H,119,139)(H,120,140)(H,121,149)(H,122,152)(H,123,142)(H,124,147)(H,125,150)(H,126,151)(H,127,148)(H4,104,105,109)(H4,106,107,110)/t56-,57-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,85-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0464 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NOC from human ORL1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 4839-41 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.060
BindingDB Entry DOI: 10.7270/Q2319WQG |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50103534
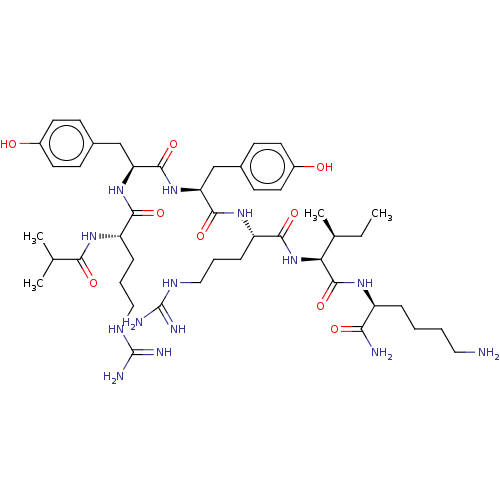
(CHEMBL3343949)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)C(C)C)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C46H74N14O9/c1-5-27(4)37(44(69)55-32(38(48)63)10-6-7-21-47)60-41(66)34(12-9-23-54-46(51)52)57-42(67)35(24-28-13-17-30(61)18-14-28)59-43(68)36(25-29-15-19-31(62)20-16-29)58-40(65)33(56-39(64)26(2)3)11-8-22-53-45(49)50/h13-20,26-27,32-37,61-62H,5-12,21-25,47H2,1-4H3,(H2,48,63)(H,55,69)(H,56,64)(H,57,67)(H,58,65)(H,59,68)(H,60,66)(H4,49,50,53)(H4,51,52,54)/t27-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]]nociceptin from human ORL1 receptor high affinity binding site expressed in African green monkey COS7 cells after 90 mins by top... |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50190308

(Ac-RYYRIK-GGG-K-(NH2)-YRFB-GGGGG | CHEMBL441930)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](-[#7])=O Show InChI InChI=1S/C85H130N28O19/c1-3-49(2)72(113-79(129)62(20-13-38-100-85(94)95)108-80(130)65(43-53-25-31-56(116)32-26-53)112-81(131)64(42-52-23-29-55(115)30-24-52)110-74(124)57(87)16-11-36-98-83(90)91)82(132)109-60(18-7-9-34-86)76(126)105-47-70(121)103-46-69(120)104-48-71(122)106-59(73(89)123)17-8-10-35-96-67(118)44-102-68(119)45-101-66(117)33-39-97-77(127)63(41-50-14-5-4-6-15-50)111-78(128)61(19-12-37-99-84(92)93)107-75(125)58(88)40-51-21-27-54(114)28-22-51/h4-6,14-15,21-32,49,57-65,72,114-116H,3,7-13,16-20,33-48,86-88H2,1-2H3,(H2,89,123)(H,96,118)(H,97,127)(H,101,117)(H,102,119)(H,103,121)(H,104,120)(H,105,126)(H,106,122)(H,107,125)(H,108,130)(H,109,132)(H,110,124)(H,111,128)(H,112,131)(H,113,129)(H4,90,91,98)(H4,92,93,99)(H4,94,95,100)/t49-,57-,58-,59-,60-,61+,62-,63-,64-,65-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0803 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NOC from human ORL1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 4839-41 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.060
BindingDB Entry DOI: 10.7270/Q2319WQG |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Binding affinity to human ORL1 receptor expressed in African green monkey COS7 cells assessed per mg protein after 90 mins by Scatchard plot analysis |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50181395

(1-benzyl-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-pip...)Show SMILES O=C(NCCCN1CCC2(CCc3ccccc23)CC1)[C@H]1CCCN1Cc1ccccc1 Show InChI InChI=1S/C28H37N3O/c32-27(26-12-6-19-31(26)22-23-8-2-1-3-9-23)29-17-7-18-30-20-15-28(16-21-30)14-13-24-10-4-5-11-25(24)28/h1-5,8-11,26H,6-7,12-22H2,(H,29,32)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cells |
J Med Chem 49: 847-9 (2006)
Article DOI: 10.1021/jm0509851
BindingDB Entry DOI: 10.7270/Q2DJ5F78 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50106478

(CHEMBL443591 | FGGFTGARKCARKC)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC1=O)C(N)=O Show InChI InChI=1S/C64H103N23O15S2/c1-35(78-49(90)32-77-62(102)51(37(3)88)87-60(100)45(29-39-18-8-5-9-19-39)80-50(91)31-75-48(89)30-76-55(95)40(67)28-38-16-6-4-7-17-38)53(93)81-43(22-14-26-73-63(69)70)56(96)84-42(21-11-13-25-66)59(99)86-47-34-104-103-33-46(52(68)92)85-58(98)41(20-10-12-24-65)83-57(97)44(23-15-27-74-64(71)72)82-54(94)36(2)79-61(47)101/h4-9,16-19,35-37,40-47,51,88H,10-15,20-34,65-67H2,1-3H3,(H2,68,92)(H,75,89)(H,76,95)(H,77,102)(H,78,90)(H,79,101)(H,80,91)(H,81,93)(H,82,94)(H,83,97)(H,84,96)(H,85,98)(H,86,99)(H,87,100)(H4,69,70,73)(H4,71,72,74)/t35-,36-,37+,40-,41-,42-,43-,44-,45-,46-,47-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NC from human ORL1 receptor expressing HEK-293 cell membrane |
J Med Chem 44: 4015-8 (2001)
BindingDB Entry DOI: 10.7270/Q25H7FJJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50181392
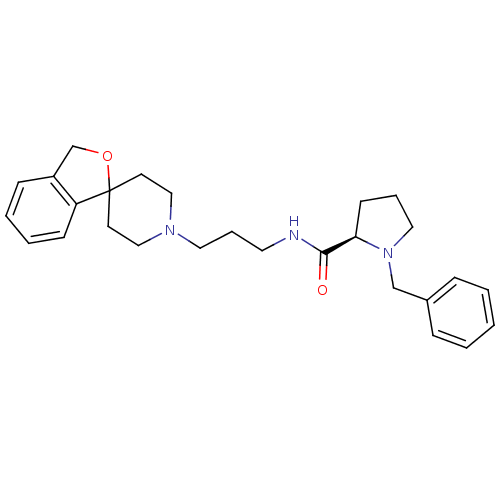
((R)-N-(3-(3H-spiro[isobenzofuran-1,4'-piperidine]-...)Show SMILES O=C(NCCCN1CCC2(CC1)OCc1ccccc21)[C@H]1CCCN1Cc1ccccc1 Show InChI InChI=1S/C27H35N3O2/c31-26(25-12-6-17-30(25)20-22-8-2-1-3-9-22)28-15-7-16-29-18-13-27(14-19-29)24-11-5-4-10-23(24)21-32-27/h1-5,8-11,25H,6-7,12-21H2,(H,28,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity on nociceptin-induced [35S]GTPgammaS binding to ORL1 expressed in CHO cells |
J Med Chem 49: 847-9 (2006)
Article DOI: 10.1021/jm0509851
BindingDB Entry DOI: 10.7270/Q2DJ5F78 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50333104
(CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H130N28O21/c1-41(2)33-54(73(124)96-44(5)67(118)104-56(36-59(84)111)74(125)99-49(64(85)115)27-28-58(83)110)105-71(122)50(23-13-15-29-80)102-70(121)53(26-18-32-91-79(88)89)101-66(117)43(4)97-76(127)57(40-108)106-72(123)51(24-14-16-30-81)103-69(120)52(25-17-31-90-78(86)87)100-65(116)42(3)95-61(113)39-94-77(128)63(45(6)109)107-75(126)55(35-47-21-11-8-12-22-47)98-62(114)38-92-60(112)37-93-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,108-109H,13-18,23-40,80-82H2,1-6H3,(H2,83,110)(H2,84,111)(H2,85,115)(H,92,112)(H,93,119)(H,94,128)(H,95,113)(H,96,124)(H,97,127)(H,98,114)(H,99,125)(H,100,116)(H,101,117)(H,102,121)(H,103,120)(H,104,118)(H,105,122)(H,106,123)(H,107,126)(H4,86,87,90)(H4,88,89,91)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from recombinant ORL1 receptor expressed in COS7 cells by competitive receptor binding assay |
Bioorg Med Chem 17: 7904-8 (2009)
Article DOI: 10.1016/j.bmc.2009.10.026
BindingDB Entry DOI: 10.7270/Q28C9X65 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50181395

(1-benzyl-N-[3-spiro(2,3-dihydro-1H-indene-1,4'-pip...)Show SMILES O=C(NCCCN1CCC2(CCc3ccccc23)CC1)[C@H]1CCCN1Cc1ccccc1 Show InChI InChI=1S/C28H37N3O/c32-27(26-12-6-19-31(26)22-23-8-2-1-3-9-23)29-17-7-18-30-20-15-28(16-21-30)14-13-24-10-4-5-11-25(24)28/h1-5,8-11,26H,6-7,12-22H2,(H,29,32)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells |
J Med Chem 49: 847-9 (2006)
Article DOI: 10.1021/jm0509851
BindingDB Entry DOI: 10.7270/Q2DJ5F78 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50181392

((R)-N-(3-(3H-spiro[isobenzofuran-1,4'-piperidine]-...)Show SMILES O=C(NCCCN1CCC2(CC1)OCc1ccccc21)[C@H]1CCCN1Cc1ccccc1 Show InChI InChI=1S/C27H35N3O2/c31-26(25-12-6-17-30(25)20-22-8-2-1-3-9-22)28-15-7-16-29-18-13-27(14-19-29)24-11-5-4-10-23(24)21-32-27/h1-5,8-11,25H,6-7,12-21H2,(H,28,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr-nociceptin from cloned human ORL1 expressed in CHO cells |
J Med Chem 49: 847-9 (2006)
Article DOI: 10.1021/jm0509851
BindingDB Entry DOI: 10.7270/Q2DJ5F78 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50106477
(CHEMBL265801 | FGGFTGARKSARKLADE | Phe-GGFTGARKSAR...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(N)=O Show InChI InChI=1S/C79H128N26O23/c1-41(2)33-54(73(124)94-44(5)67(118)102-56(36-62(113)114)74(125)97-49(64(83)115)27-28-61(111)112)103-71(122)50(23-13-15-29-80)100-70(121)53(26-18-32-89-79(86)87)99-66(117)43(4)95-76(127)57(40-106)104-72(123)51(24-14-16-30-81)101-69(120)52(25-17-31-88-78(84)85)98-65(116)42(3)93-59(109)39-92-77(128)63(45(6)107)105-75(126)55(35-47-21-11-8-12-22-47)96-60(110)38-90-58(108)37-91-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,106-107H,13-18,23-40,80-82H2,1-6H3,(H2,83,115)(H,90,108)(H,91,119)(H,92,128)(H,93,109)(H,94,124)(H,95,127)(H,96,110)(H,97,125)(H,98,116)(H,99,117)(H,100,121)(H,101,120)(H,102,118)(H,103,122)(H,104,123)(H,105,126)(H,111,112)(H,113,114)(H4,84,85,88)(H4,86,87,89)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.286 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NC from human ORL1 receptor expressing HEK-293 cell membrane |
J Med Chem 44: 4015-8 (2001)
BindingDB Entry DOI: 10.7270/Q25H7FJJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50333104

(CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H130N28O21/c1-41(2)33-54(73(124)96-44(5)67(118)104-56(36-59(84)111)74(125)99-49(64(85)115)27-28-58(83)110)105-71(122)50(23-13-15-29-80)102-70(121)53(26-18-32-91-79(88)89)101-66(117)43(4)97-76(127)57(40-108)106-72(123)51(24-14-16-30-81)103-69(120)52(25-17-31-90-78(86)87)100-65(116)42(3)95-61(113)39-94-77(128)63(45(6)109)107-75(126)55(35-47-21-11-8-12-22-47)98-62(114)38-92-60(112)37-93-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,108-109H,13-18,23-40,80-82H2,1-6H3,(H2,83,110)(H2,84,111)(H2,85,115)(H,92,112)(H,93,119)(H,94,128)(H,95,113)(H,96,124)(H,97,127)(H,98,114)(H,99,125)(H,100,116)(H,101,117)(H,102,121)(H,103,120)(H,104,118)(H,105,122)(H,106,123)(H,107,126)(H4,86,87,90)(H4,88,89,91)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Binding affinity to ORL1 receptor (unknown origin) by radioligand displacement assay |
Bioorg Med Chem 21: 2764-71 (2013)
Article DOI: 10.1016/j.bmc.2013.03.016
BindingDB Entry DOI: 10.7270/Q2N87C5Q |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM29986
(arylpyrazole, 31)Show SMILES Cc1c(CN[C@H]2CC[C@@H](F)C2)nn(c1-c1cnc(C)c(F)c1)-c1ncccc1Cl |r| Show InChI InChI=1S/C21H22ClF2N5/c1-12-19(11-27-16-6-5-15(23)9-16)28-29(21-17(22)4-3-7-25-21)20(12)14-8-18(24)13(2)26-10-14/h3-4,7-8,10,15-16,27H,5-6,9,11H2,1-2H3/t15-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr14-NC/OFQ from ORL1 receptor |
Bioorg Med Chem Lett 19: 4729-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.051
BindingDB Entry DOI: 10.7270/Q2H9958F |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50106479
(CHEMBL384755 | FGGFTGARKSARK | H-FGGFTGARKSARK-NH2...)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.353 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NC from human ORL1 receptor expressing HEK-293 cell membrane |
J Med Chem 44: 4015-8 (2001)
BindingDB Entry DOI: 10.7270/Q25H7FJJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50106471
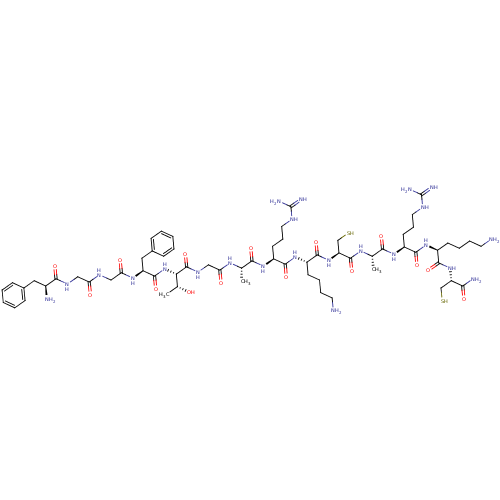
(CHEMBL406718 | FGGFTGARKCARKC)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C64H105N23O15S2/c1-35(78-49(90)32-77-62(102)51(37(3)88)87-60(100)45(29-39-18-8-5-9-19-39)80-50(91)31-75-48(89)30-76-55(95)40(67)28-38-16-6-4-7-17-38)53(93)81-43(22-14-26-73-63(69)70)56(96)84-42(21-11-13-25-66)59(99)86-47(34-104)61(101)79-36(2)54(94)82-44(23-15-27-74-64(71)72)57(97)83-41(20-10-12-24-65)58(98)85-46(33-103)52(68)92/h4-9,16-19,35-37,40-47,51,88,103-104H,10-15,20-34,65-67H2,1-3H3,(H2,68,92)(H,75,89)(H,76,95)(H,77,102)(H,78,90)(H,79,101)(H,80,91)(H,81,93)(H,82,94)(H,83,97)(H,84,96)(H,85,98)(H,86,99)(H,87,100)(H4,69,70,73)(H4,71,72,74)/t35-,36-,37+,40-,41-,42-,43-,44-,45-,46-,47-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.471 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NC from human ORL1 receptor expressing HEK-293 cell membrane |
J Med Chem 44: 4015-8 (2001)
BindingDB Entry DOI: 10.7270/Q25H7FJJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM29986

(arylpyrazole, 31)Show SMILES Cc1c(CN[C@H]2CC[C@@H](F)C2)nn(c1-c1cnc(C)c(F)c1)-c1ncccc1Cl |r| Show InChI InChI=1S/C21H22ClF2N5/c1-12-19(11-27-16-6-5-15(23)9-16)28-29(21-17(22)4-3-7-25-21)20(12)14-8-18(24)13(2)26-10-14/h3-4,7-8,10,15-16,27H,5-6,9,11H2,1-2H3/t15-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr14-NC/OFQ from ORL1 receptor |
Bioorg Med Chem Lett 19: 4729-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.051
BindingDB Entry DOI: 10.7270/Q2H9958F |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50106480

(CHEMBL437915 | FGGFTGARKSARKL)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O Show InChI InChI=1S/C67H111N23O16/c1-37(2)30-48(55(71)96)88-61(102)44(22-12-14-26-68)86-60(101)47(25-17-29-77-67(74)75)85-57(98)39(4)82-64(105)50(36-91)89-62(103)45(23-13-15-27-69)87-59(100)46(24-16-28-76-66(72)73)84-56(97)38(3)81-52(94)35-80-65(106)54(40(5)92)90-63(104)49(32-42-20-10-7-11-21-42)83-53(95)34-78-51(93)33-79-58(99)43(70)31-41-18-8-6-9-19-41/h6-11,18-21,37-40,43-50,54,91-92H,12-17,22-36,68-70H2,1-5H3,(H2,71,96)(H,78,93)(H,79,99)(H,80,106)(H,81,94)(H,82,105)(H,83,95)(H,84,97)(H,85,98)(H,86,101)(H,87,100)(H,88,102)(H,89,103)(H,90,104)(H4,72,73,76)(H4,74,75,77)/t38-,39-,40+,43-,44-,45-,46-,47-,48-,49-,50-,54-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NC from human ORL1 receptor expressing HEK-293 cell membrane |
J Med Chem 44: 4015-8 (2001)
BindingDB Entry DOI: 10.7270/Q25H7FJJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM29986

(arylpyrazole, 31)Show SMILES Cc1c(CN[C@H]2CC[C@@H](F)C2)nn(c1-c1cnc(C)c(F)c1)-c1ncccc1Cl |r| Show InChI InChI=1S/C21H22ClF2N5/c1-12-19(11-27-16-6-5-15(23)9-16)28-29(21-17(22)4-3-7-25-21)20(12)14-8-18(24)13(2)26-10-14/h3-4,7-8,10,15-16,27H,5-6,9,11H2,1-2H3/t15-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | 0.310 | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co.
| Assay Description
Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... |
Bioorg Med Chem Lett 19: 3627-31 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.116
BindingDB Entry DOI: 10.7270/Q289145G |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50333104

(CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H130N28O21/c1-41(2)33-54(73(124)96-44(5)67(118)104-56(36-59(84)111)74(125)99-49(64(85)115)27-28-58(83)110)105-71(122)50(23-13-15-29-80)102-70(121)53(26-18-32-91-79(88)89)101-66(117)43(4)97-76(127)57(40-108)106-72(123)51(24-14-16-30-81)103-69(120)52(25-17-31-90-78(86)87)100-65(116)42(3)95-61(113)39-94-77(128)63(45(6)109)107-75(126)55(35-47-21-11-8-12-22-47)98-62(114)38-92-60(112)37-93-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,108-109H,13-18,23-40,80-82H2,1-6H3,(H2,83,110)(H2,84,111)(H2,85,115)(H,92,112)(H,93,119)(H,94,128)(H,95,113)(H,96,124)(H,97,127)(H,98,114)(H,99,125)(H,100,116)(H,101,117)(H,102,121)(H,103,120)(H,104,118)(H,105,122)(H,106,123)(H,107,126)(H4,86,87,90)(H4,88,89,91)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human ORL1-Galpha fusion receptor in COS7 cells |
Bioorg Med Chem 16: 2635-44 (2008)
Article DOI: 10.1016/j.bmc.2007.11.043
BindingDB Entry DOI: 10.7270/Q2416XW9 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50296580
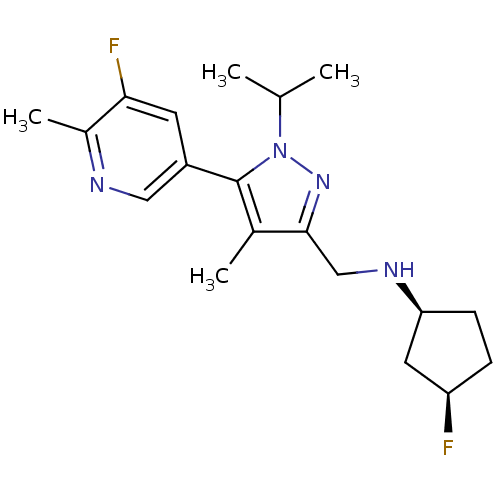
((1S,3R)-3-fluoro-N-((5-(5-fluoro-6-methylpyridin-3...)Show SMILES CC(C)n1nc(CN[C@H]2CC[C@@H](F)C2)c(C)c1-c1cnc(C)c(F)c1 |r| Show InChI InChI=1S/C19H26F2N4/c1-11(2)25-19(14-7-17(21)13(4)22-9-14)12(3)18(24-25)10-23-16-6-5-15(20)8-16/h7,9,11,15-16,23H,5-6,8,10H2,1-4H3/t15-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr14-NC/OFQ from ORL1 receptor |
Bioorg Med Chem Lett 19: 4729-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.051
BindingDB Entry DOI: 10.7270/Q2H9958F |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50296581

((1S,3R)-N-((1-ethyl-5-(5-fluoro-6-methylpyridin-3-...)Show SMILES CCn1nc(CN[C@H]2CC[C@@H](F)C2)c(C)c1-c1cnc(C)c(F)c1 |r| Show InChI InChI=1S/C18H24F2N4/c1-4-24-18(13-7-16(20)12(3)21-9-13)11(2)17(23-24)10-22-15-6-5-14(19)8-15/h7,9,14-15,22H,4-6,8,10H2,1-3H3/t14-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr14-NC/OFQ from ORL1 receptor |
Bioorg Med Chem Lett 19: 4729-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.051
BindingDB Entry DOI: 10.7270/Q2H9958F |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50533346
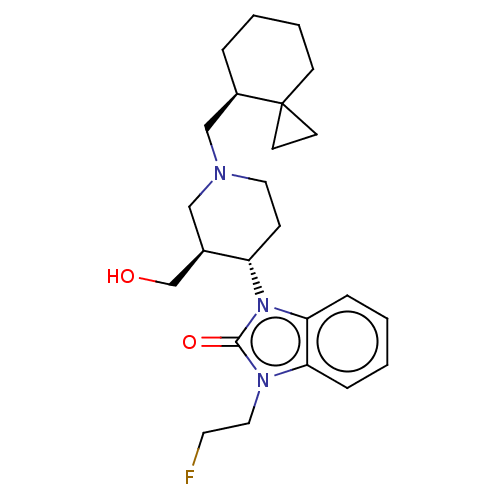
(CHEMBL4454908)Show SMILES OC[C@H]1CN(C[C@H]2CCCCC22CC2)CC[C@@H]1n1c2ccccc2n(CC[18F])c1=O |r| Show InChI InChI=1S/C24H34FN3O2/c25-12-14-27-21-6-1-2-7-22(21)28(23(27)30)20-8-13-26(15-18(20)17-29)16-19-5-3-4-9-24(19)10-11-24/h1-2,6-7,18-20,29H,3-5,8-17H2/t18-,19-,20+/m1/s1/i25-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin/orphanin FQ from human nociceptin opioid receptor expressed in CHO cell membranes after 1 hr by microplate sc... |
J Med Chem 59: 7011-28 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01499
BindingDB Entry DOI: 10.7270/Q2057KDD |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]]nociceptin from human ORL1 receptor expressed in African green monkey COS7 cells after 90 mins by topcount analysis |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM29995
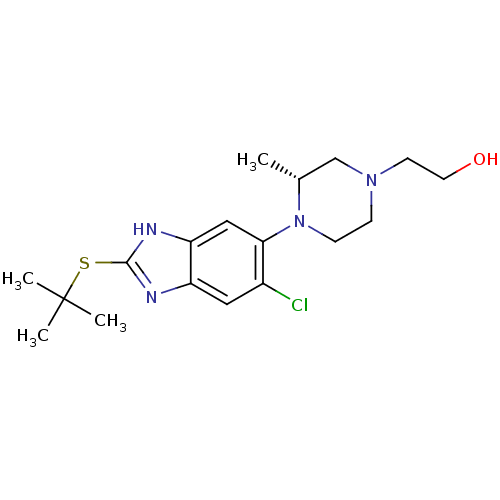
(CHEMBL494350 | benzimidazole-based antagonist, 1)Show SMILES C[C@@H]1CN(CCO)CCN1c1cc2[nH]c(SC(C)(C)C)nc2cc1Cl |r| Show InChI InChI=1S/C18H27ClN4OS/c1-12-11-22(7-8-24)5-6-23(12)16-10-15-14(9-13(16)19)20-17(21-15)25-18(2,3)4/h9-10,12,24H,5-8,11H2,1-4H3,(H,20,21)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human ORL1 receptor by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 3282-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.037
BindingDB Entry DOI: 10.7270/Q2542NDJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50372279
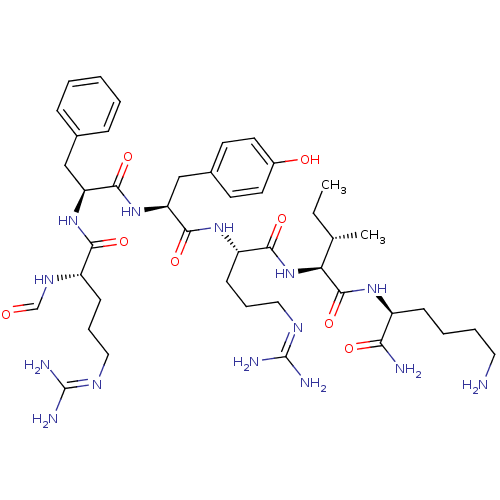
(CHEMBL271478)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6]=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C43H68N14O8/c1-3-26(2)35(41(65)53-30(36(45)60)13-7-8-20-44)57-38(62)32(15-10-22-51-43(48)49)54-39(63)34(24-28-16-18-29(59)19-17-28)56-40(64)33(23-27-11-5-4-6-12-27)55-37(61)31(52-25-58)14-9-21-50-42(46)47/h4-6,11-12,16-19,25-26,30-35,59H,3,7-10,13-15,20-24,44H2,1-2H3,(H2,45,60)(H,52,58)(H,53,65)(H,54,63)(H,55,61)(H,56,64)(H,57,62)(H4,46,47,50)(H4,48,49,51)/t26-,30-,31-,32-,33-,34-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human ORL1-Galpha fusion receptor in COS7 cells |
Bioorg Med Chem 16: 2635-44 (2008)
Article DOI: 10.1016/j.bmc.2007.11.043
BindingDB Entry DOI: 10.7270/Q2416XW9 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50239749

(2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...)Show SMILES CCC(C)(CC)Sc1nc2cc(Cl)c(cc2[nH]1)N1CCN(CCO)CC1 Show InChI InChI=1S/C19H29ClN4OS/c1-4-19(3,5-2)26-18-21-15-12-14(20)17(13-16(15)22-18)24-8-6-23(7-9-24)10-11-25/h12-13,25H,4-11H2,1-3H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human ORL1 receptor expressed in CHO cell membrane by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 18: 3278-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.054
BindingDB Entry DOI: 10.7270/Q2HT2Q57 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50239749

(2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...)Show SMILES CCC(C)(CC)Sc1nc2cc(Cl)c(cc2[nH]1)N1CCN(CCO)CC1 Show InChI InChI=1S/C19H29ClN4OS/c1-4-19(3,5-2)26-18-21-15-12-14(20)17(13-16(15)22-18)24-8-6-23(7-9-24)10-11-25/h12-13,25H,4-11H2,1-3H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human ORL1 receptor by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 3282-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.037
BindingDB Entry DOI: 10.7270/Q2542NDJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50333104

(CHEMBL389521 | H-FGGFTGARKSARKLANQ-NH2 | N/OFQ-NH2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#7])=O Show InChI InChI=1S/C79H130N28O21/c1-41(2)33-54(73(124)96-44(5)67(118)104-56(36-59(84)111)74(125)99-49(64(85)115)27-28-58(83)110)105-71(122)50(23-13-15-29-80)102-70(121)53(26-18-32-91-79(88)89)101-66(117)43(4)97-76(127)57(40-108)106-72(123)51(24-14-16-30-81)103-69(120)52(25-17-31-90-78(86)87)100-65(116)42(3)95-61(113)39-94-77(128)63(45(6)109)107-75(126)55(35-47-21-11-8-12-22-47)98-62(114)38-92-60(112)37-93-68(119)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,108-109H,13-18,23-40,80-82H2,1-6H3,(H2,83,110)(H2,84,111)(H2,85,115)(H,92,112)(H,93,119)(H,94,128)(H,95,113)(H,96,124)(H,97,127)(H,98,114)(H,99,125)(H,100,116)(H,101,117)(H,102,121)(H,103,120)(H,104,118)(H,105,122)(H,106,123)(H,107,126)(H4,86,87,90)(H4,88,89,91)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from recombinant ORL1 receptor expressed in COS7 cells by competitive receptor binding assay |
Bioorg Med Chem 17: 7904-8 (2009)
Article DOI: 10.1016/j.bmc.2009.10.026
BindingDB Entry DOI: 10.7270/Q28C9X65 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50190305
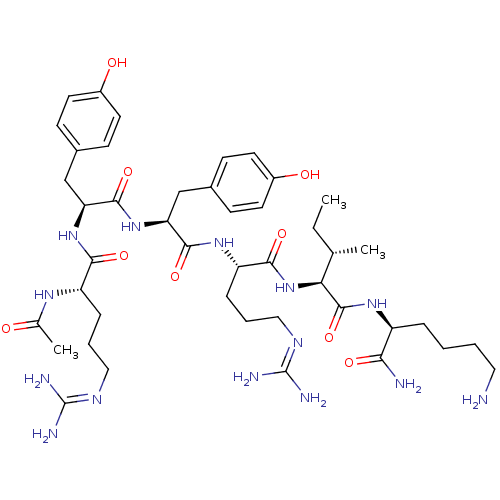
(Ac-RYYRIK-NH2 | CHEMBL437723)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C44H70N14O9/c1-4-25(2)36(42(67)54-31(37(46)62)9-5-6-20-45)58-39(64)33(11-8-22-52-44(49)50)55-40(65)34(23-27-12-16-29(60)17-13-27)57-41(66)35(24-28-14-18-30(61)19-15-28)56-38(63)32(53-26(3)59)10-7-21-51-43(47)48/h12-19,25,31-36,60-61H,4-11,20-24,45H2,1-3H3,(H2,46,62)(H,53,59)(H,54,67)(H,55,65)(H,56,63)(H,57,66)(H,58,64)(H4,47,48,51)(H4,49,50,52)/t25-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.767 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NOC from human ORL1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 4839-41 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.060
BindingDB Entry DOI: 10.7270/Q2319WQG |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50190309
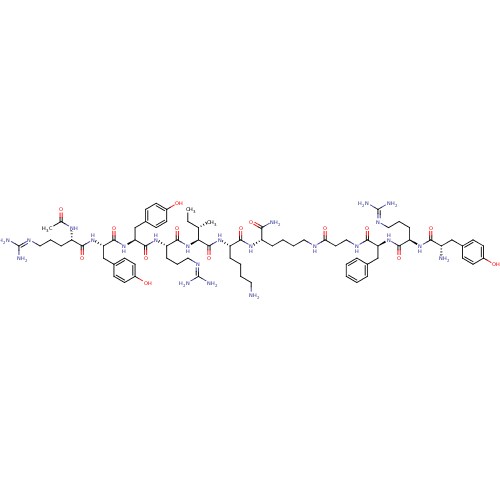
(Ac-RYYRIK-K-(NH2)-YRFB | CHEMBL412939)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](-[#7])=O Show InChI InChI=1S/C77H117N23O15/c1-4-45(2)64(100-71(112)59(21-14-39-91-77(85)86)95-72(113)61(43-49-24-30-52(103)31-25-49)99-73(114)62(44-50-26-32-53(104)33-27-50)98-68(109)56(92-46(3)101)19-12-37-89-75(81)82)74(115)96-57(18-8-10-35-78)69(110)93-55(65(80)106)17-9-11-36-87-63(105)34-40-88-67(108)60(42-47-15-6-5-7-16-47)97-70(111)58(20-13-38-90-76(83)84)94-66(107)54(79)41-48-22-28-51(102)29-23-48/h5-7,15-16,22-33,45,54-62,64,102-104H,4,8-14,17-21,34-44,78-79H2,1-3H3,(H2,80,106)(H,87,105)(H,88,108)(H,92,101)(H,93,110)(H,94,107)(H,95,113)(H,96,115)(H,97,111)(H,98,109)(H,99,114)(H,100,112)(H4,81,82,89)(H4,83,84,90)(H4,85,86,91)/t45-,54-,55-,56-,57-,58+,59-,60-,61-,62-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.789 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NOC from human ORL1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 4839-41 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.060
BindingDB Entry DOI: 10.7270/Q2319WQG |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50372278
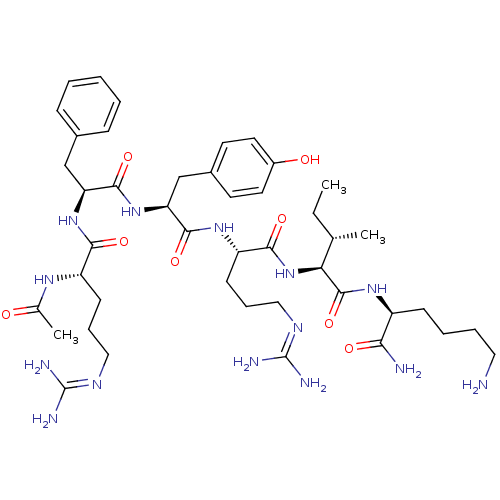
(CHEMBL256055)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C44H70N14O8/c1-4-26(2)36(42(66)54-31(37(46)61)14-8-9-21-45)58-39(63)33(16-11-23-52-44(49)50)55-40(64)35(25-29-17-19-30(60)20-18-29)57-41(65)34(24-28-12-6-5-7-13-28)56-38(62)32(53-27(3)59)15-10-22-51-43(47)48/h5-7,12-13,17-20,26,31-36,60H,4,8-11,14-16,21-25,45H2,1-3H3,(H2,46,61)(H,53,59)(H,54,66)(H,55,64)(H,56,62)(H,57,65)(H,58,63)(H4,47,48,51)(H4,49,50,52)/t26-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human ORL1-Galpha fusion receptor in COS7 cells |
Bioorg Med Chem 16: 2635-44 (2008)
Article DOI: 10.1016/j.bmc.2007.11.043
BindingDB Entry DOI: 10.7270/Q2416XW9 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM29994

(2-Cyclohexylcarbonylbenzimidazole, 7e)Show SMILES CCN1CCN(CC1)c1cc2[nH]c(nc2cc1Cl)C(=O)[C@]1(CC)CC[C@@](C)(O)CC1 |r,wU:20.23,25.29,wD:20.22,25.28,(-10.04,.77,;-8.71,,;-7.38,.77,;-7.38,2.31,;-6.04,3.08,;-4.71,2.31,;-4.71,.77,;-6.04,,;-3.38,3.08,;-2.04,2.31,;-.71,3.08,;.76,2.6,;1.66,3.85,;.76,5.1,;-.71,4.62,;-2.04,5.39,;-3.38,4.62,;-4.71,5.39,;3.2,3.85,;3.97,5.18,;4.29,2.76,;4.84,4.2,;6.37,4.44,;5.83,2.84,;6.66,1.54,;5.96,.17,;5.96,-1.37,;7.45,-.23,;4.42,.1,;3.59,1.39,)| Show InChI InChI=1S/C23H33ClN4O2/c1-4-23(8-6-22(3,30)7-9-23)20(29)21-25-17-14-16(24)19(15-18(17)26-21)28-12-10-27(5-2)11-13-28/h14-15,30H,4-13H2,1-3H3,(H,25,26)/t22-,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | 2.40 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co.
| Assay Description
Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... |
Bioorg Med Chem Lett 19: 3096-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.023
BindingDB Entry DOI: 10.7270/Q24J0CFR |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM29988

(benzimidazole analogue, 7h | benzimidazole derivat...)Show SMILES CCN1CCN(CC1)c1cc2[nH]c(S[C@@]3(C)CC[C@@H](CC3)N(C)C(C)=O)nc2cc1Cl |r,wU:14.15,18.22,wD:14.14,(-10.04,.77,;-8.71,,;-7.38,.77,;-7.38,2.31,;-6.04,3.08,;-4.71,2.31,;-4.71,.77,;-6.04,,;-3.38,3.08,;-2.04,2.31,;-.71,3.08,;.76,2.6,;1.66,3.85,;3.2,3.85,;4.29,2.76,;4.84,4.2,;3.59,1.39,;4.42,.1,;5.96,.17,;6.66,1.54,;5.83,2.84,;6.79,-1.12,;6.09,-2.49,;8.33,-1.05,;9.17,-2.34,;9.04,.32,;.76,5.1,;-.71,4.62,;-2.04,5.39,;-3.38,4.62,;-4.71,5.39,)| Show InChI InChI=1S/C23H34ClN5OS/c1-5-28-10-12-29(13-11-28)21-15-20-19(14-18(21)24)25-22(26-20)31-23(3)8-6-17(7-9-23)27(4)16(2)30/h14-15,17H,5-13H2,1-4H3,(H,25,26)/t17-,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.810 | n/a | 1.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co.
| Assay Description
Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... |
Bioorg Med Chem Lett 19: 3100-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.022
BindingDB Entry DOI: 10.7270/Q20R9MRX |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM29988

(benzimidazole analogue, 7h | benzimidazole derivat...)Show SMILES CCN1CCN(CC1)c1cc2[nH]c(S[C@@]3(C)CC[C@@H](CC3)N(C)C(C)=O)nc2cc1Cl |r,wU:14.15,18.22,wD:14.14,(-10.04,.77,;-8.71,,;-7.38,.77,;-7.38,2.31,;-6.04,3.08,;-4.71,2.31,;-4.71,.77,;-6.04,,;-3.38,3.08,;-2.04,2.31,;-.71,3.08,;.76,2.6,;1.66,3.85,;3.2,3.85,;4.29,2.76,;4.84,4.2,;3.59,1.39,;4.42,.1,;5.96,.17,;6.66,1.54,;5.83,2.84,;6.79,-1.12,;6.09,-2.49,;8.33,-1.05,;9.17,-2.34,;9.04,.32,;.76,5.1,;-.71,4.62,;-2.04,5.39,;-3.38,4.62,;-4.71,5.39,)| Show InChI InChI=1S/C23H34ClN5OS/c1-5-28-10-12-29(13-11-28)21-15-20-19(14-18(21)24)25-22(26-20)31-23(3)8-6-17(7-9-23)27(4)16(2)30/h14-15,17H,5-13H2,1-4H3,(H,25,26)/t17-,23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co.
| Assay Description
Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... |
Bioorg Med Chem Lett 19: 3096-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.023
BindingDB Entry DOI: 10.7270/Q24J0CFR |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50106469
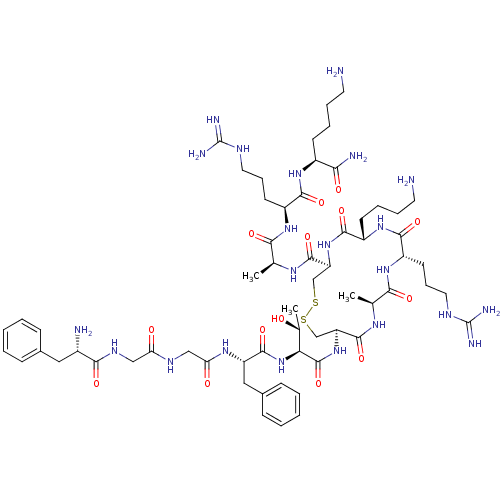
(CHEMBL269029 | FGGFTCARKCARK | cyclo[Cys6,Cys10]N/...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC1=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C62H100N22O14S2/c1-34(51(89)79-42(22-14-26-71-61(67)68)54(92)78-40(50(66)88)20-10-12-24-63)75-58(96)45-32-99-100-33-46(59(97)76-35(2)52(90)80-43(23-15-27-72-62(69)70)55(93)81-41(56(94)82-45)21-11-13-25-64)83-60(98)49(36(3)85)84-57(95)44(29-38-18-8-5-9-19-38)77-48(87)31-73-47(86)30-74-53(91)39(65)28-37-16-6-4-7-17-37/h4-9,16-19,34-36,39-46,49,85H,10-15,20-33,63-65H2,1-3H3,(H2,66,88)(H,73,86)(H,74,91)(H,75,96)(H,76,97)(H,77,87)(H,78,92)(H,79,89)(H,80,90)(H,81,93)(H,82,94)(H,83,98)(H,84,95)(H4,67,68,71)(H4,69,70,72)/t34-,35-,36+,39-,40-,41-,42-,43-,44-,45-,46-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.833 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NC from human ORL1 receptor expressing HEK-293 cell membrane |
J Med Chem 44: 4015-8 (2001)
BindingDB Entry DOI: 10.7270/Q25H7FJJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50296580

((1S,3R)-3-fluoro-N-((5-(5-fluoro-6-methylpyridin-3...)Show SMILES CC(C)n1nc(CN[C@H]2CC[C@@H](F)C2)c(C)c1-c1cnc(C)c(F)c1 |r| Show InChI InChI=1S/C19H26F2N4/c1-11(2)25-19(14-7-17(21)13(4)22-9-14)12(3)18(24-25)10-23-16-6-5-15(20)8-16/h7,9,11,15-16,23H,5-6,8,10H2,1-4H3/t15-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr14-NC/OFQ from ORL1 receptor |
Bioorg Med Chem Lett 19: 4729-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.051
BindingDB Entry DOI: 10.7270/Q2H9958F |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM392076

(US10301272, Example 7/9)Show SMILES CC(C)(C)NS(=O)(=O)c1ccc(-c2sc(nc2CC2CCCCC2)C(=O)N[C@H]2C[C@@H](C2)C(O)=O)c2ccccc12 |r,wU:27.28,wD:29.33,(8.76,1.15,;7.67,.06,;6.19,.46,;7.28,1.55,;8.07,-1.42,;6.98,-2.51,;6.59,-4,;8.07,-3.6,;5.5,-2.11,;5.1,-.63,;3.61,-.23,;2.52,-1.32,;1.03,-.92,;.56,.55,;-.98,.55,;-1.46,-.92,;-.21,-1.82,;-.21,-3.36,;-1.55,-4.13,;-2.88,-3.36,;-4.21,-4.13,;-4.21,-5.67,;-2.88,-6.44,;-1.55,-5.67,;-2.07,1.64,;-1.67,3.12,;-3.56,1.24,;-4.65,2.33,;-6.19,2.33,;-6.19,3.87,;-4.65,3.87,;-7.28,4.96,;-6.88,6.44,;-8.76,4.56,;2.92,-2.8,;1.83,-3.89,;2.23,-5.38,;3.72,-5.78,;4.81,-4.69,;4.41,-3.2,)| Show InChI InChI=1S/C30H37N3O5S2/c1-30(2,3)33-40(37,38)25-14-13-23(21-11-7-8-12-22(21)25)26-24(15-18-9-5-4-6-10-18)32-28(39-26)27(34)31-20-16-19(17-20)29(35)36/h7-8,11-14,18-20,33H,4-6,9-10,15-17H2,1-3H3,(H,31,34)(H,35,36)/t19-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human recombinant NOP receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 28: 1446-1455 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.093
BindingDB Entry DOI: 10.7270/Q27W6FPC |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM30012

(benzimidazole analogue, 7k)Show SMILES CCN1CCN(CC1)c1cc2[nH]c(S[C@@]3(C)CC[C@@H](CC3)C(=O)NC)nc2cc1Cl |r,wU:14.15,18.22,wD:14.14,(-10.04,.77,;-8.71,,;-7.38,.77,;-7.38,2.31,;-6.04,3.08,;-4.71,2.31,;-4.71,.77,;-6.04,,;-3.38,3.08,;-2.04,2.31,;-.71,3.08,;.76,2.6,;1.66,3.85,;3.2,3.85,;3.97,2.52,;4.74,3.85,;3.48,1.06,;4.51,-.1,;6.01,.21,;6.5,1.67,;5.48,2.82,;7.04,-.94,;6.55,-2.4,;8.54,-.63,;9.57,-1.79,;.76,5.1,;-.71,4.62,;-2.04,5.39,;-3.38,4.62,;-4.71,5.39,)| Show InChI InChI=1S/C22H32ClN5OS/c1-4-27-9-11-28(12-10-27)19-14-18-17(13-16(19)23)25-21(26-18)30-22(2)7-5-15(6-8-22)20(29)24-3/h13-15H,4-12H2,1-3H3,(H,24,29)(H,25,26)/t15-,22- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | 1.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co.
| Assay Description
Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... |
Bioorg Med Chem Lett 19: 3100-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.022
BindingDB Entry DOI: 10.7270/Q20R9MRX |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM557925

(US11365191, Example 158)Show SMILES Fc1ccc([C@@H]2CC[C@H](CCNC3CCNC(=O)C3)CC2)c(Cl)c1 |r,wD:5.4,8.8,(-8.67,2.69,;-7.34,1.92,;-7.34,.38,;-6,-.38,;-4.67,.38,;-3.33,-.39,;-2,.38,;-.67,-.39,;-.67,-1.93,;.67,-2.7,;2,-1.93,;3.33,-2.7,;4.67,-1.93,;4.67,-.39,;6,.39,;7.34,-.39,;7.34,-1.93,;8.67,-2.69,;6,-2.7,;-2,-2.7,;-3.33,-1.93,;-4.67,1.92,;-3.33,2.7,;-6,2.69,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2TF01J4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50296590
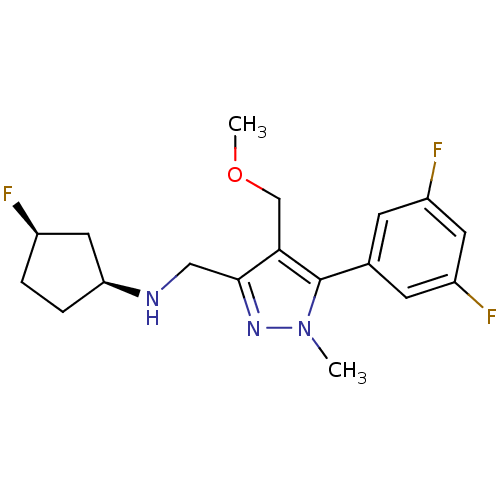
((1S,3R)-N-((5-(3,5-difluorophenyl)-4-(methoxymethy...)Show SMILES COCc1c(CN[C@H]2CC[C@@H](F)C2)nn(C)c1-c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C18H22F3N3O/c1-24-18(11-5-13(20)7-14(21)6-11)16(10-25-2)17(23-24)9-22-15-4-3-12(19)8-15/h5-7,12,15,22H,3-4,8-10H2,1-2H3/t12-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]Tyr14-NC/OFQ from ORL1 receptor |
Bioorg Med Chem Lett 19: 4729-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.051
BindingDB Entry DOI: 10.7270/Q2H9958F |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50190305

(Ac-RYYRIK-NH2 | CHEMBL437723)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C44H70N14O9/c1-4-25(2)36(42(67)54-31(37(46)62)9-5-6-20-45)58-39(64)33(11-8-22-52-44(49)50)55-40(65)34(23-27-12-16-29(60)17-13-27)57-41(66)35(24-28-14-18-30(61)19-15-28)56-38(63)32(53-26(3)59)10-7-21-51-43(47)48/h12-19,25,31-36,60-61H,4-11,20-24,45H2,1-3H3,(H2,46,62)(H,53,59)(H,54,67)(H,55,65)(H,56,63)(H,57,66)(H,58,64)(H4,47,48,51)(H4,49,50,52)/t25-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University
Curated by ChEMBL
| Assay Description
Displacement of [3H]]nociceptin from human ORL1 receptor expressed in African green monkey COS7 cells after 90 mins by topcount analysis |
Bioorg Med Chem 22: 5902-9 (2014)
Article DOI: 10.1016/j.bmc.2014.09.018
BindingDB Entry DOI: 10.7270/Q2WD42BN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM557923

(N-(2-((trans)-4-(3-chloro-5-fluoropyridin-2-yl)cyc...)Show SMILES Fc1cnc([C@@H]2CC[C@H](CCNC3CCOCC3)CC2)c(Cl)c1 |r,wD:5.4,8.8,(-8,8.11,;-6.67,7.34,;-6.67,5.8,;-5.33,5.03,;-4,5.8,;-2.67,5.03,;-1.33,5.8,;,5.03,;,3.49,;1.33,2.72,;2.67,3.49,;4,2.72,;5.33,3.49,;6.67,2.72,;8,3.49,;8,5.03,;6.67,5.8,;5.33,5.03,;-1.33,2.72,;-2.67,3.49,;-4,7.34,;-2.67,8.11,;-5.33,8.11,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2TF01J4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM557922

(US11365191, Example 76)Show SMILES CNC(=O)C1(CCOCC1)NCC[C@H]1CC[C@H](CC1)c1ccc(F)cc1Cl |r,wD:16.20,13.13,(8,-2.54,;6.67,-3.31,;5.33,-2.54,;4.25,-3.62,;5.33,-1,;6.67,-1.77,;8,-1,;8,.54,;6.67,1.31,;5.33,.54,;4,-1.77,;2.67,-1,;1.33,-1.77,;,-1,;,.54,;-1.33,1.31,;-2.67,.54,;-2.67,-1,;-1.33,-1.77,;-4,1.31,;-5.33,.54,;-6.67,1.31,;-6.67,2.85,;-8,3.62,;-5.33,3.62,;-4,2.85,;-2.67,3.62,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2TF01J4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM557921

(US11365191, Example 64)Show SMILES Fc1ccc([C@@H]2CC[C@H](CCNC3CCCC3)CC2)c(Cl)c1 |r,wD:5.4,8.8,(-7.89,2.69,;-6.55,1.93,;-5.22,2.69,;-3.89,1.93,;-3.89,.38,;-2.55,-.38,;-1.22,.38,;.11,-.38,;.11,-1.93,;1.45,-2.69,;2.78,-1.93,;4.12,-2.69,;5.45,-1.93,;6.86,-2.55,;7.89,-1.41,;7.12,-.07,;5.61,-.39,;-1.22,-2.69,;-2.55,-1.93,;-5.22,-.38,;-5.22,-1.93,;-6.55,.38,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2TF01J4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM557920

(US11365191, Example 61)Show SMILES Fc1ccc([C@@H]2CC[C@H](CCNC3CCOCC3)CC2)c(Cl)c1 |r,wD:5.4,8.8,(-8,2.69,;-6.67,1.93,;-6.67,.38,;-5.33,-.38,;-4,.38,;-2.67,-.38,;-1.33,.38,;,-.38,;,-1.93,;1.33,-2.69,;2.67,-1.93,;4,-2.69,;5.33,-1.93,;6.67,-2.69,;8,-1.93,;8,-.38,;6.67,.39,;5.33,-.38,;-1.33,-2.69,;-2.67,-1.93,;-4,1.93,;-2.67,2.69,;-5.33,2.69,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2TF01J4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM557919

(US11365191, Example 57)Show SMILES FC1COCCC1NCC[C@H]1CC[C@@H](CC1)c1ccc(F)cc1Cl |r,wU:10.10,wD:13.17,(6.67,-8.68,;6.67,-7.14,;8,-6.37,;8,-4.83,;6.67,-4.06,;5.33,-4.83,;5.33,-6.37,;4,-7.14,;2.67,-6.37,;1.33,-7.14,;,-6.37,;-1.33,-7.14,;-2.67,-6.37,;-2.67,-4.83,;-1.33,-4.06,;,-4.83,;-4,-4.06,;-5.33,-4.83,;-6.67,-4.06,;-6.67,-2.52,;-8,-1.75,;-5.33,-1.75,;-4,-2.52,;-2.67,-1.75,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2TF01J4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM557918

(N-(2-((cis)-4-(2-chloro-4-fluorophenyl)cyclohexyl)...)Show SMILES FC1COCCC1NCC[C@H]1CC[C@H](CC1)c1ccc(F)cc1Cl |r,wD:13.17,10.10,(6.67,1.75,;6.67,3.29,;8,4.06,;8,5.6,;6.67,6.37,;5.33,5.6,;5.33,4.06,;4,3.29,;2.67,4.06,;1.33,3.29,;,4.06,;,5.6,;-1.33,6.37,;-2.67,5.6,;-2.67,4.06,;-1.33,3.29,;-4,6.37,;-5.33,5.6,;-6.67,6.37,;-6.67,7.91,;-8,8.68,;-5.33,8.68,;-4,7.91,;-2.67,8.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2TF01J4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM557917

(US11365191, Example 55)Show SMILES FC1COCCC1NCC[C@H]1CC[C@H](CC1)c1ccc(F)cc1Cl |r,wD:13.17,10.10,(6.67,-3.47,;6.67,-1.93,;8,-1.15,;8,.38,;6.67,1.16,;5.33,.38,;5.33,-1.15,;4,-1.93,;2.67,-1.15,;1.33,-1.93,;,-1.15,;,.38,;-1.33,1.15,;-2.67,.38,;-2.67,-1.15,;-1.33,-1.92,;-4,1.15,;-5.33,.38,;-6.67,1.15,;-6.67,2.69,;-8,3.47,;-5.33,3.47,;-4,2.69,;-2.67,3.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2TF01J4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM557916

(US11365191, Example 54)Show SMILES Fc1ccc([C@@H]2CC[C@H](CCNC3CCC(F)(F)C3)CC2)c(Cl)c1 |r,wD:5.4,8.8,(-8.55,2.69,;-7.22,1.93,;-7.22,.38,;-5.89,-.38,;-4.55,.38,;-3.22,-.38,;-1.89,.38,;-.55,-.38,;-.55,-1.93,;.78,-2.69,;2.12,-1.93,;3.45,-2.69,;4.78,-1.93,;4.94,-.39,;6.45,-.07,;7.22,-1.41,;8.55,-2.18,;8.55,-.64,;6.19,-2.55,;-1.89,-2.69,;-3.22,-1.93,;-4.55,1.93,;-3.22,2.69,;-5.89,2.69,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2TF01J4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data



















































