

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50195673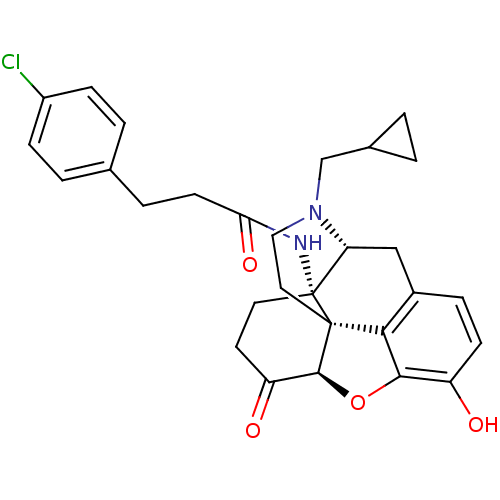 (CHEMBL267027 | N-cyclopropylmethyl-14beta-[3'-(4'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Activity at human recombinant kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50195666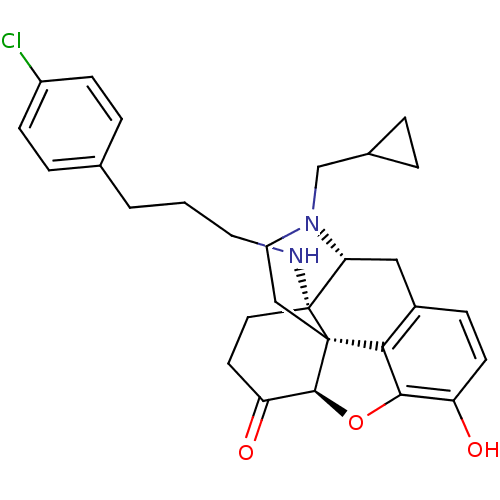 (CHEMBL443311 | N-cyclopropylmethyl-14beta-[3'-(4'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Activity at human recombinant kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027433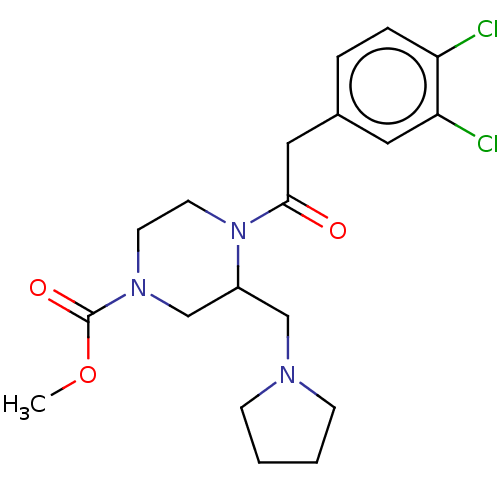 (CHEMBL603370 | GR-89696) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro agonist activity at kappa opioid receptor in rabbit vas deferens. | J Med Chem 36: 2075-83 (1993) BindingDB Entry DOI: 10.7270/Q2D50NKJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045179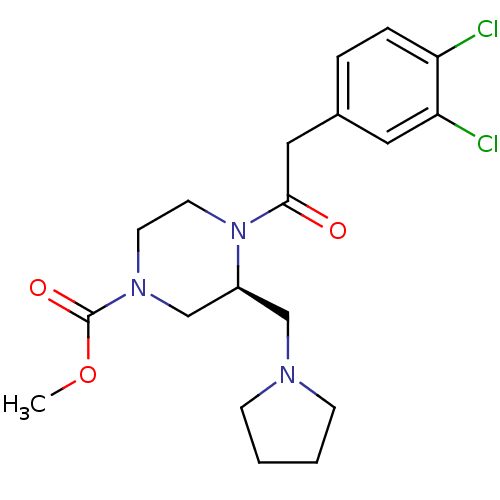 ((R)-4-[2-(3,4-Dichloro-phenyl)-acetyl]-3-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonistic activity at kappa opioid receptor of rabbit vas deferens | Bioorg Med Chem Lett 2: 1275-1278 (1992) Article DOI: 10.1016/S0960-894X(00)80229-2 BindingDB Entry DOI: 10.7270/Q2125SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045192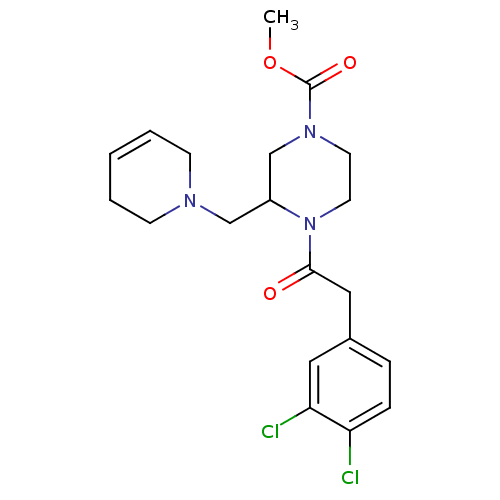 (4-[2-(3,4-Dichloro-phenyl)-acetyl]-3-(3,6-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro agonist activity at kappa opioid receptor in rabbit vas deferens. | J Med Chem 36: 2075-83 (1993) BindingDB Entry DOI: 10.7270/Q2D50NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027433 (CHEMBL603370 | GR-89696) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro agonist activity at kappa opioid receptor in rabbit vas deferens. | J Med Chem 36: 2075-83 (1993) BindingDB Entry DOI: 10.7270/Q2D50NKJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027433 (CHEMBL603370 | GR-89696) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro agonist activity at kappa opioid receptor in rabbit vas deferens. | J Med Chem 36: 2075-83 (1993) BindingDB Entry DOI: 10.7270/Q2D50NKJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50010532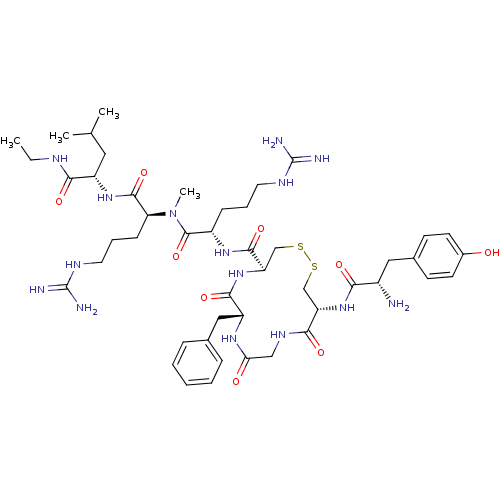 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Opioid receptor kappa 1 of rabbit vas deferens (RVD) | J Med Chem 33: 206-12 (1990) BindingDB Entry DOI: 10.7270/Q2QV3KG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50225844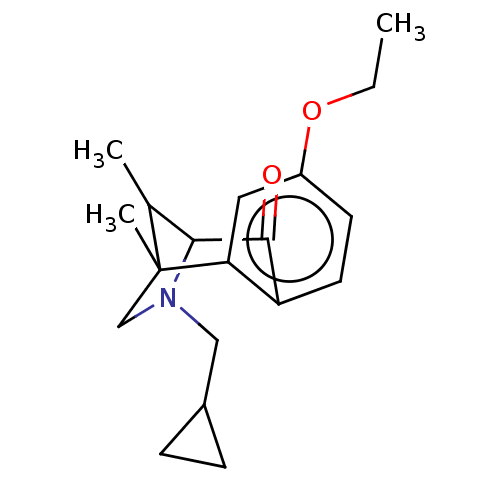 (CHEMBL56347) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for antagonistic activity against opioid receptor in electrically stimulated longitudinal muscle of guinea pig ileum | J Med Chem 29: 1861-4 (1986) BindingDB Entry DOI: 10.7270/Q2HX1FW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM209923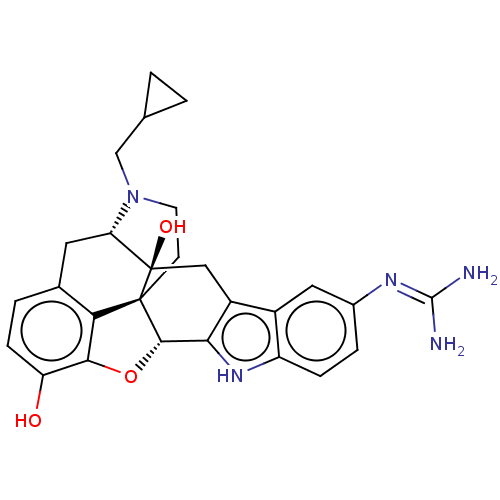 (5'-Guanidinonaltrindole (5'-GNTI)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description For antagonist experiments, protein was preincubated with test compounds for 15 min prior to the addition of 100 nM U69,593 and [35S]GTPγS. Reac... | J Biol Chem 288: 22387-98 (2013) Article DOI: 10.1074/jbc.M113.476234 BindingDB Entry DOI: 10.7270/Q25Q4TXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000293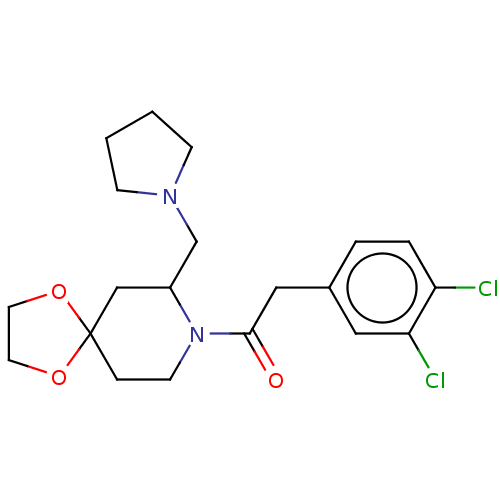 (2-(3,4-Dichloro-phenyl)-1-(7-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro kappa-opioid receptor agonist activity in isolated rabbit vas deferens assay | J Med Chem 37: 2138-44 (1994) BindingDB Entry DOI: 10.7270/Q2668C78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000293 (2-(3,4-Dichloro-phenyl)-1-(7-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro agonist activity at kappa opioid receptor in rabbit vas deferens. | J Med Chem 36: 2075-83 (1993) BindingDB Entry DOI: 10.7270/Q2D50NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50195656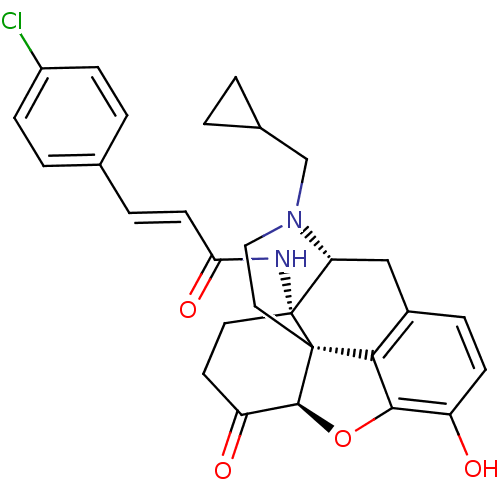 (14beta-4'-Chlorocinnamoylaminodihydronormorphinone...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Activity at human recombinant kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000293 (2-(3,4-Dichloro-phenyl)-1-(7-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50040123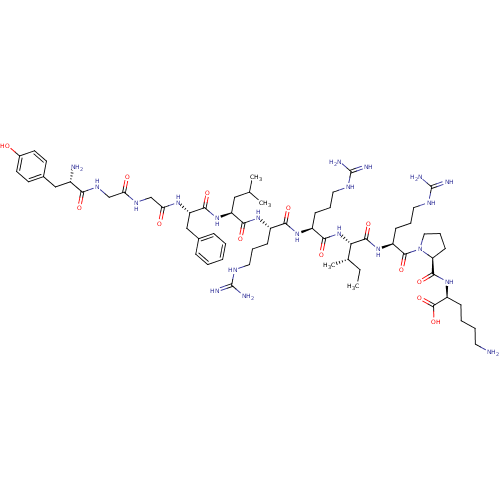 (CHEMBL438223 | HTry-Gly-Gly-Phe-Leu-Arg-Arg-lle-Ar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZG6WWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001107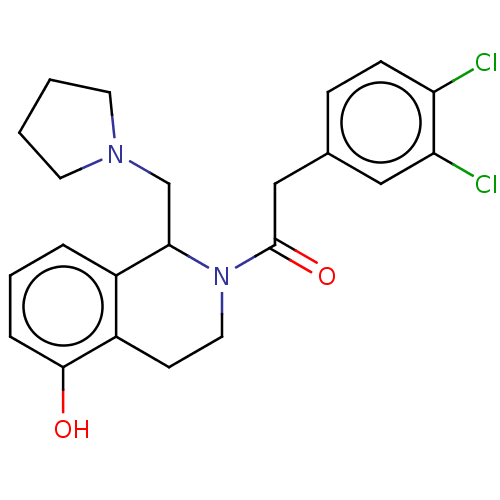 (2-(3,4-Dichloro-phenyl)-1-(5-hydroxy-1-pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro kappa-opioid receptor agonist activity in isolated rabbit vas deferens assay | J Med Chem 37: 2138-44 (1994) BindingDB Entry DOI: 10.7270/Q2668C78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000288 ((1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50558721 (CHEMBL4784791) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.132 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZG6WWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM214798 (Dynorphin A (1-17) | YGGFLRRIRPKLK) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZG6WWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50010704 (CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human KOR expressed in mouse HN9.10 cell membranes incubated for 2 hrs by liquid scintillation counting based radiol... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZG6WWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM581036 (RFCX0139-43 | US11492374, ID 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.157 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma... | Citation and Details BindingDB Entry DOI: 10.7270/Q26977FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000271 (1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50002346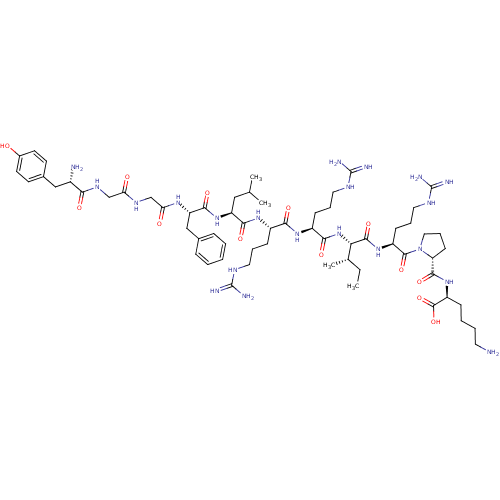 (CHEMBL384584 | [D-pro10]Dynorphin A(1-11)) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Tested for opioid receptor agonistic activity in guinea pig ileum | J Med Chem 35: 4638-9 (1993) BindingDB Entry DOI: 10.7270/Q2416XPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50280147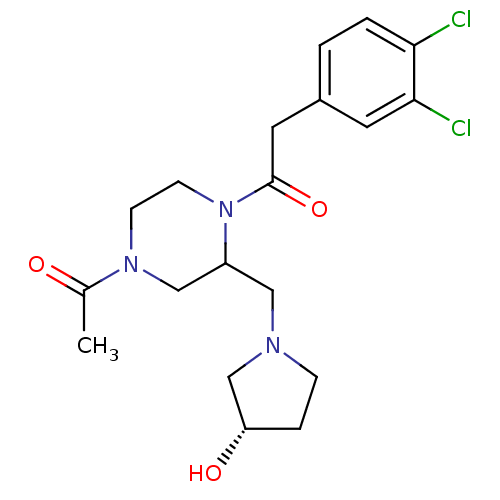 (1-[4-Acetyl-2-((S)-3-hydroxy-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonistic activity at kappa opioid receptor of rabbit vas deferens | Bioorg Med Chem Lett 2: 1275-1278 (1992) Article DOI: 10.1016/S0960-894X(00)80229-2 BindingDB Entry DOI: 10.7270/Q2125SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50340646 (CHEMBL4159127) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 assessed as inhibition forskolin-induced cAMP accumulation after 15 mins by EIA | J Med Chem 61: 5751-5757 (2018) Article DOI: 10.1021/acs.jmedchem.8b00296 BindingDB Entry DOI: 10.7270/Q2NV9MTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM581037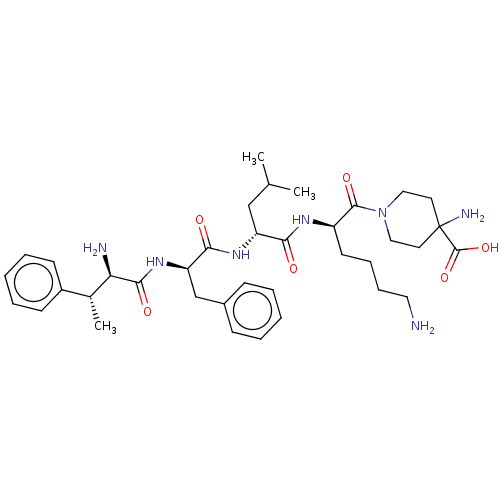 (RFCX0139-45 | US11492374, ID 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.253 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma... | Citation and Details BindingDB Entry DOI: 10.7270/Q26977FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM581034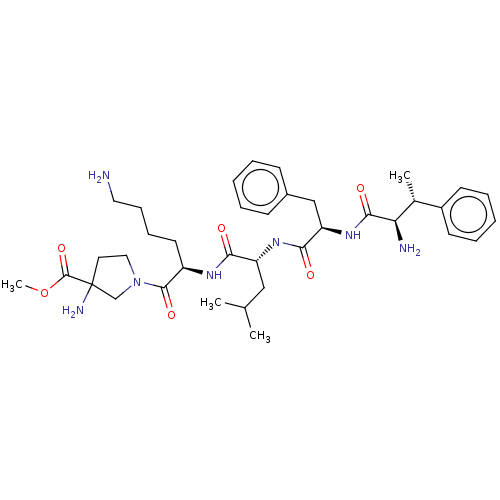 (US11492374, ID 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.255 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma... | Citation and Details BindingDB Entry DOI: 10.7270/Q26977FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531921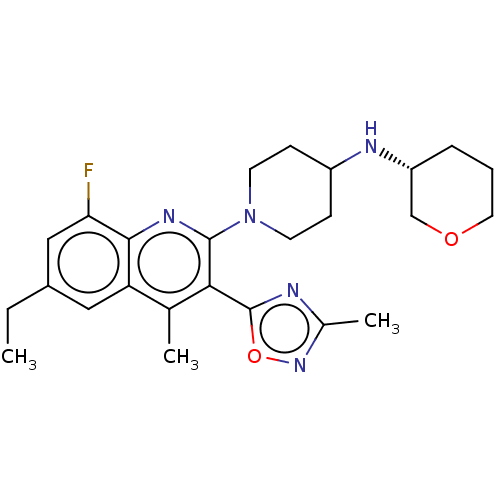 (CHEMBL4544914 | US10676469, Compound 148 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 BindingDB Entry DOI: 10.7270/Q2CV4N6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Specialized Chemistry Center Curated by ChEMBL | Assay Description Antagonist activity against HA-tagged human recombinant kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by [35S]GTPgammaS bi... | Bioorg Med Chem 23: 3948-56 (2015) Article DOI: 10.1016/j.bmc.2014.12.033 BindingDB Entry DOI: 10.7270/Q2R49SJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50226283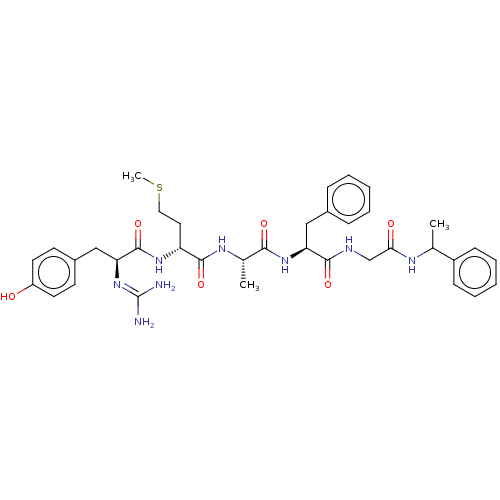 (CHEMBL172293) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against opioid activity in guinea pig ileum | J Med Chem 29: 889-94 (1986) BindingDB Entry DOI: 10.7270/Q2959KSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045186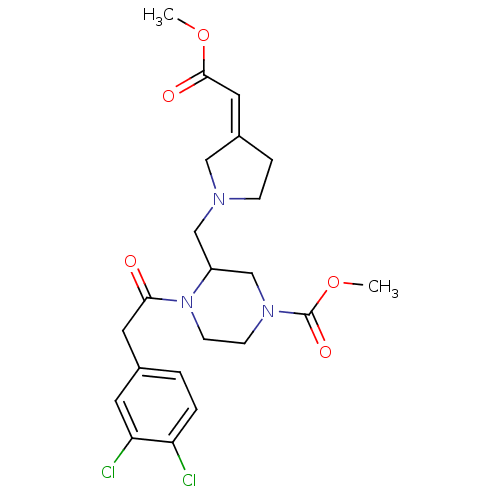 (4-[2-(3,4-Dichloro-phenyl)-acetyl]-3-(3-methoxycar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro agonist activity at kappa opioid receptor in rabbit vas deferens. | J Med Chem 36: 2075-83 (1993) BindingDB Entry DOI: 10.7270/Q2D50NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50195659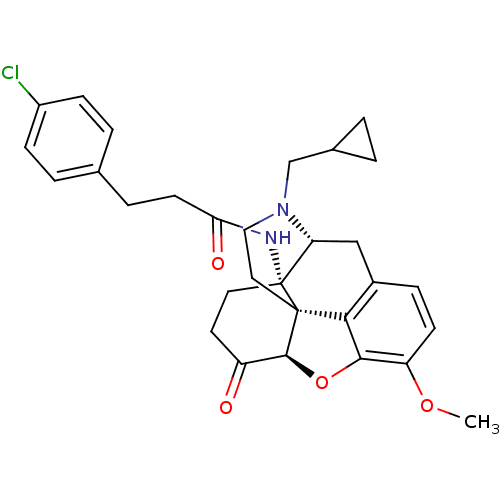 (CHEMBL217395 | N-cyclopropylmethyl-14beta-[3'-(4'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Activity at human recombinant kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in HEK293 cells assessed as inhibition of forskolin-induced cAMP production after 30 mins b... | Bioorg Med Chem 22: 4694-703 (2014) Article DOI: 10.1016/j.bmc.2014.07.012 BindingDB Entry DOI: 10.7270/Q2H133NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123599 (ETORPHINE) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated the ability to protect against the irreversible antagonism of morphines effects by beta-FNA in guinea pig ileal longitudinal muscle. | J Med Chem 26: 1341-3 (1983) BindingDB Entry DOI: 10.7270/Q2VH5R2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50195663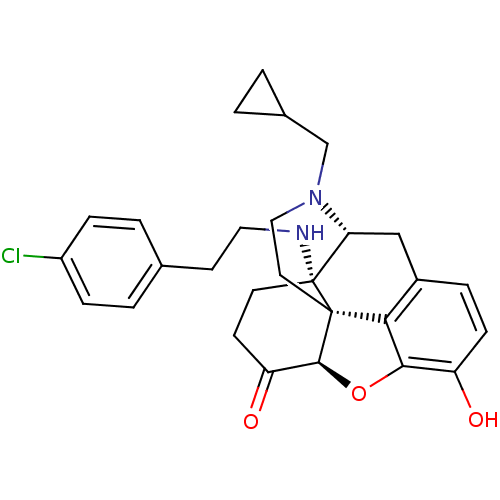 (CHEMBL217658 | N-cyclopropylmethyl-14beta-[2'-(4''...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Activity at human recombinant kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045193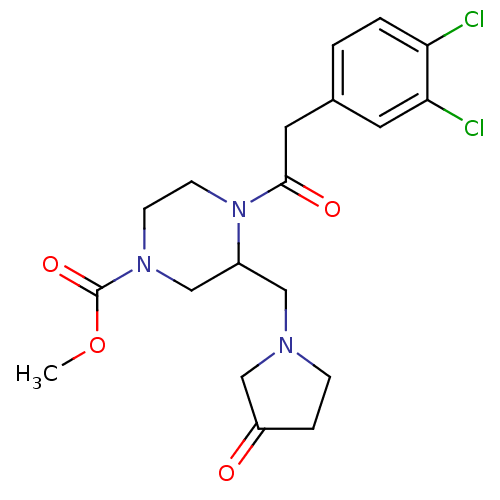 (4-[2-(3,4-Dichloro-phenyl)-acetyl]-3-(3-oxo-pyrrol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro agonist activity at kappa opioid receptor in rabbit vas deferens. | J Med Chem 36: 2075-83 (1993) BindingDB Entry DOI: 10.7270/Q2D50NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045196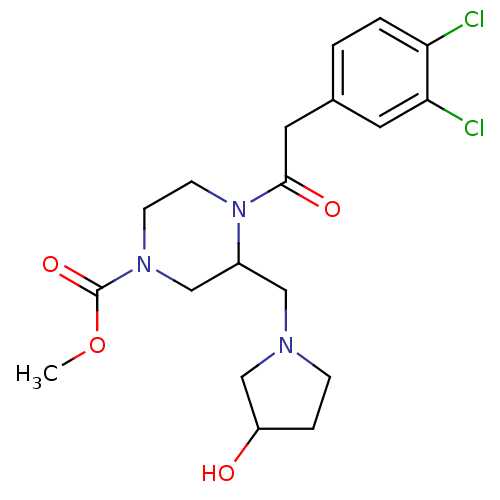 (4-[2-(3,4-Dichloro-phenyl)-acetyl]-3-(3-hydroxy-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro agonist activity at kappa opioid receptor in rabbit vas deferens. | J Med Chem 36: 2075-83 (1993) BindingDB Entry DOI: 10.7270/Q2D50NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50039101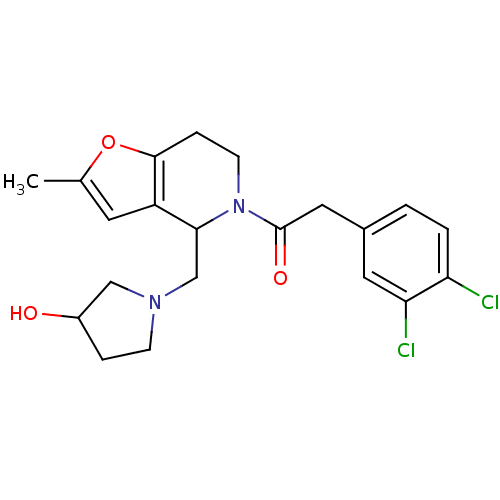 (2-(3,4-Dichloro-phenyl)-1-[4-(3-hydroxy-pyrrolidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro kappa-opioid receptor agonist activity in isolated rabbit vas deferens assay | J Med Chem 37: 2138-44 (1994) BindingDB Entry DOI: 10.7270/Q2668C78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM581035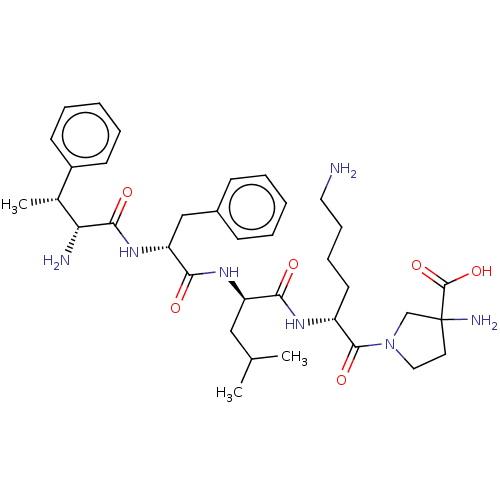 (US11492374, ID 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma... | Citation and Details BindingDB Entry DOI: 10.7270/Q26977FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045189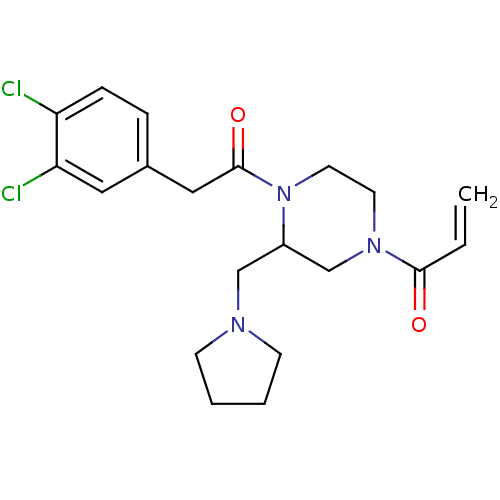 (1-{4-[2-(3,4-Dichloro-phenyl)-acetyl]-3-pyrrolidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro agonist activity at kappa opioid receptor in rabbit vas deferens. | J Med Chem 36: 2075-83 (1993) BindingDB Entry DOI: 10.7270/Q2D50NKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM209922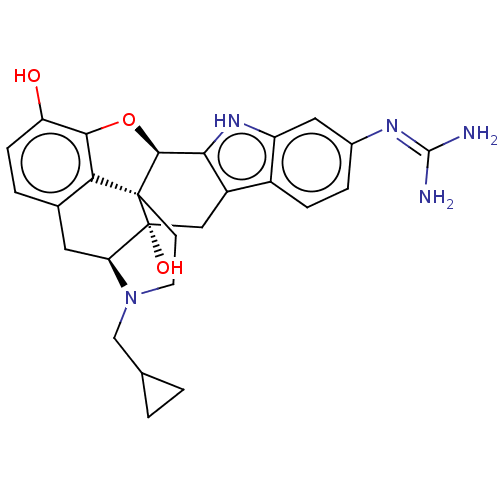 (6'-Guanidinonaltrindole (6'-GNTI)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description For antagonist experiments, protein was preincubated with test compounds for 15 min prior to the addition of 100 nM U69,593 and [35S]GTPγS. Reac... | J Biol Chem 288: 22387-98 (2013) Article DOI: 10.1074/jbc.M113.476234 BindingDB Entry DOI: 10.7270/Q25Q4TXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50195675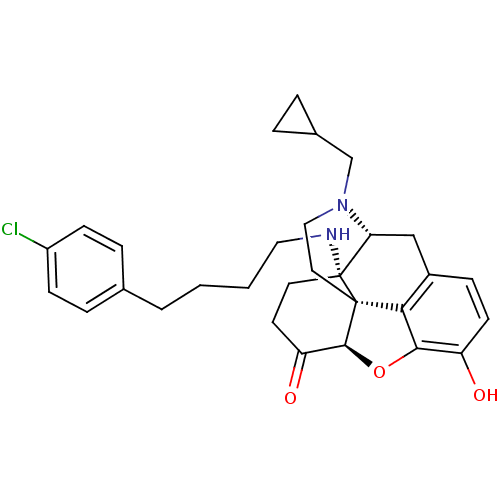 (CHEMBL217102 | N-cyclopropylmethyl-14beta-[4'-(4''...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Activity at human recombinant kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM209921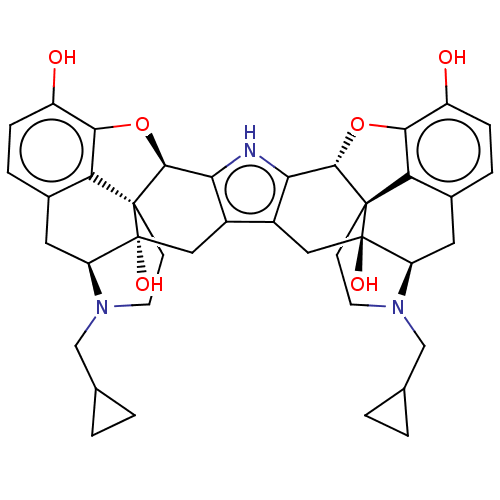 (Norbinaltorphimine (nor-BNI)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description For antagonist experiments, protein was preincubated with test compounds for 15 min prior to the addition of 100 nM U69,593 and [35S]GTPγS. Reac... | J Biol Chem 288: 22387-98 (2013) Article DOI: 10.1074/jbc.M113.476234 BindingDB Entry DOI: 10.7270/Q25Q4TXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50039104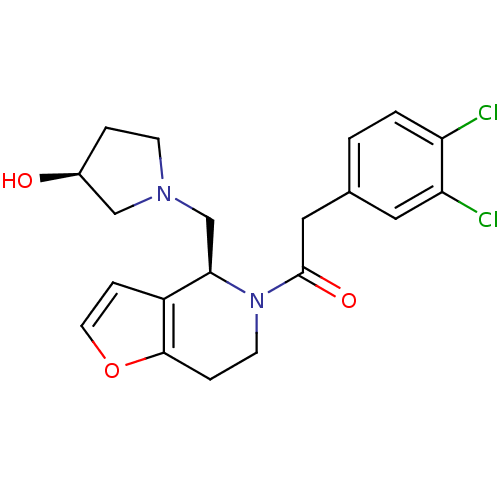 ((SS)-2-(3,4-Dichloro-phenyl)-1-[4-(3-hydroxy-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description In vitro kappa-opioid receptor agonist activity in isolated rabbit vas deferens assay | J Med Chem 37: 2138-44 (1994) BindingDB Entry DOI: 10.7270/Q2668C78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM156523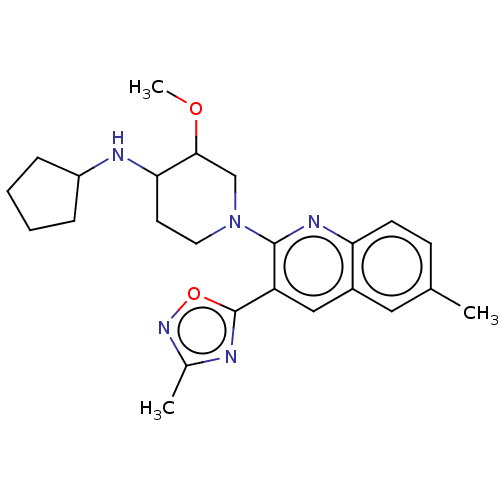 (US10118915, Compound 309 | US9682966, 309) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute US Patent | Assay Description Well-known assay for kappa opioid receptor | US Patent US10118915 (2018) BindingDB Entry DOI: 10.7270/Q23J3G00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50013388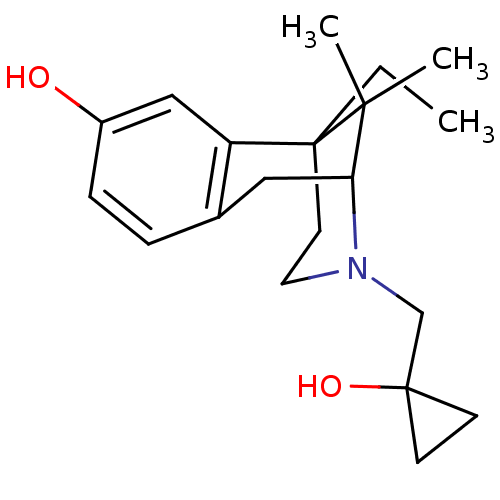 (6-Ethyl-3-(1-hydroxy-cyclopropylmethyl)-11,11-dime...) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description In vitro binding affinity against Opioid receptors from bovine caudate nucleus determined in presence of [3H]- bremazocine (0.5 nM) | J Med Chem 33: 737-41 (1990) BindingDB Entry DOI: 10.7270/Q2SN09J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50130561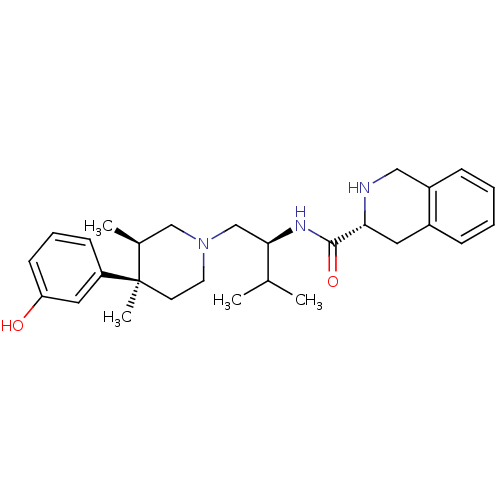 ((R)-N-((S)-1-((3R,4R)-4-(3-hydroxyphenyl)-3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 20: 5847-52 (2010) Article DOI: 10.1016/j.bmcl.2010.07.113 BindingDB Entry DOI: 10.7270/Q23F4PV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM156516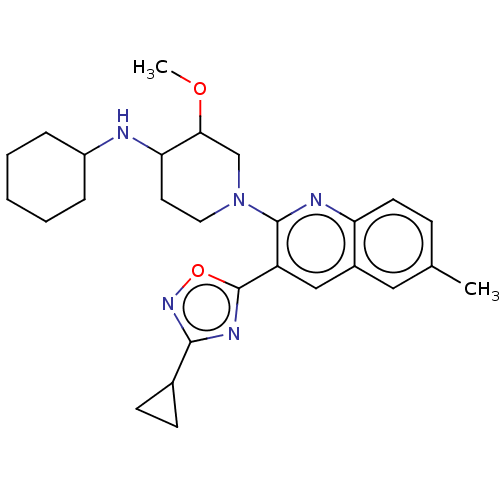 (US10118915, Compound 303 | US9682966, 303) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute US Patent | Assay Description Well-known assay for kappa opioid receptor | US Patent US10118915 (2018) BindingDB Entry DOI: 10.7270/Q23J3G00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM156523 (US10118915, Compound 309 | US9682966, 309) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute US Patent | Assay Description The cell line for the OPRK1 antagonist assay stably expresses the following elements. The carboxy terminus of the OPRK1 receptor has a 7 amino acid l... | US Patent US9682966 (2017) BindingDB Entry DOI: 10.7270/Q2610XHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM156516 (US10118915, Compound 303 | US9682966, 303) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute US Patent | Assay Description The cell line for the OPRK1 antagonist assay stably expresses the following elements. The carboxy terminus of the OPRK1 receptor has a 7 amino acid l... | US Patent US9682966 (2017) BindingDB Entry DOI: 10.7270/Q2610XHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3131 total ) | Next | Last >> |