

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50195666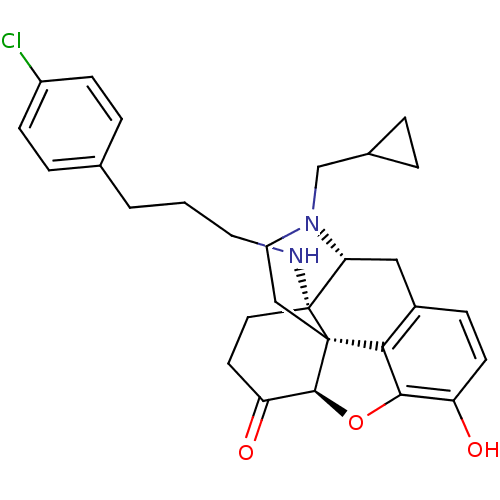 (CHEMBL443311 | N-cyclopropylmethyl-14beta-[3'-(4'-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50340644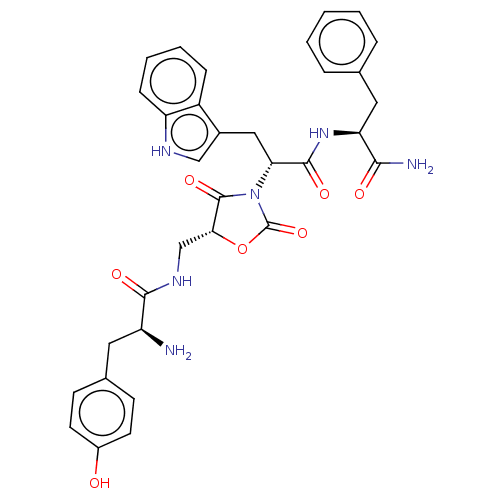 (CHEMBL4169199) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in HEK293 assessed as inhibition forskolin-induced cAMP accumulation after 15 mins by EIA | J Med Chem 61: 5751-5757 (2018) Article DOI: 10.1021/acs.jmedchem.8b00296 BindingDB Entry DOI: 10.7270/Q2NV9MTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50195673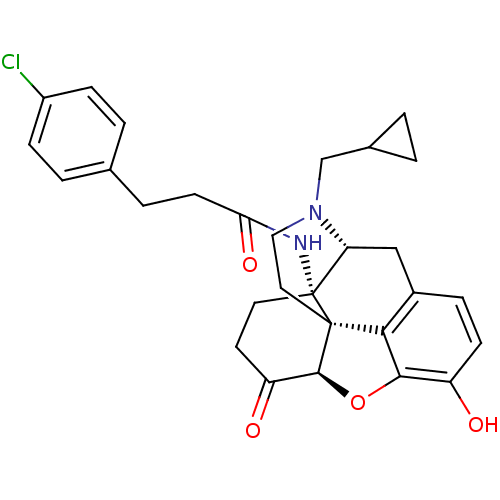 (CHEMBL267027 | N-cyclopropylmethyl-14beta-[3'-(4'-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50195659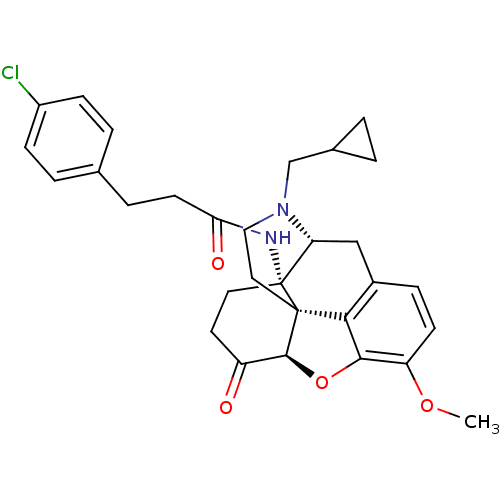 (CHEMBL217395 | N-cyclopropylmethyl-14beta-[3'-(4'-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017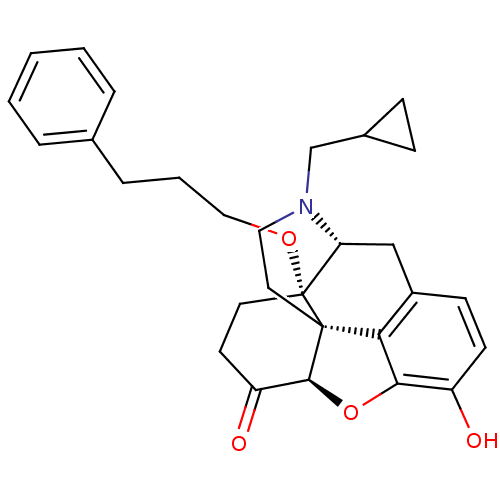 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM456904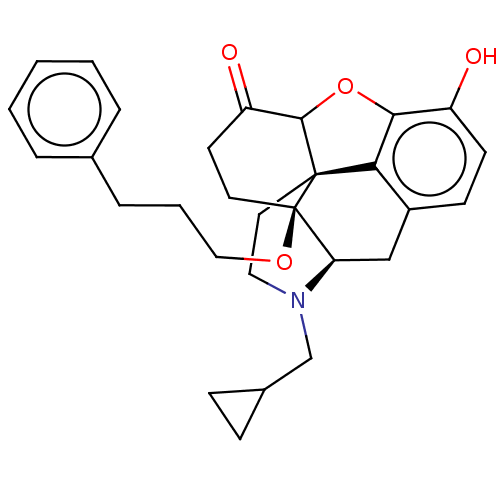 (US10736890, Compound TABLE B.14) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50249017 (14-O-phenylpropylnaltrexone | 4-cyclopropylmethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300357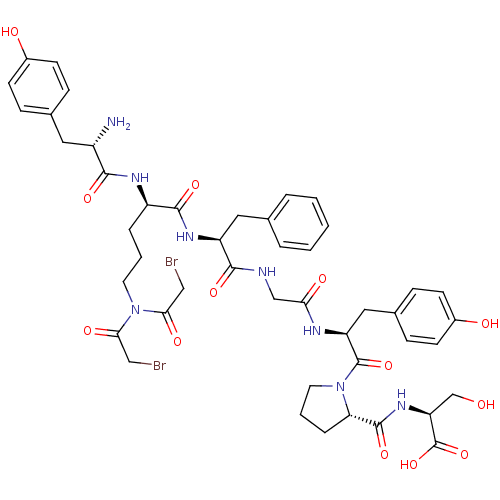 ((S)-2-((S)-1-((2S,8S,11R)-11-((S)-2-amino-3-(4-hyd...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in CHO cells after 90 mins by scintillation counting | J Med Chem 52: 7372-5 (2009) Article DOI: 10.1021/jm9007592 BindingDB Entry DOI: 10.7270/Q2K64J5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50195660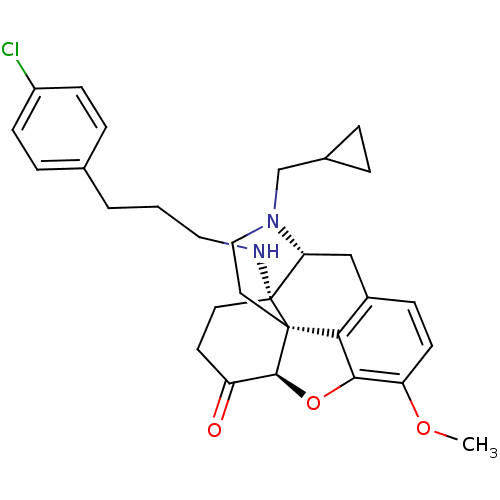 (CHEMBL384497 | N-cyclopropylmethyl-14beta-[3'-(4'-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50017233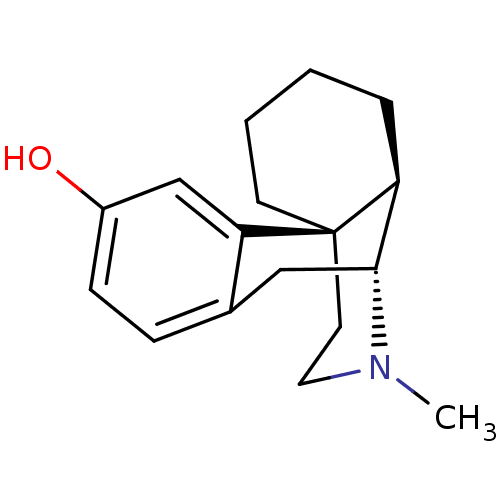 (CHEMBL592 | LEVO-DROMORAN | LEVORPHANOL) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Tested for effective concentration against cloned human Opioid receptor mu 1 | J Med Chem 46: 34-48 (2002) Article DOI: 10.1021/jm020164l BindingDB Entry DOI: 10.7270/Q2TM7BTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM309410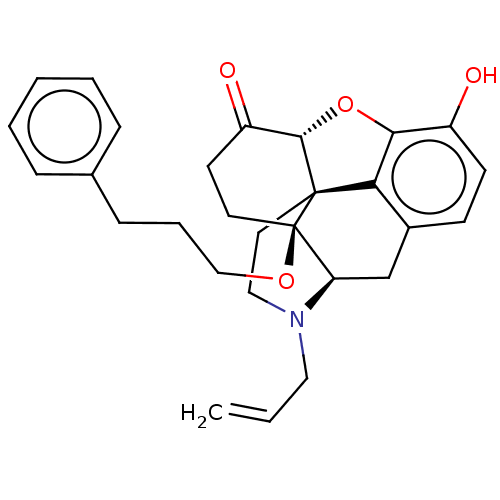 (US10231963, Table B.13 | US11534436, Compound Tabl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM309410 (US10231963, Table B.13 | US11534436, Compound Tabl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM456903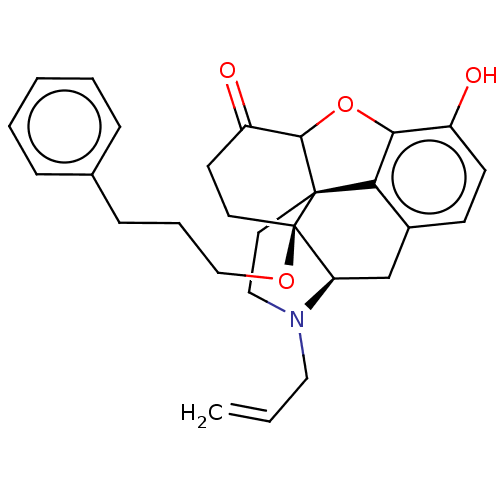 (US10736890, Compound TABLE B.13) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM309410 (US10231963, Table B.13 | US11534436, Compound Tabl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50386678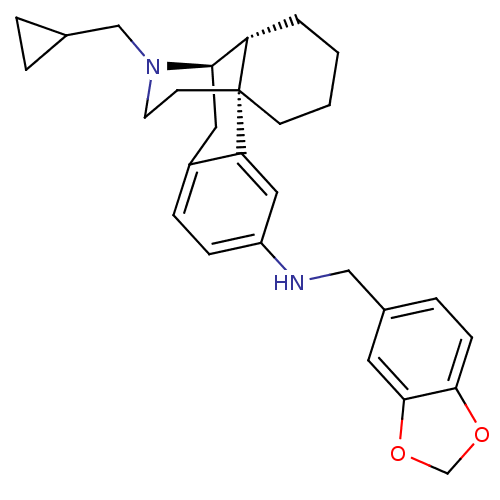 (CHEMBL2048766) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-induced [33S]GTPgammaS binding after 60 mins b... | J Med Chem 55: 3878-90 (2012) Article DOI: 10.1021/jm3001086 BindingDB Entry DOI: 10.7270/Q28053P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50195655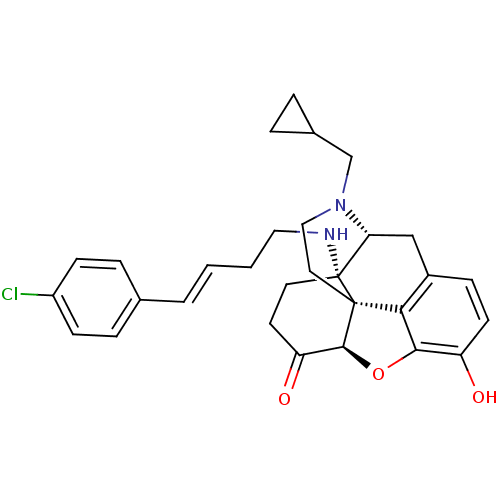 (CHEMBL383896 | N-cyclopropylmethyl-14beta-[4'(4''-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50195665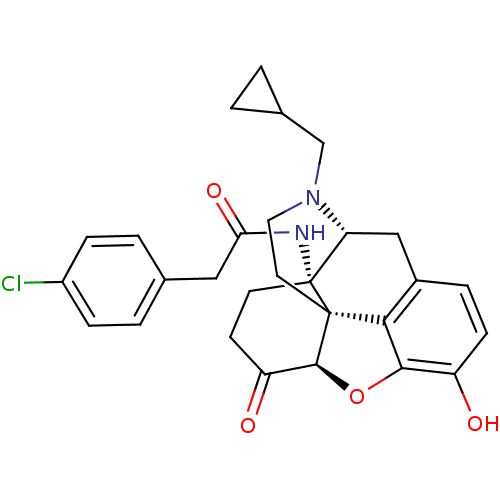 (CHEMBL448428 | N-cyclopropylmethyl-14beta-[2'-(4''...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Activity at human recombinant mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM587295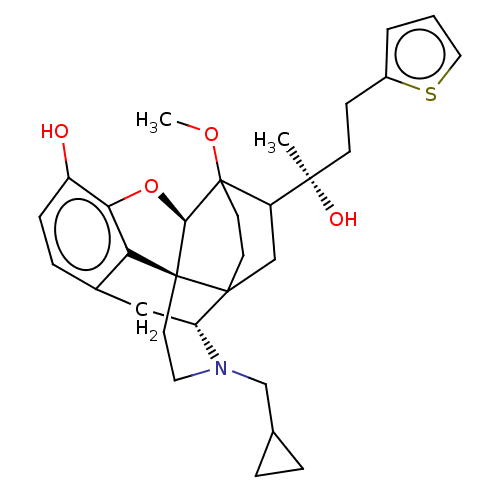 (US11534436, Compound Table B.4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM456894 (US10736890, Compound TABLE B.4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM309336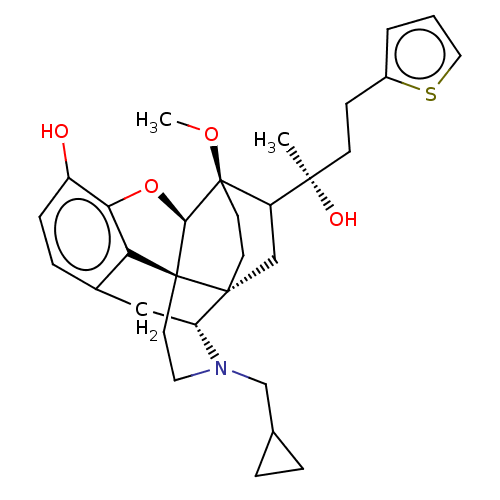 (US10231963, Table B.4 | US9656961, Example 00121) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | J Med Chem 50: 4214-21 (2007) BindingDB Entry DOI: 10.7270/Q2W66P26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM309336 (US10231963, Table B.4 | US9656961, Example 00121) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal of ... | US Patent US9656961 (2017) BindingDB Entry DOI: 10.7270/Q2PR7Z13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50474150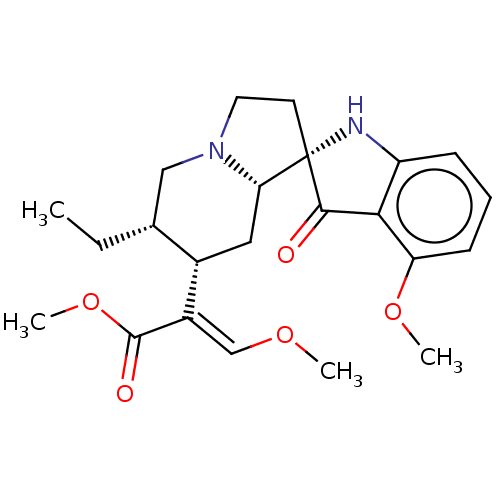 (CHEMBL58362) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor (unknown origin) in presence of DPDPE by [35S]-GTPgammaS binding assay | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111701 BindingDB Entry DOI: 10.7270/Q2668HN9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50195675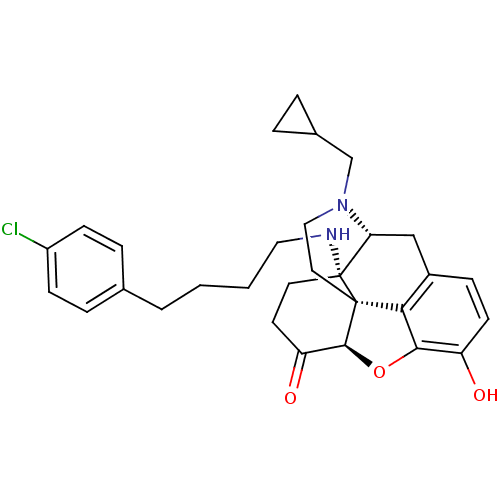 (CHEMBL217102 | N-cyclopropylmethyl-14beta-[4'-(4''...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in CHO cells | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015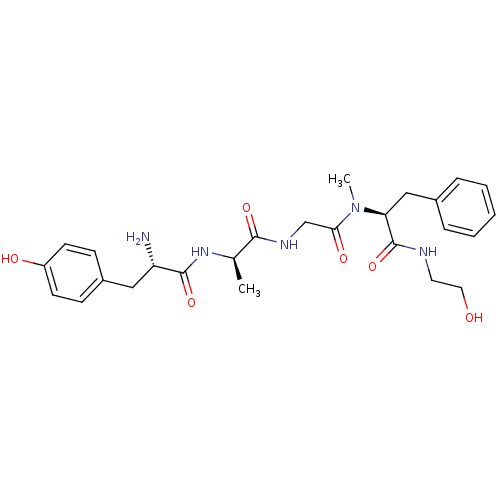 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.294 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma... | Citation and Details BindingDB Entry DOI: 10.7270/Q26977FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50272031 ((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in human SHSY5Y cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem 16: 6286-96 (2008) Article DOI: 10.1016/j.bmc.2008.04.020 BindingDB Entry DOI: 10.7270/Q2ZG6S2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300359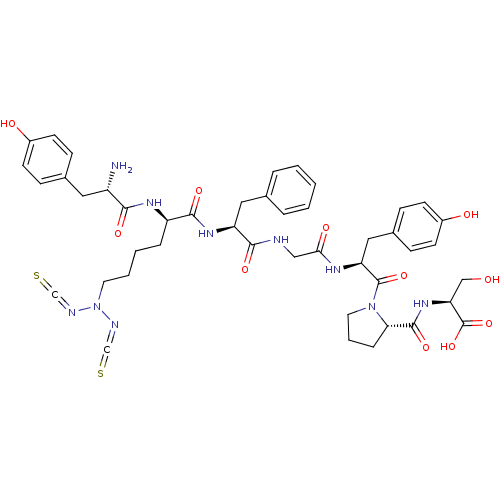 ((S)-2-((S)-1-((2S,8S,11R,14S)-14-amino-8-benzyl-11...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in CHO cells after 90 mins by scintillation counting | J Med Chem 52: 7372-5 (2009) Article DOI: 10.1021/jm9007592 BindingDB Entry DOI: 10.7270/Q2K64J5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant Mu-type opioid receptor expressed in CHO cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu-opioid receptor expressed in HEK293 cells measured after 120 mins by scintillation counting metho... | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199865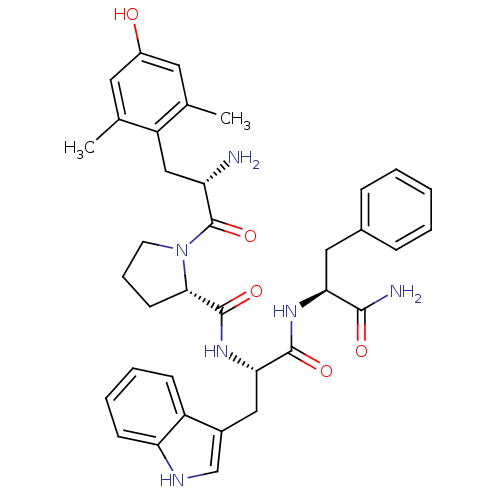 ((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in human SHSY5Y cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem 16: 6286-96 (2008) Article DOI: 10.1016/j.bmc.2008.04.020 BindingDB Entry DOI: 10.7270/Q2ZG6S2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179909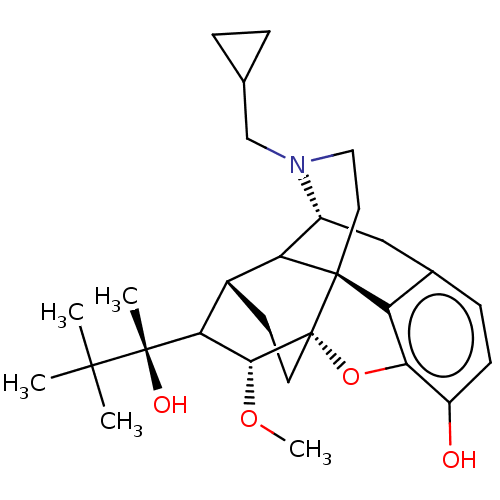 (US9133125, Table D, buprenorphine) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.410 | -12.8 | 0.450 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for u opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal o... | US Patent US9133125 (2015) BindingDB Entry DOI: 10.7270/Q2736PPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q2125W08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10752592 (2020) BindingDB Entry DOI: 10.7270/Q23R0WX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199896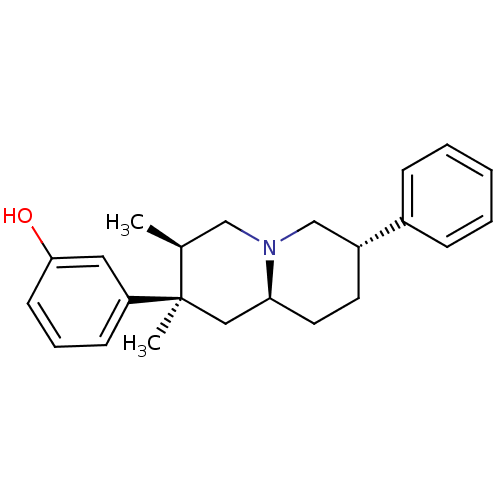 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.570 | -12.6 | 0.530 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Adolor Corporation US Patent | Assay Description The receptor binding method (DeHaven and DeHaven-Hudkins, 1998) was a modification of the method of Raynor et al. (1994). | US Patent US8580788 (2013) BindingDB Entry DOI: 10.7270/Q2N29VKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50195656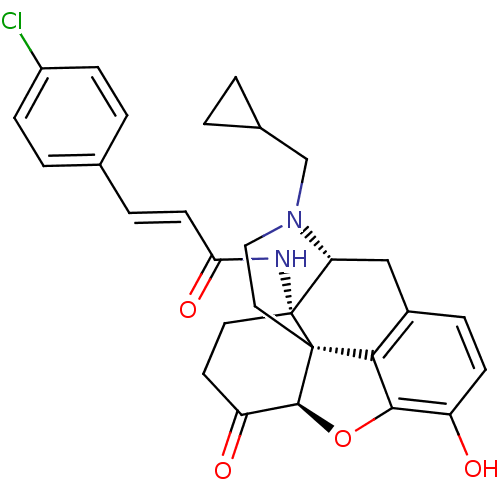 (14beta-4'-Chlorocinnamoylaminodihydronormorphinone...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Activity at human recombinant mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity assessed as inhibition of loperamide-stimulated [35S]GTPgammaS binding to human mu opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199896 (3-((2R,3R,7S,9-alpha-S)-2,3-dimethyl-7-phenyl-octa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity against human cloned mu opioid receptor expressed in CHO cells assessed as inhibition of loperamide-stimulated [35S]GTP-gamma-S b... | J Med Chem 49: 7278-89 (2006) Article DOI: 10.1021/jm060486f BindingDB Entry DOI: 10.7270/Q27P906S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000092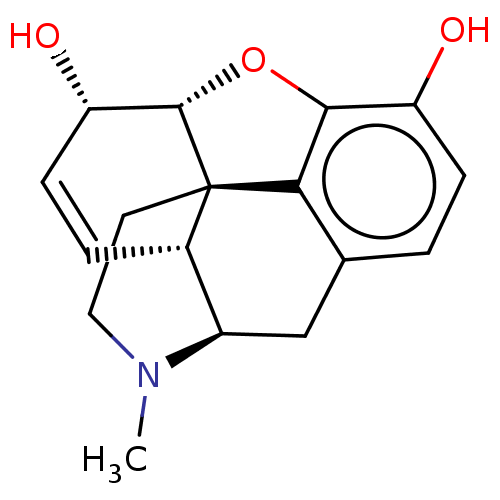 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Tested for effective concentration against cloned human Opioid receptor mu 1 | J Med Chem 46: 34-48 (2002) Article DOI: 10.1021/jm020164l BindingDB Entry DOI: 10.7270/Q2TM7BTT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50026603 (Buprenorphine | CHEBI:3216) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Activity at human cloned mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 203-8 (2008) Article DOI: 10.1016/j.bmcl.2008.10.134 BindingDB Entry DOI: 10.7270/Q2RX9D1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212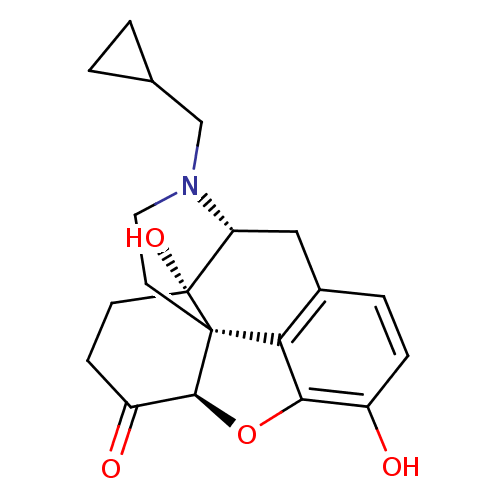 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Activity at human recombinant mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 49: 6104-10 (2006) Article DOI: 10.1021/jm060595u BindingDB Entry DOI: 10.7270/Q24X58MF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50176702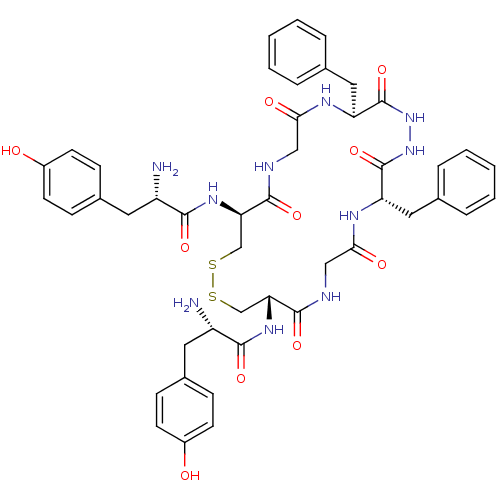 ((S)-2-amino-N-{(4S,10S,15S,21S)-21-[(S)-2-amino-3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor | Bioorg Med Chem Lett 16: 367-72 (2005) Article DOI: 10.1016/j.bmcl.2005.09.080 BindingDB Entry DOI: 10.7270/Q24X57BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50199946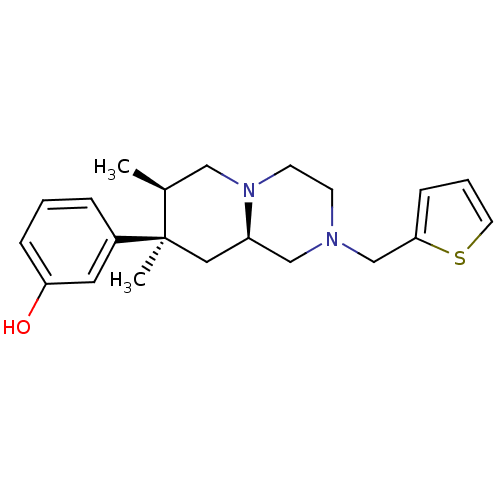 (3-((7R,8R,9alphaR)-7,8-dimethyl-2-(thiophen-2-ylme...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Antagonist activity assessed as inhibition of loperamide-stimulated [35S]GTPgammaS binding to human mu opioid receptor expressed in CHO cells | J Med Chem 49: 7290-306 (2006) Article DOI: 10.1021/jm0604878 BindingDB Entry DOI: 10.7270/Q2CF9QW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179898 (US10231963, Table C.2 | US10287250, Compound 2 | U...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | -13.4 | 0.650 | n/a | n/a | n/a | n/a | 7.5 | 25 |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for u opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Journal o... | US Patent US9133125 (2015) BindingDB Entry DOI: 10.7270/Q2736PPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179898 (US10231963, Table C.2 | US10287250, Compound 2 | U...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CJ8JBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179898 (US10231963, Table C.2 | US10287250, Compound 2 | U...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10752592 (2020) BindingDB Entry DOI: 10.7270/Q23R0WX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179898 (US10231963, Table C.2 | US10287250, Compound 2 | U...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
ALKERMES PHARMA IRELAND LIMITED US Patent | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | US Patent US10736890 (2020) BindingDB Entry DOI: 10.7270/Q2F47S5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM179898 (US10231963, Table C.2 | US10287250, Compound 2 | U...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The Ki (binding affinity) for μ opioid receptors was determined using a competitive displacement assay as previously described in Neumeyer (Jour... | Bioorg Med Chem Lett 17: 4284-9 (2007) BindingDB Entry DOI: 10.7270/Q2125W08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101628 (2-Amino-1-[3-(benzylamino-methyl)-3,4-dihydro-1H-i...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Agonist potency using GTP-gamma [35S]- binding assay for mu-opioid receptor | J Med Chem 44: 2387-90 (2001) BindingDB Entry DOI: 10.7270/Q25B01RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2309 total ) | Next | Last >> |