

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168435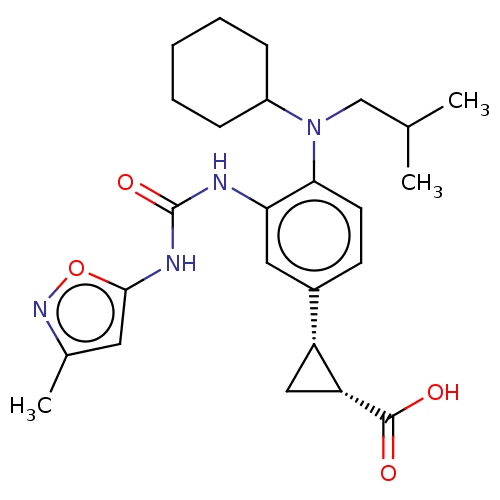 (US9675571, 129) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | 25 |
SRMLSC US Patent | Assay Description Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... | US Patent US9675571 (2017) BindingDB Entry DOI: 10.7270/Q2BK19HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168360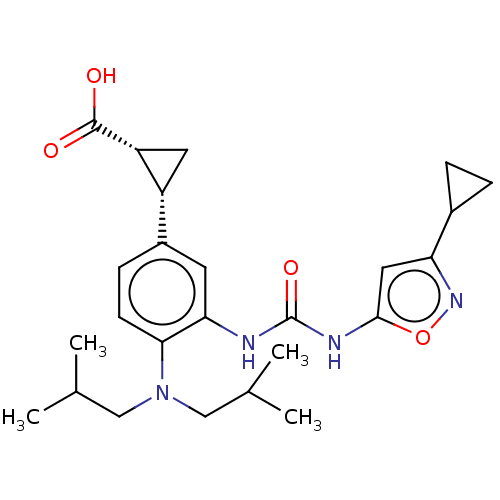 (US9675571, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | 25 |
SRMLSC US Patent | Assay Description Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... | US Patent US9675571 (2017) BindingDB Entry DOI: 10.7270/Q2BK19HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168315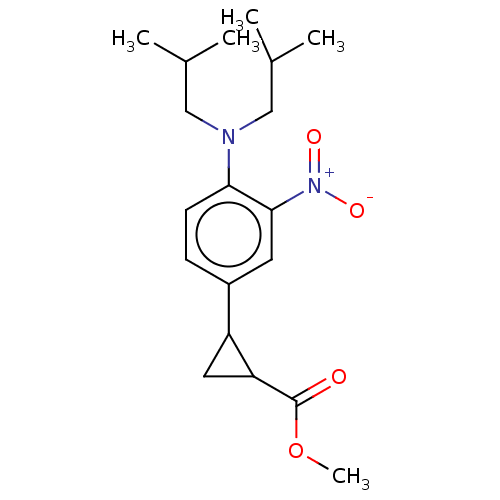 (US9675571, ent-1J) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | 25 |
SRMLSC US Patent | Assay Description Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... | US Patent US9675571 (2017) BindingDB Entry DOI: 10.7270/Q2BK19HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168358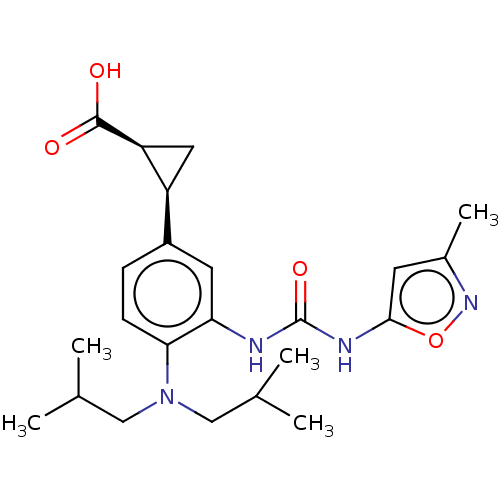 (US9675571, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | 25 |
SRMLSC US Patent | Assay Description Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... | US Patent US9675571 (2017) BindingDB Entry DOI: 10.7270/Q2BK19HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168337 (US9675571, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | 25 |
SRMLSC US Patent | Assay Description Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... | US Patent US9675571 (2017) BindingDB Entry DOI: 10.7270/Q2BK19HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168378 (US9675571, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | 25 |
SRMLSC US Patent | Assay Description Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... | US Patent US9675571 (2017) BindingDB Entry DOI: 10.7270/Q2BK19HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168377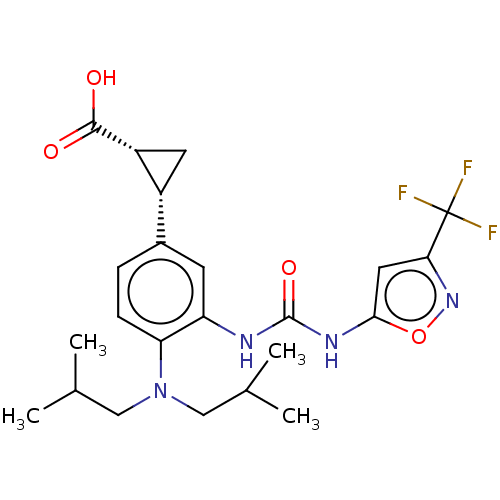 (US9675571, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | 25 |
SRMLSC US Patent | Assay Description Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... | US Patent US9675571 (2017) BindingDB Entry DOI: 10.7270/Q2BK19HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate incubated f... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127038 BindingDB Entry DOI: 10.7270/Q2D79G0Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168314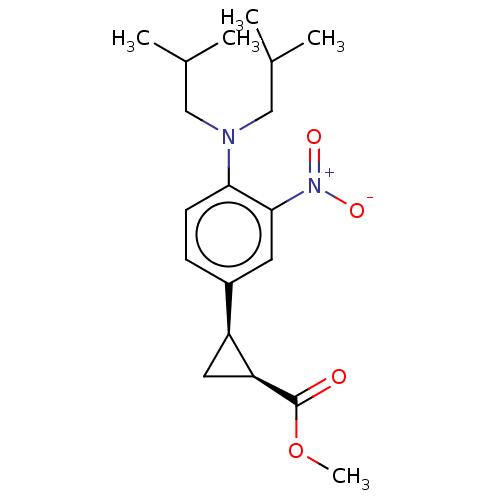 (US9675571, 1J) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | 25 |
SRMLSC US Patent | Assay Description Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... | US Patent US9675571 (2017) BindingDB Entry DOI: 10.7270/Q2BK19HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of human IDO1 expressed in HEK293 cells assessed as reduction in kynurenine production after 30 mins | ACS Med Chem Lett 9: 131-136 (2018) Article DOI: 10.1021/acsmedchemlett.7b00488 BindingDB Entry DOI: 10.7270/Q2FR0041 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50046104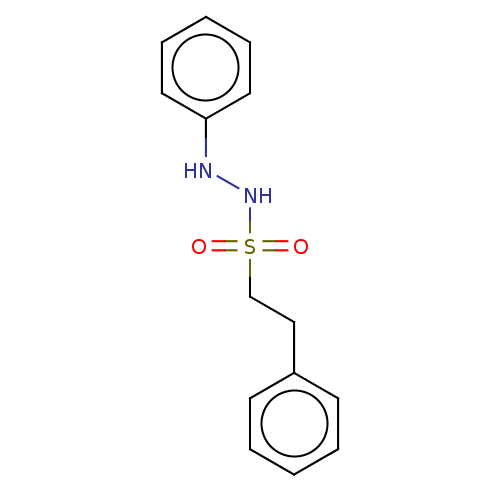 (CHEMBL3310297) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >10 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 3403-6 (2014) Article DOI: 10.1016/j.bmcl.2014.05.084 BindingDB Entry DOI: 10.7270/Q23R0VH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50545137 (CHEMBL4644903 | US11591301, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate incubated f... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127038 BindingDB Entry DOI: 10.7270/Q2D79G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of IFNgamma-stimulated IDO1 in human HeLa cells | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126901 BindingDB Entry DOI: 10.7270/Q2F76H3W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM526970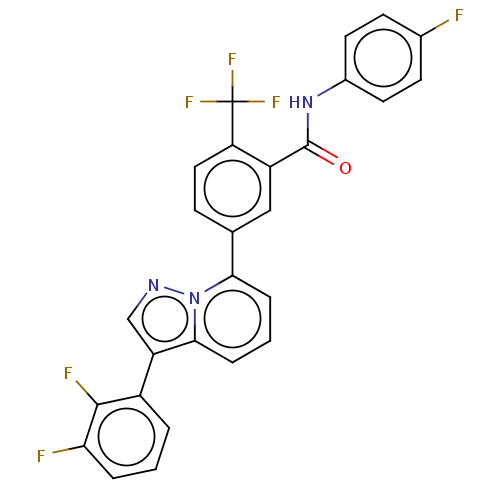 (5-(3-(2,3-difluorophenyl)pyrazolo[1,5-a]pyridin 7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure IDO1 inhibition in tissue culture, HeLa cells were treated with a test compound in the presence of IFNγ, which induces IDO1 expressio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM526969 (5-(1-(2,3-difluorophenyl)imidazo[1,5-a]pyridin-5- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure IDO1 inhibition in tissue culture, HeLa cells were treated with a test compound in the presence of IFNγ, which induces IDO1 expressio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM526968 (5-(3-(2,3-difluorophenyl)imidazo[1,2- b]pyridazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure IDO1 inhibition in tissue culture, HeLa cells were treated with a test compound in the presence of IFNγ, which induces IDO1 expressio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50545146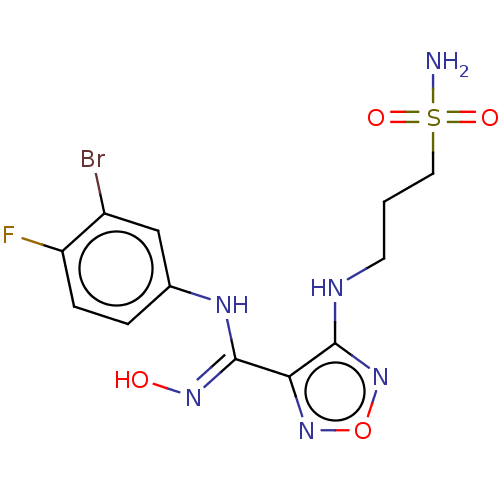 (CHEMBL4639448 | US11591301, Compound 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate incubated f... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127038 BindingDB Entry DOI: 10.7270/Q2D79G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50545138 (CHEMBL4645780 | US11591301, Compound 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate incubated f... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127038 BindingDB Entry DOI: 10.7270/Q2D79G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM526967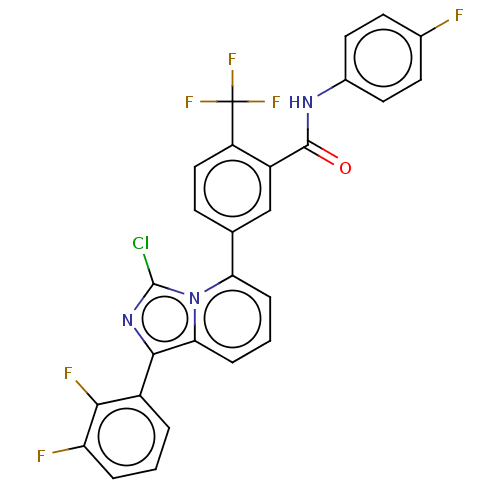 (5-(3-chloro-1-(2,3-difluorophenyl)imidazo[1,5- a]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure IDO1 inhibition in tissue culture, HeLa cells were treated with a test compound in the presence of IFNγ, which induces IDO1 expressio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50545147 (CHEMBL4642406) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate incubated f... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127038 BindingDB Entry DOI: 10.7270/Q2D79G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM526966 (N-(4-fluorophenyl)-5-(3-(pyridin-3- yl)imidazo[1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure IDO1 inhibition in tissue culture, HeLa cells were treated with a test compound in the presence of IFNγ, which induces IDO1 expressio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168399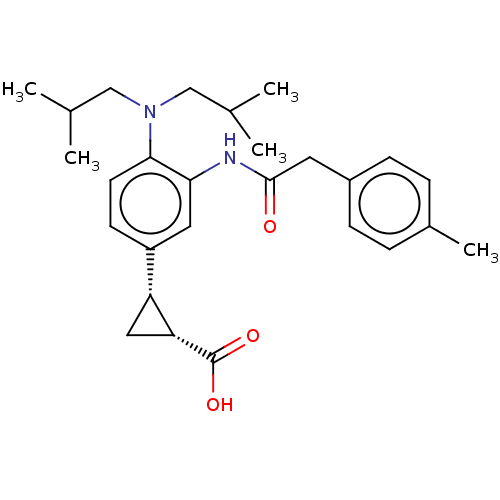 (US9675571, 60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | 25 |
SRMLSC US Patent | Assay Description Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... | US Patent US9675571 (2017) BindingDB Entry DOI: 10.7270/Q2BK19HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168413 (US9675571, 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | 25 |
SRMLSC US Patent | Assay Description Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... | US Patent US9675571 (2017) BindingDB Entry DOI: 10.7270/Q2BK19HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM526965 (5-(3-(2,3-difluorophenyl)imidazo[1,2- b]pyridazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure IDO1 inhibition in tissue culture, HeLa cells were treated with a test compound in the presence of IFNγ, which induces IDO1 expressio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50545148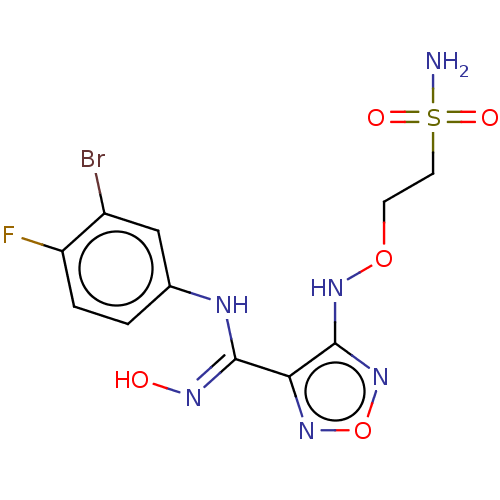 (CHEMBL4637369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate incubated f... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127038 BindingDB Entry DOI: 10.7270/Q2D79G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584087 (CHEMBL5093619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incu... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145159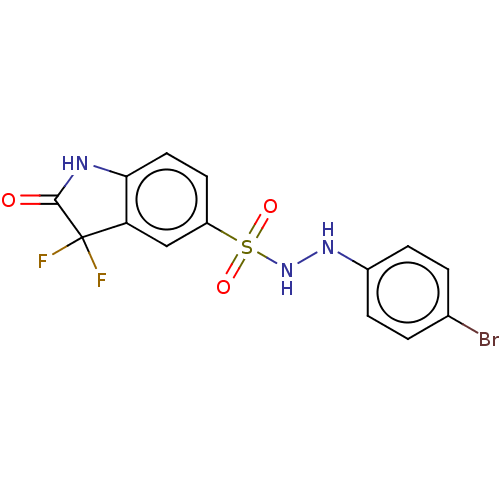 (CHEMBL3765205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584085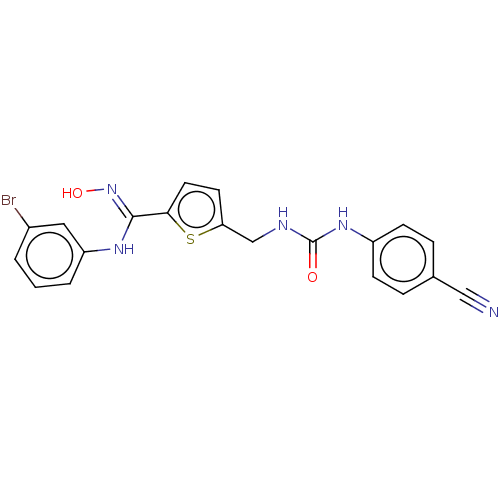 (CHEMBL5074542) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incu... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50545142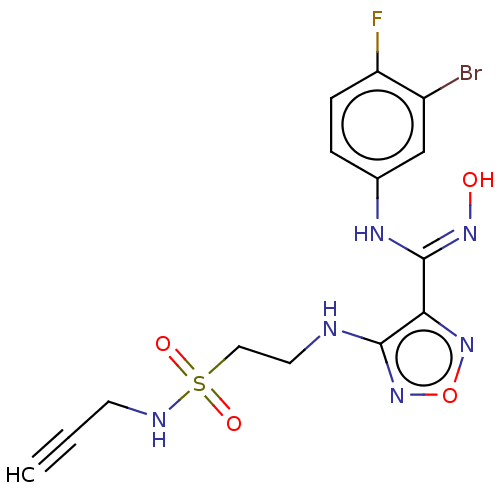 (CHEMBL4646789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate incubated f... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127038 BindingDB Entry DOI: 10.7270/Q2D79G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50145160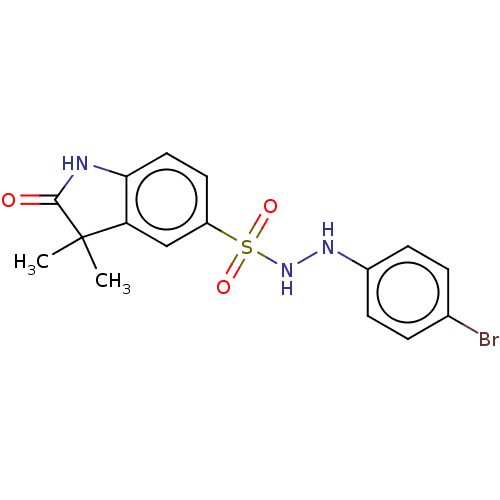 (CHEMBL3764846) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of Indoleamine 2,3-dioxygenase in human HeLa cells in presence of L-tryptophan as substrate after 24 hrs in presence of L-tryptophan | J Med Chem 59: 419-30 (2016) Article DOI: 10.1021/acs.jmedchem.5b01640 BindingDB Entry DOI: 10.7270/Q2FF3V69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM526964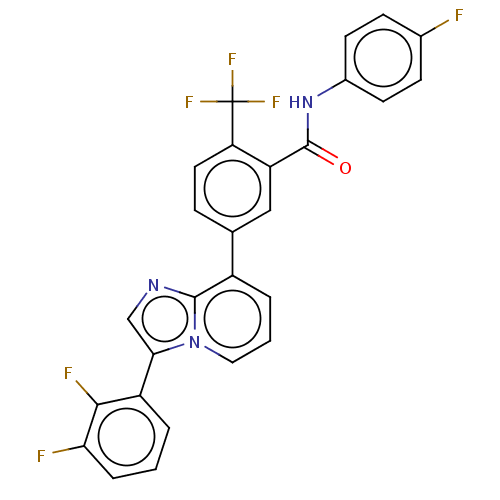 (5-(3-(2,3-difluorophenyl)imidazo[1,2-a]pyridin-8- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure IDO1 inhibition in tissue culture, HeLa cells were treated with a test compound in the presence of IFNγ, which induces IDO1 expressio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM526963 (5-(3-(2,3-difluorophenyl)-2- (hydroxymethyl)imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure IDO1 inhibition in tissue culture, HeLa cells were treated with a test compound in the presence of IFNγ, which induces IDO1 expressio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584084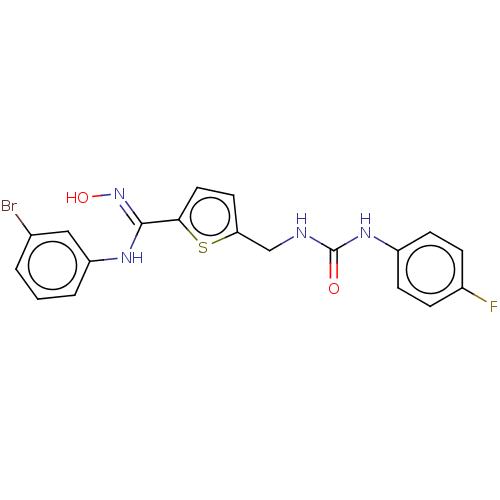 (CHEMBL5080101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incu... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50545140 (CHEMBL4644666 | US11591301, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate incubated f... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127038 BindingDB Entry DOI: 10.7270/Q2D79G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM526962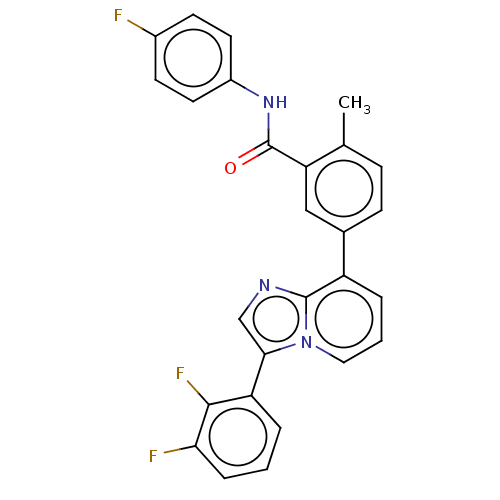 (5-(3-(2,3-difluorophenyl)imidazo[1,2-a]pyridin-8- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure IDO1 inhibition in tissue culture, HeLa cells were treated with a test compound in the presence of IFNγ, which induces IDO1 expressio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50203249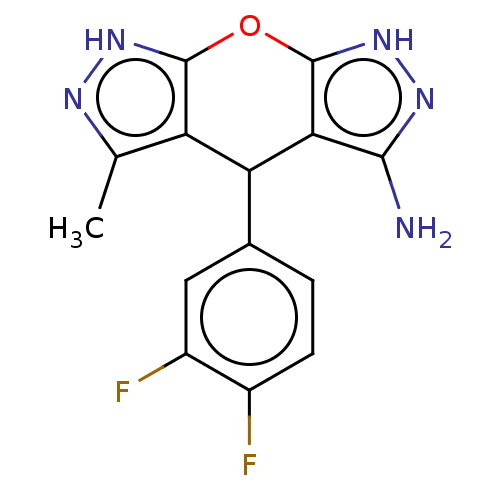 (CHEMBL3945146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
Indian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-induced human MDA-MB-231 cells using tryptophan as substrate preincubated for 4 hrs followed by substrate addition for... | ACS Med Chem Lett 7: 1167-1172 (2016) Article DOI: 10.1021/acsmedchemlett.6b00359 BindingDB Entry DOI: 10.7270/Q2TF009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584073 (CHEMBL5081219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incu... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50203269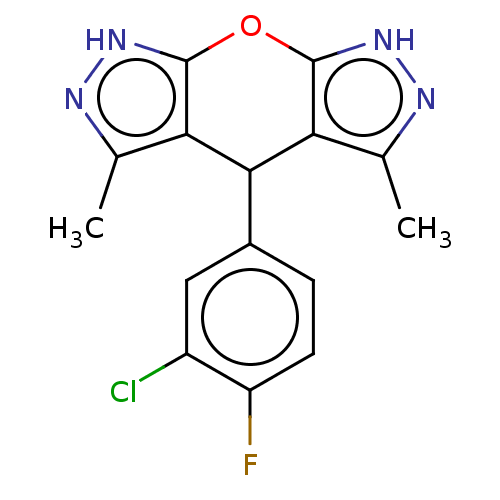 (CHEMBL3913434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Indian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-induced human MDA-MB-231 cells using tryptophan as substrate preincubated for 4 hrs followed by substrate addition for... | ACS Med Chem Lett 7: 1167-1172 (2016) Article DOI: 10.1021/acsmedchemlett.6b00359 BindingDB Entry DOI: 10.7270/Q2TF009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50203281 (CHEMBL3971895) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Indian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-induced human MDA-MB-231 cells using tryptophan as substrate preincubated for 4 hrs followed by substrate addition for... | ACS Med Chem Lett 7: 1167-1172 (2016) Article DOI: 10.1021/acsmedchemlett.6b00359 BindingDB Entry DOI: 10.7270/Q2TF009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50545136 (CHEMBL4639516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate incubated f... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127038 BindingDB Entry DOI: 10.7270/Q2D79G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584076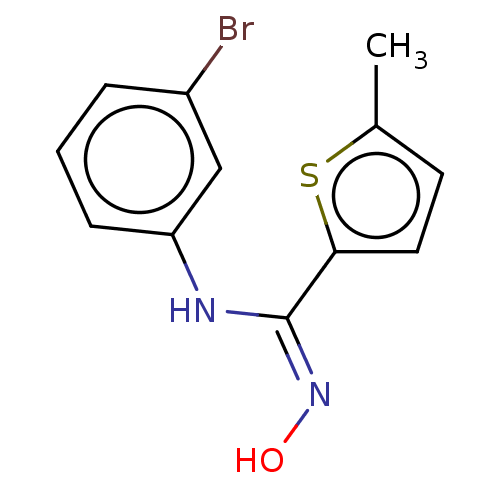 (CHEMBL5083059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incu... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50138819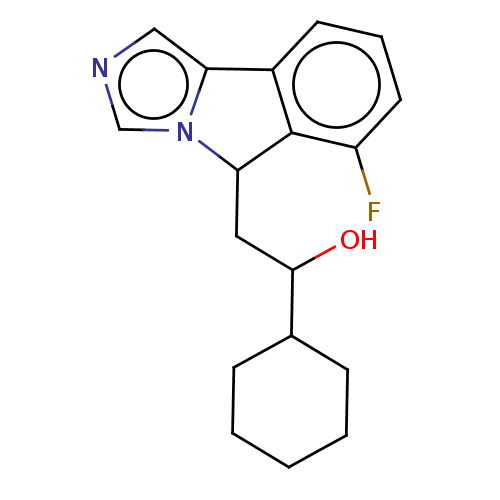 (CHEMBL3753837 | US10233190, Example 1357) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells incubated for 24 hrs | J Med Chem 59: 282-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01390 BindingDB Entry DOI: 10.7270/Q28917QW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM526961 (N-(4-fluorophenyl)-5-(3-(5-fluoropyridin-3- yl)imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure IDO1 inhibition in tissue culture, HeLa cells were treated with a test compound in the presence of IFNγ, which induces IDO1 expressio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM370526 (4-(1-hydroxy-2-(5H-imidazo[5,1-a]isoindol-5- yl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
NewLink Genetics Corporation Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO1 expressed in T-REx-293 cells assessed as reduction in kynurenine level measured after 16 hrs | J Med Chem 62: 6705-6733 (2019) Article DOI: 10.1021/acs.jmedchem.9b00662 BindingDB Entry DOI: 10.7270/Q21G0QNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM526960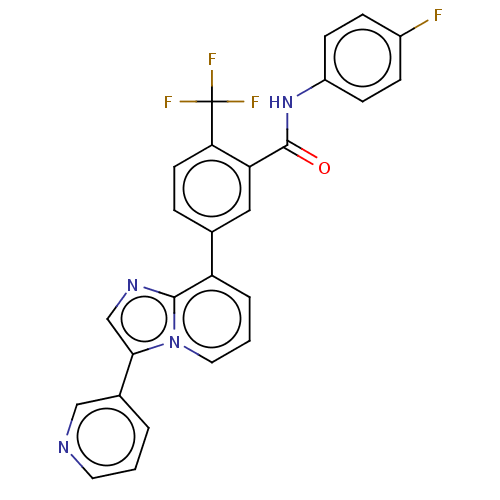 (N-(4-fluorophenyl)-5-(3-(pyridin-3- yl)imidazo[1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
TBA | Assay Description To measure IDO1 inhibition in tissue culture, HeLa cells were treated with a test compound in the presence of IFNγ, which induces IDO1 expressio... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JQ146J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584082 (CHEMBL5094486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incu... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50545135 (CHEMBL4645599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells assessed as reduction in kynurenine formation using L-tryptophan as substrate incubated f... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127038 BindingDB Entry DOI: 10.7270/Q2D79G0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300305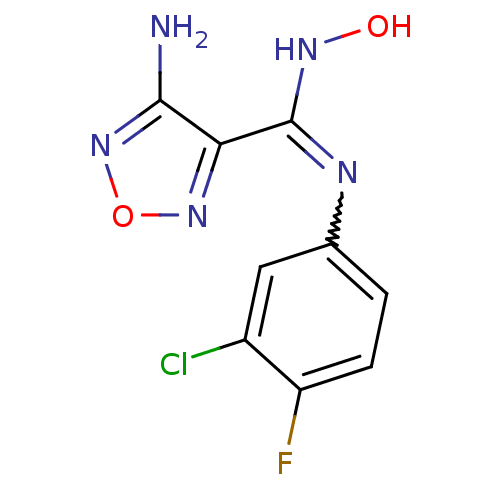 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
Indian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-induced human MDA-MB-231 cells using tryptophan as substrate preincubated for 4 hrs followed by substrate addition for... | ACS Med Chem Lett 7: 1167-1172 (2016) Article DOI: 10.1021/acsmedchemlett.6b00359 BindingDB Entry DOI: 10.7270/Q2TF009K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50584075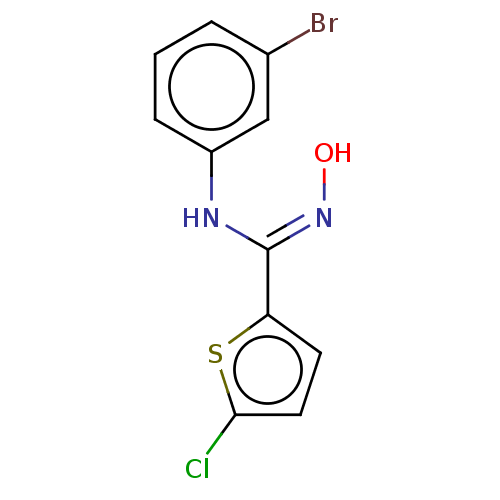 (CHEMBL5076745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incu... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114043 BindingDB Entry DOI: 10.7270/Q2SX6J39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126144 (CHEMBL3629569 | US10155972, Compound NewLink 1 | U...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells incubated for 24 hrs | J Med Chem 59: 282-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01390 BindingDB Entry DOI: 10.7270/Q28917QW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 311 total ) | Next | Last >> |