 Found 5731 hits of ic50 data for polymerid = 2230
Found 5731 hits of ic50 data for polymerid = 2230 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50442991

(CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...)Show SMILES [O-][N+](=O)c1ccc-2c(c1)C(=O)c1nc3ccccc3c(=O)n-21 Show InChI InChI=1S/C15H7N3O4/c19-13-10-7-8(18(21)22)5-6-12(10)17-14(13)16-11-4-2-1-3-9(11)15(17)20/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 expressed in HEK293 cells assessed as kynurenine release after 5 hrs by spectrophotometry |
J Med Chem 56: 8321-31 (2013)
Article DOI: 10.1021/jm401195n
BindingDB Entry DOI: 10.7270/Q2X34ZWS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021
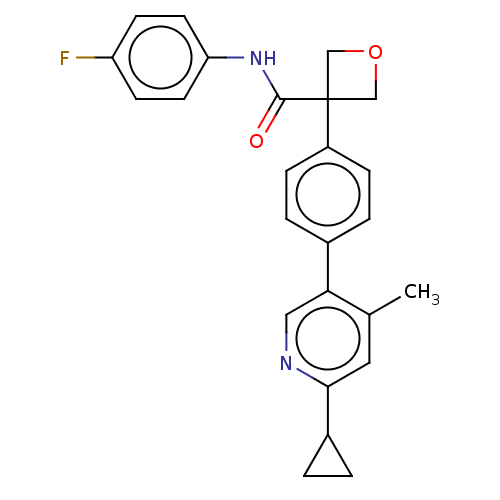
(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503
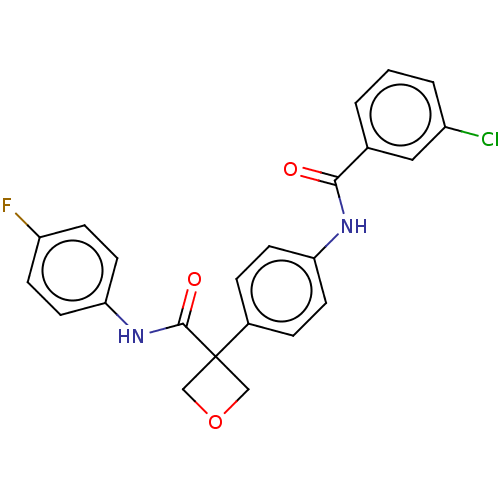
(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567132
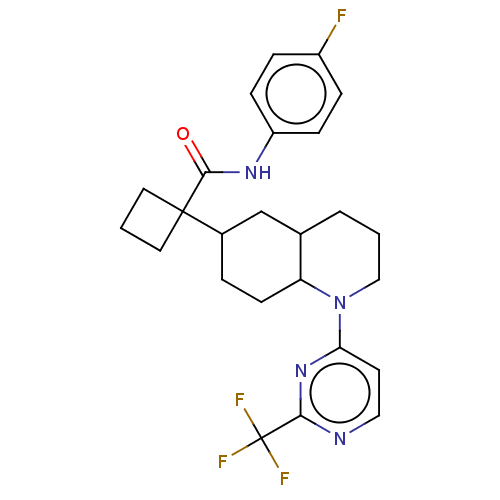
(CHEMBL4863767)Show SMILES Fc1ccc(NC(=O)C2(CCC2)C2CCC3C(CCCN3c3ccnc(n3)C(F)(F)F)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM309529

(3-(5-fluoro-1H-indol-3- yl)pyrrolidine-2,5-dione |...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114524
BindingDB Entry DOI: 10.7270/Q2PZ5DW0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567128
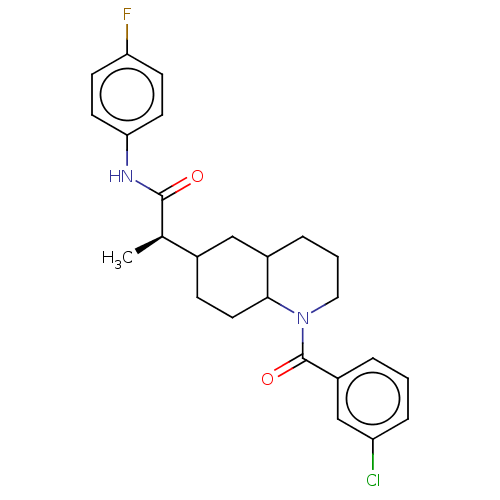
(CHEMBL4855797)Show SMILES C[C@H](C1CCC2C(CCCN2C(=O)c2cccc(Cl)c2)C1)C(=O)Nc1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567131

(CHEMBL4848630)Show SMILES Cc1nccc(n1)N1CCCC2CC(CCC12)C1(CCC1)C(=O)Nc1ccc(F)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568395

(CHEMBL4848098)Show SMILES Clc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3n2)c2ccccn2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568383

(CHEMBL4872023)Show SMILES Fc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3c2)C(=O)OC2CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50590772
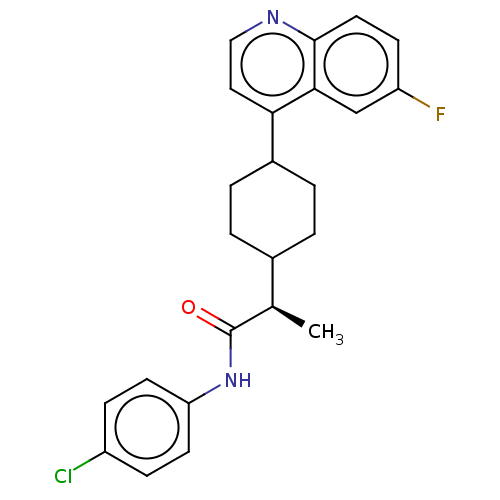
(BMS 986205 | BMS-986205 | Bms-986205 | F 001287 | ...)Show SMILES C[C@H](C1CCC(CC1)c1ccnc2ccc(F)cc12)C(=O)Nc1ccc(Cl)cc1 |r,wD:1.0,(-6.99,-1.78,;-6.99,-.24,;-8.32,.53,;-9.66,-.24,;-10.99,.53,;-10.99,2.07,;-9.66,2.84,;-8.32,2.07,;-12.35,2.8,;-12.35,4.34,;-13.68,5.11,;-15.02,4.34,;-15.02,2.8,;-16.35,2.03,;-16.35,.49,;-15.02,-.28,;-15.01,-1.82,;-13.68,.49,;-13.68,2.03,;-5.66,.53,;-5.66,2.07,;-4.32,-.24,;-2.99,.53,;-2.99,2.07,;-1.66,2.84,;-.32,2.07,;1.05,2.8,;-.32,.53,;-1.66,-.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114625
BindingDB Entry DOI: 10.7270/Q2JW8JVD |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM168435
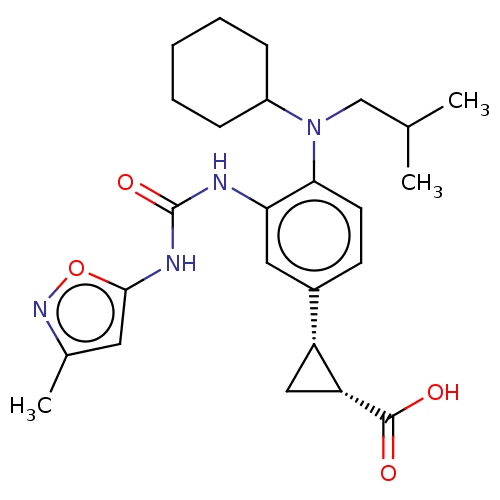
(US9675571, 129)Show SMILES CC(C)CN(C1CCCCC1)c1ccc(cc1NC(=O)Nc1cc(C)no1)[C@H]1C[C@H]1C(O)=O |r| Show InChI InChI=1S/C25H34N4O4/c1-15(2)14-29(18-7-5-4-6-8-18)22-10-9-17(19-13-20(19)24(30)31)12-21(22)26-25(32)27-23-11-16(3)28-33-23/h9-12,15,18-20H,4-8,13-14H2,1-3H3,(H,30,31)(H2,26,27,32)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 expressed in HEK293 cells incubated for 20 mins by Ehrlich's reagent based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00925
BindingDB Entry DOI: 10.7270/Q28D010N |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416
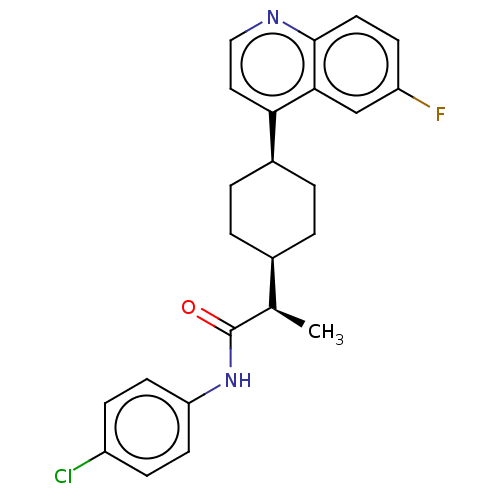
(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113967
BindingDB Entry DOI: 10.7270/Q26W9G4F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416

(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells preincubated for 1 hr followed by IFN-gamma stimulation and measured after 20 hrs by Ehrlich colorimetric reag... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00925
BindingDB Entry DOI: 10.7270/Q28D010N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM168435

(US9675571, 129)Show SMILES CC(C)CN(C1CCCCC1)c1ccc(cc1NC(=O)Nc1cc(C)no1)[C@H]1C[C@H]1C(O)=O |r| Show InChI InChI=1S/C25H34N4O4/c1-15(2)14-29(18-7-5-4-6-8-18)22-10-9-17(19-13-20(19)24(30)31)12-21(22)26-25(32)27-23-11-16(3)28-33-23/h9-12,15,18-20H,4-8,13-14H2,1-3H3,(H,30,31)(H2,26,27,32)/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416

(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) by cell-based assay |
J Med Chem 62: 9161-9174 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01079
BindingDB Entry DOI: 10.7270/Q27W6GJ0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50575244

(CHEMBL4851857)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)n(CC(=O)N2CCN(CC2)c2cccc(C)c2C)nc1C(=O)N1CCC(CC1)OCCO |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells incubated for 48 hrs by fluorescence microplate reader assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00531
BindingDB Entry DOI: 10.7270/Q2W66QKB |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50575243

(CHEMBL4851825)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)n(CC(=O)N2CCN(CC2)c2cccc(C)c2C)nc1C(=O)N1CCC(=O)CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells incubated for 48 hrs by fluorescence microplate reader assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00531
BindingDB Entry DOI: 10.7270/Q2W66QKB |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50575245

(CHEMBL4862164)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)n(CC(=O)N2CCN(CC2)c2cccc(C)c2C)nc1C(=O)N1CCC2(CO)CC1C2 |r,wD:3.4,1.0,(39.86,-6.8,;41.4,-6.81,;41.25,-5.28,;42.65,-5.91,;43.04,-4.42,;43.89,-6.81,;43.41,-8.28,;41.87,-8.27,;44.66,-9.18,;44.66,-10.72,;45.99,-11.49,;47.33,-10.72,;45.99,-13.03,;44.66,-13.81,;44.66,-15.35,;45.99,-16.11,;47.32,-15.35,;47.32,-13.81,;45.99,-17.65,;44.66,-18.42,;44.66,-19.96,;45.99,-20.73,;47.33,-19.95,;48.66,-20.71,;47.32,-18.41,;48.65,-17.64,;45.9,-8.28,;45.43,-6.81,;46.33,-5.56,;45.7,-4.16,;47.86,-5.72,;48.49,-7.14,;50.01,-7.3,;50.92,-6.06,;52.45,-6.28,;53.02,-7.7,;50.3,-4.65,;48.76,-4.48,;49.52,-5.8,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells incubated for 48 hrs by fluorescence microplate reader assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00531
BindingDB Entry DOI: 10.7270/Q2W66QKB |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506627

(US11046649, Ex. 5)Show SMILES Fc1cc2ncn([C@@H]3C[C@H]4[C@H](CNC(c5ccc(Cl)cc5)C(F)(F)F)[C@H]4C3)c2cc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00679
BindingDB Entry DOI: 10.7270/Q2RJ4P9S |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50575248

(CHEMBL4861457)Show SMILES CC(=O)NC1CCN(CC1)C(=O)c1nn(CC(=O)N2CCN(CC2)c2cccc(C)c2Cl)c2CC3CCCCC3c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells incubated for 48 hrs by fluorescence microplate reader assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00531
BindingDB Entry DOI: 10.7270/Q2W66QKB |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50575246

(CHEMBL4879235)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)n(CC(=O)N2CCN(CC2)c2cccc(C)c2C)nc1C(=O)N1CC[C@@H](O)[C@H](F)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells incubated for 48 hrs by fluorescence microplate reader assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00531
BindingDB Entry DOI: 10.7270/Q2W66QKB |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50578624
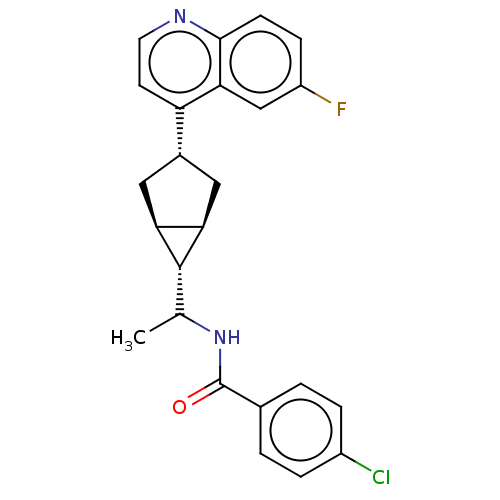
(CHEMBL4856710)Show SMILES [H][C@@]12C[C@H](C[C@]1([H])[C@H]2C(C)NC(=O)c1ccc(Cl)cc1)c1ccnc2ccc(F)cc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00679
BindingDB Entry DOI: 10.7270/Q2RJ4P9S |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50543295
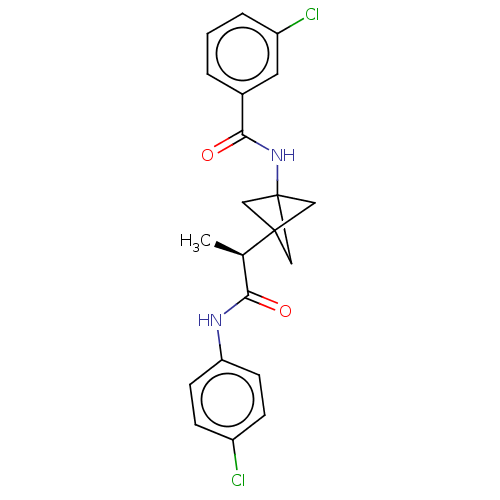
(CHEMBL4642946)Show SMILES C[C@H](C(=O)Nc1ccc(Cl)cc1)C12CC(C1)(C2)NC(=O)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C21H20Cl2N2O2/c1-13(18(26)24-17-7-5-15(22)6-8-17)20-10-21(11-20,12-20)25-19(27)14-3-2-4-16(23)9-14/h2-9,13H,10-12H2,1H3,(H,24,26)(H,25,27)/t13-,20?,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells using L-tryptophan as substrate incubated for 48 hrs by fluorescence assay |
ACS Med Chem Lett 11: 1548-1554 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00195
BindingDB Entry DOI: 10.7270/Q2RB786J |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506733

(4-(((1R,3s,5S,6r)-6-(1-(5- chloro-1H-benzo[d]- imi...)Show SMILES CC([C@@H]1[C@H]2C[C@@H](C[C@@H]12)Oc1ccnc2ccc(F)cc12)c1nc2cc(Cl)ccc2[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568397
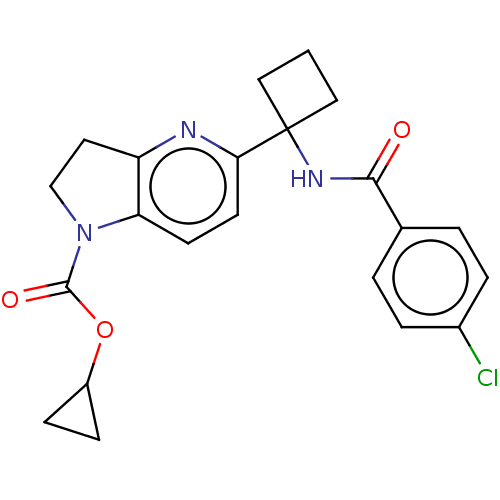
(CHEMBL4869600)Show SMILES Clc1ccc(cc1)C(=O)NC1(CCC1)c1ccc2N(CCc2n1)C(=O)OC1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50575247

(CHEMBL4867998)Show SMILES [H][C@]12C[C@@]1([H])c1c(C2)n(CC(=O)N2CCN(CC22CC2)c2ccc(F)c(C)c2C)nc1C(=O)N1CCC(O)C(CO)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells incubated for 48 hrs by fluorescence microplate reader assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00531
BindingDB Entry DOI: 10.7270/Q2W66QKB |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506769
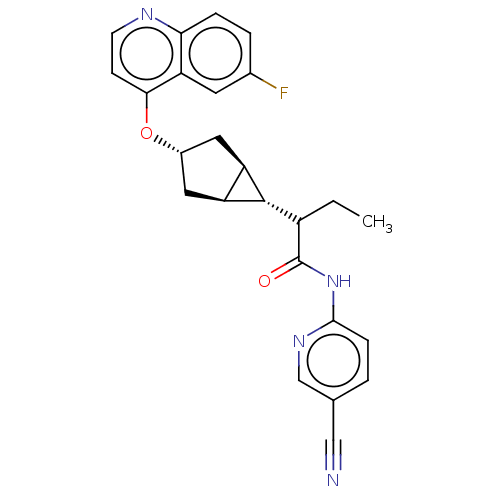
(N-(5-cyanopyridin-2-yl)-2- ((1R,3s,5S,6r)-3-((6- f...)Show SMILES CCC([C@@H]1[C@H]2C[C@@H](C[C@@H]12)Oc1ccnc2ccc(F)cc12)C(=O)Nc1ccc(cn1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506768

(N-(5-cyanopyrazin-2-yl)-2- ((1R,3s,5S,6r)-3-((6- f...)Show SMILES CCC([C@@H]1[C@H]2C[C@@H](C[C@@H]12)Oc1ccnc2ccc(F)cc12)C(=O)Nc1cnc(cn1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50578623
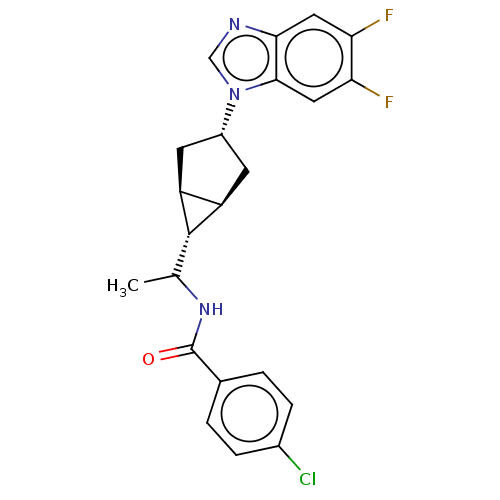
(CHEMBL4858888)Show SMILES [H][C@@]12C[C@H](C[C@]1([H])[C@H]2C(C)NC(=O)c1ccc(Cl)cc1)n1cnc2cc(F)c(F)cc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00679
BindingDB Entry DOI: 10.7270/Q2RJ4P9S |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50573448
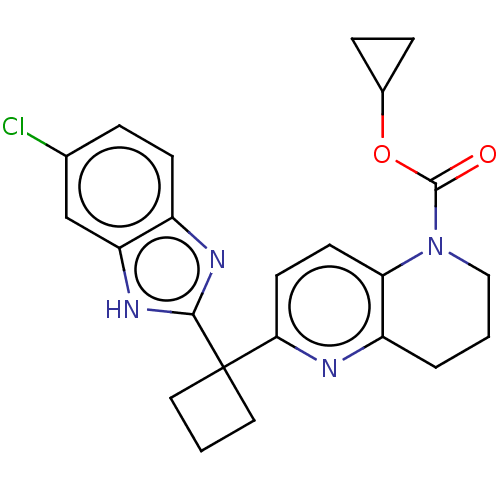
(CHEMBL4862659)Show SMILES Clc1ccc2nc([nH]c2c1)C1(CCC1)c1ccc2N(CCCc2n1)C(=O)OC1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.693 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma induced human HeLa cells measured after 48 hrs by fluorescence assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00469
BindingDB Entry DOI: 10.7270/Q2Q52TDX |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM317549
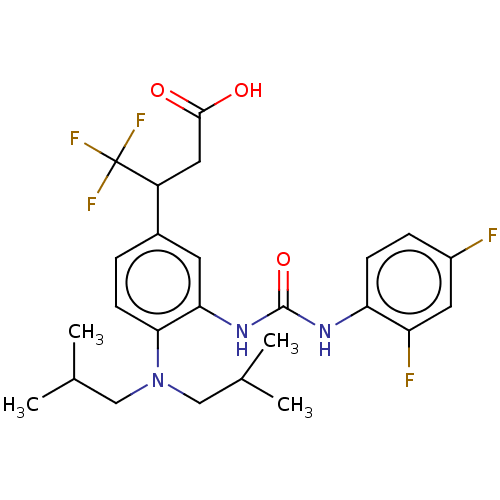
(3-(3-(3-(2,4- difluorophenyl)ureido)-4- (diisobuty...)Show SMILES CC(C)CN(CC(C)C)c1ccc(cc1NC(=O)Nc1ccc(F)cc1F)C(CC(O)=O)C(F)(F)F Show InChI InChI=1S/C25H30F5N3O3/c1-14(2)12-33(13-15(3)4)22-8-5-16(18(11-23(34)35)25(28,29)30)9-21(22)32-24(36)31-20-7-6-17(26)10-19(20)27/h5-10,14-15,18H,11-13H2,1-4H3,(H,34,35)(H2,31,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Human IDO1/HEK293 cells were seeded at 10,000 cells per 50 uL per well with RPMI/phenol red free media contains 10% FBS in a 384-well black wall clea... |
US Patent US9624188 (2017)
BindingDB Entry DOI: 10.7270/Q2GF0WKT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567134

(CHEMBL4848366)Show SMILES CC(N1CCC2C(CCCN2C(=O)OC2CC2)C1)C(=O)Nc1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506721

(N-(4-fluorophenyl)-2- ((1R,3s,5S,6r)-3-((6- fluoro...)Show SMILES CC([C@@H]1[C@H]2C[C@@H](C[C@@H]12)Oc1ccnc2ccc(F)cc12)C(=O)Nc1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506767
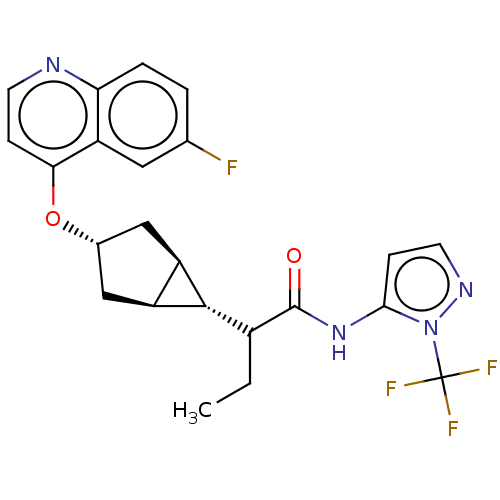
(2-((1R,3s,5S,6r)-3-((6- fluoroquinolin-4-yl)oxy)- ...)Show SMILES CCC([C@@H]1[C@H]2C[C@@H](C[C@@H]12)Oc1ccnc2ccc(F)cc12)C(=O)Nc1ccnn1C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604025
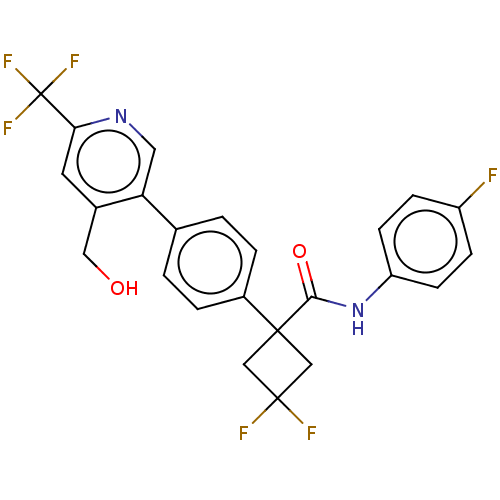
(CHEMBL5173861)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CC(F)(F)C1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552941

(CHEMBL4764710)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1CCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506758
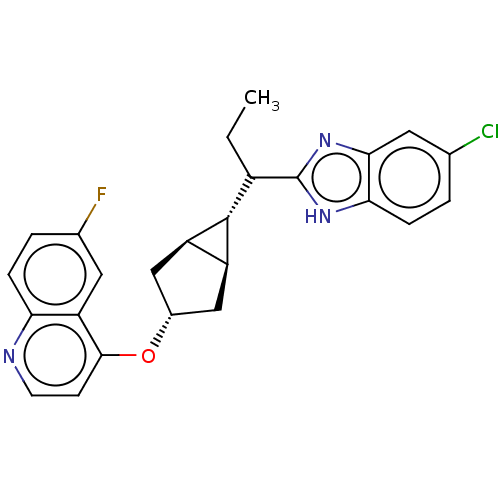
(4-(((1R,3s,5S,6r)-6-(1-(5- chloro-1H-benzo[d]- imi...)Show SMILES CCC([C@@H]1[C@H]2C[C@@H](C[C@@H]12)Oc1ccnc2ccc(F)cc12)c1nc2cc(Cl)ccc2[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506730

(N-(4-chlorophenyl)-2- cyclopropyl-2- ((1R,3s,5S,6r...)Show SMILES Fc1ccc2nccc(O[C@H]3C[C@H]4[C@@H](C3)[C@@H]4C(C3CC3)C(=O)Nc3ccc(Cl)cc3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568391
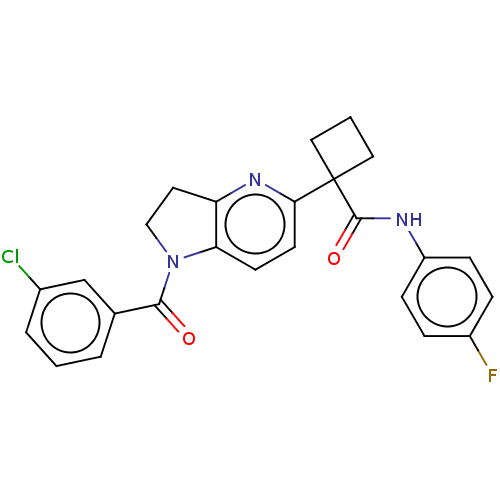
(CHEMBL4855740)Show SMILES Fc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3n2)C(=O)c2cccc(Cl)c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506634

(US11046649, Ex. 12)Show SMILES CCC([C@@H]1[C@H]2C[C@@H](C[C@@H]12)Oc1ccnc2ccc(F)cc12)c1nc2cc(ccc2[nH]1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567130

(CHEMBL4858599)Show SMILES Fc1ccc(NC(=O)C2(CCC2)C2CCC3C(CCCN3C(=O)C3CC3)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506736
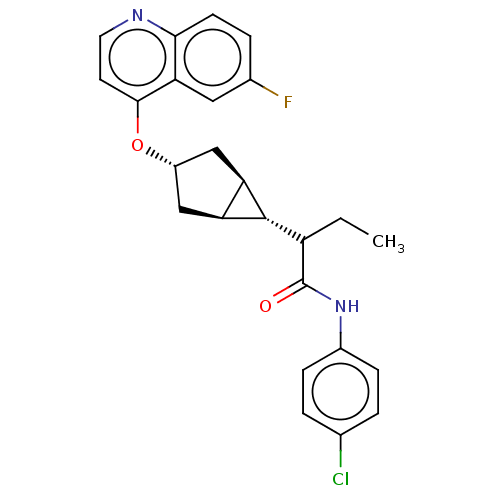
(N-(4-chlorophenyl)-2- ((1R,3s,5S,6r)-3-((6- fluoro...)Show SMILES CCC([C@@H]1[C@H]2C[C@@H](C[C@@H]12)Oc1ccnc2ccc(F)cc12)C(=O)Nc1ccc(Cl)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50578677

(CHEMBL4849690)Show SMILES [H][C@@]12C[C@H](C[C@]1([H])[C@H]2C(CC)c1nc2cc(Cl)ccc2[nH]1)c1ccnc2ccc(F)cc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human HeLa cells using tryptophan as substrate incubated for 24 hrs by RFMS assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00679
BindingDB Entry DOI: 10.7270/Q2RJ4P9S |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506731

(2-cyclopropyl-2- ((1R,3s,5S,6r)-3-((6- fluoroquino...)Show SMILES Fc1ccc2nccc(O[C@H]3C[C@H]4[C@@H](C3)[C@@H]4C(C3CC3)C(=O)Nc3cc([nH]n3)C(F)(F)F)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50573449

(CHEMBL4848644)Show SMILES FC(F)(F)c1cnc2nc([nH]c2c1)C1(CCC1)c1ccc2N(CCCc2n1)C(=O)c1ccccc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.958 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma induced human HeLa cells measured after 48 hrs by fluorescence assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00469
BindingDB Entry DOI: 10.7270/Q2Q52TDX |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506770

(N-(1-(difluoromethyl)-1H- pyrazol-4-yl)-2- ((1R,3s...)Show SMILES CCC([C@@H]1[C@H]2C[C@@H](C[C@@H]12)Oc1ccnc2ccc(F)cc12)C(=O)Nc1cnn(c1)C(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM506737

(2-((1R,3s,5S,6r)-3-((6- fluoroquinolin-4-yl)oxy)- ...)Show SMILES CCC([C@@H]1[C@H]2C[C@@H](C[C@@H]12)Oc1ccnc2ccc(F)cc12)C(=O)Nc1cc([nH]n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
HeLa cells were obtained from the American Type Culture Collection (ATCC) and maintained in DMEM media containing 10% FBS. Cells (7,000/well) were se... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2474F05 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50567129
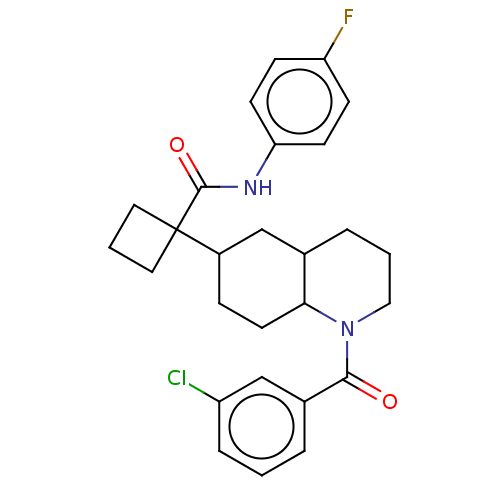
(CHEMBL4849622)Show SMILES Fc1ccc(NC(=O)C2(CCC2)C2CCC3C(CCCN3C(=O)c3cccc(Cl)c3)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in human whole blood assessed as unbound concentration |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128314
BindingDB Entry DOI: 10.7270/Q2TT4VP5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50573452
(CHEMBL4865877)Show SMILES C[C@H](c1nc2ccc(Cl)cc2[nH]1)c1ccc2N(CCCc2n1)C(=O)OC1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.982 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma induced human HeLa cells measured after 48 hrs by fluorescence assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00469
BindingDB Entry DOI: 10.7270/Q2Q52TDX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data