 Found 143 hits of ic50 for UniProtKB: P00491
Found 143 hits of ic50 for UniProtKB: P00491 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046227
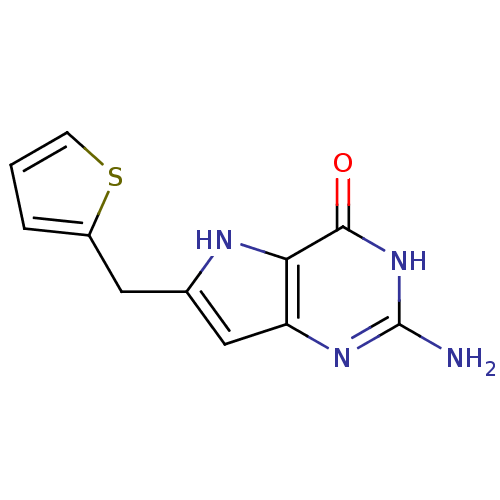
(2-Amino-6-thiophen-2-ylmethyl-3,7-dihydro-pyrrolo[...)Show InChI InChI=1S/C11H10N4OS/c12-11-14-8-5-6(4-7-2-1-3-17-7)13-9(8)10(16)15-11/h1-3,5,13H,4H2,(H3,12,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046231
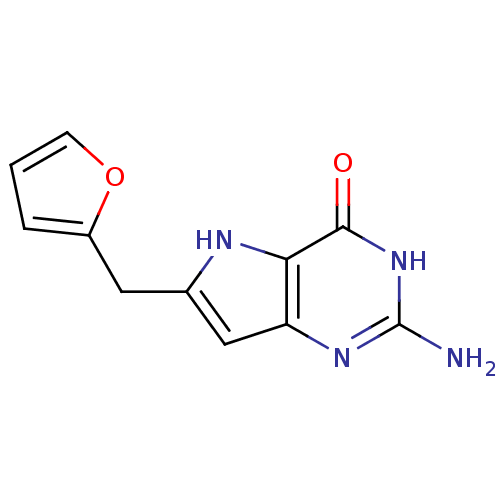
(2-Amino-6-furan-2-ylmethyl-3,7-dihydro-pyrrolo[3,2...)Show InChI InChI=1S/C11H10N4O2/c12-11-14-8-5-6(4-7-2-1-3-17-7)13-9(8)10(16)15-11/h1-3,5,13H,4H2,(H3,12,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046249
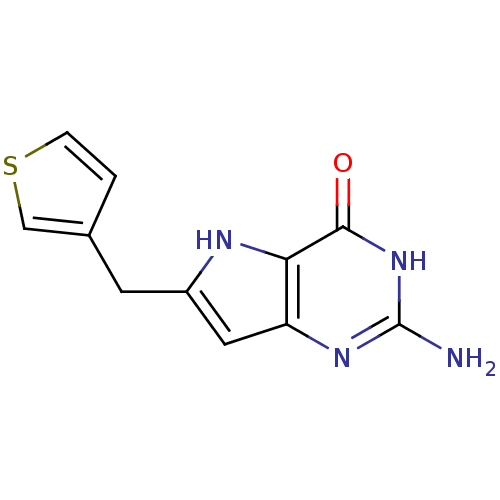
(2-Amino-6-thiophen-3-ylmethyl-3,7-dihydro-pyrrolo[...)Show InChI InChI=1S/C11H10N4OS/c12-11-14-8-4-7(3-6-1-2-17-5-6)13-9(8)10(16)15-11/h1-2,4-5,13H,3H2,(H3,12,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046252
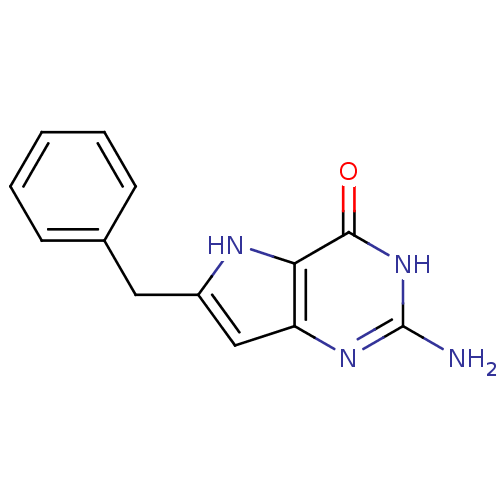
(2-Amino-6-benzyl-3,7-dihydro-pyrrolo[3,2-d]pyrimid...)Show InChI InChI=1S/C13H12N4O/c14-13-16-10-7-9(15-11(10)12(18)17-13)6-8-4-2-1-3-5-8/h1-5,7,15H,6H2,(H3,14,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046224
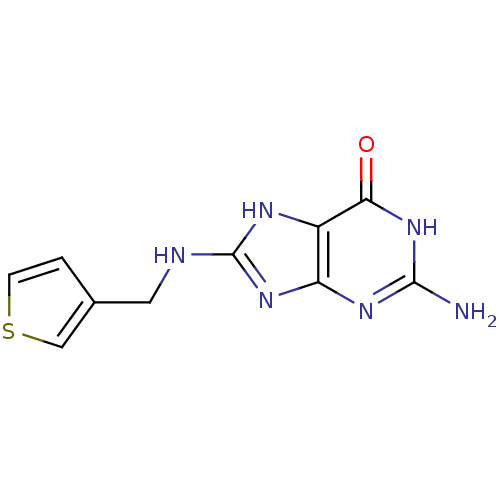
(2-Amino-8-[(thiophen-3-ylmethyl)-amino]-1,9-dihydr...)Show InChI InChI=1S/C10H10N6OS/c11-9-14-7-6(8(17)16-9)13-10(15-7)12-3-5-1-2-18-4-5/h1-2,4H,3H2,(H5,11,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046246
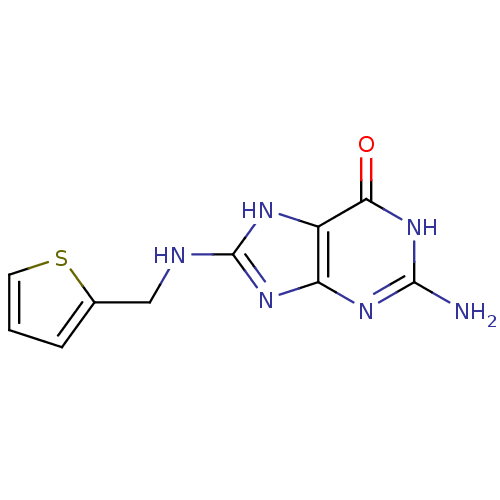
(2-Amino-8-[(thiophen-2-ylmethyl)-amino]-1,9-dihydr...)Show InChI InChI=1S/C10H10N6OS/c11-9-14-7-6(8(17)16-9)13-10(15-7)12-4-5-2-1-3-18-5/h1-3H,4H2,(H5,11,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046241

(2-Amino-8-[(furan-2-ylmethyl)-amino]-1,9-dihydro-p...)Show InChI InChI=1S/C10H10N6O2/c11-9-14-7-6(8(17)16-9)13-10(15-7)12-4-5-2-1-3-18-5/h1-3H,4H2,(H5,11,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046251
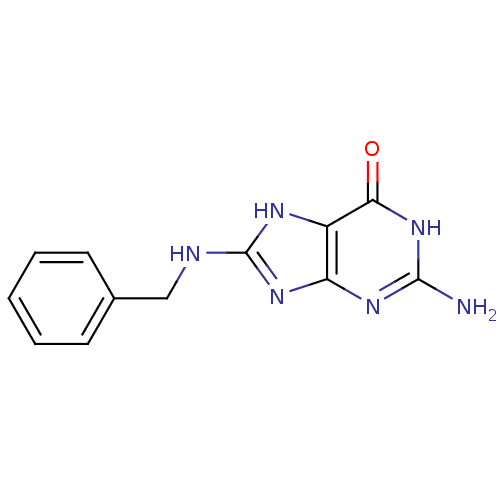
(2-Amino-8-benzylamino-1,9-dihydro-purin-6-one | CH...)Show InChI InChI=1S/C12H12N6O/c13-11-16-9-8(10(19)18-11)15-12(17-9)14-6-7-4-2-1-3-5-7/h1-5H,6H2,(H5,13,14,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046221
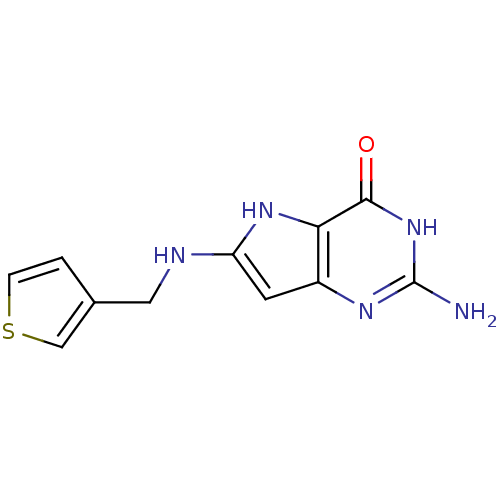
(2-Amino-6-[(thiophen-3-ylmethyl)-amino]-3,7-dihydr...)Show InChI InChI=1S/C11H11N5OS/c12-11-14-7-3-8(15-9(7)10(17)16-11)13-4-6-1-2-18-5-6/h1-3,5,13,15H,4H2,(H3,12,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046216

(2-Amino-6-[(furan-2-ylmethyl)-amino]-3,7-dihydro-p...)Show InChI InChI=1S/C11H11N5O2/c12-11-14-7-4-8(15-9(7)10(17)16-11)13-5-6-2-1-3-18-6/h1-4,13,15H,5H2,(H3,12,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046223

(2-Amino-6-benzylamino-3,7-dihydro-pyrrolo[3,2-d]py...)Show InChI InChI=1S/C13H13N5O/c14-13-16-9-6-10(17-11(9)12(19)18-13)15-7-8-4-2-1-3-5-8/h1-6,15,17H,7H2,(H3,14,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | CHEMBL5282677

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | CHEMBL5273669
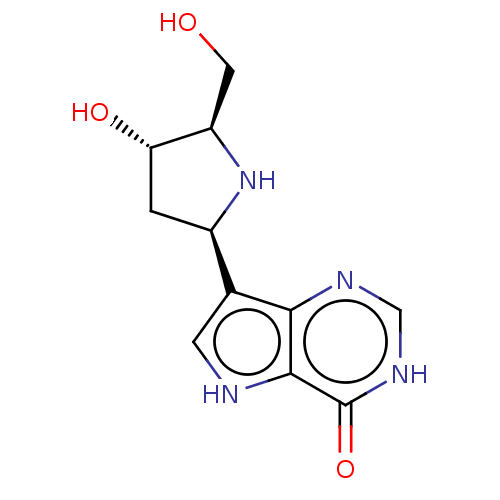
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046243

(2-Amino-6-[(thiophen-2-ylmethyl)-amino]-3,7-dihydr...)Show InChI InChI=1S/C11H11N5OS/c12-11-14-7-4-8(15-9(7)10(17)16-11)13-5-6-2-1-3-18-6/h1-4,13,15H,5H2,(H3,12,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | CHEMBL5280395
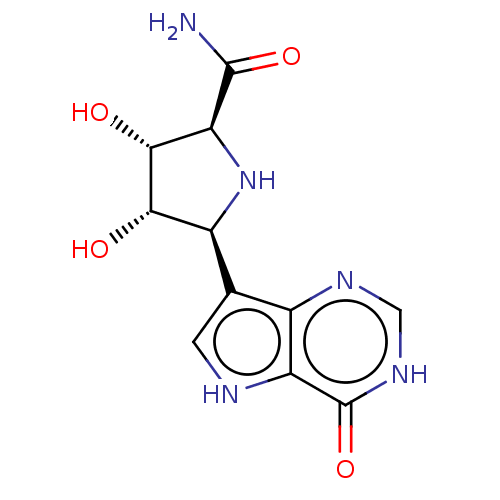
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195589
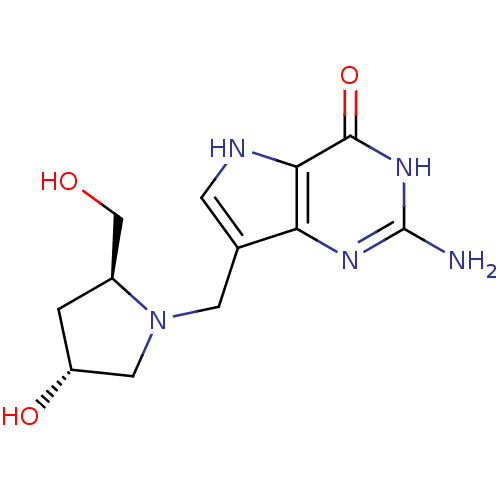
(2-amino-1,5-dihydro-7-[[(2S,4R)-2-(hydroxymethyl)-...)Show SMILES Nc1nc2c(CN3C[C@H](O)C[C@H]3CO)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C12H17N5O3/c13-12-15-9-6(2-14-10(9)11(20)16-12)3-17-4-8(19)1-7(17)5-18/h2,7-8,14,18-19H,1,3-5H2,(H3,13,15,16,20)/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human PNP activity |
J Med Chem 49: 6037-45 (2006)
Article DOI: 10.1021/jm060547+
BindingDB Entry DOI: 10.7270/Q2NV9K11 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195587

(1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...)Show SMILES OC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H14N4O4/c16-2-5-9(17)10(18)7(15-5)4-1-12-8-6(4)13-3-14-11(8)19/h1,3,5,7,9-10,12,15-18H,2H2,(H,13,14,19)/t5-,7+,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human PNP activity |
J Med Chem 49: 6037-45 (2006)
Article DOI: 10.1021/jm060547+
BindingDB Entry DOI: 10.7270/Q2NV9K11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195593
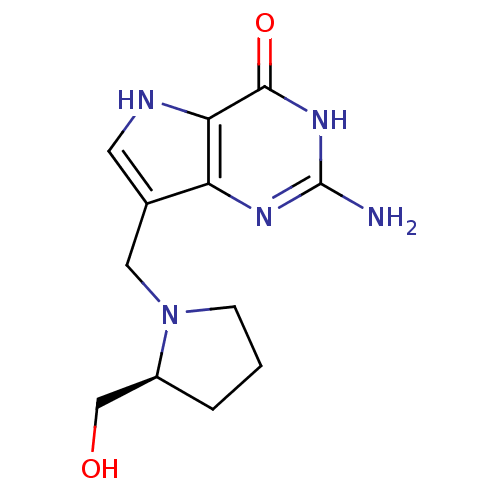
(2-amino-1,5-dihydro-7-[[(2S)-2-(hydroxymethyl)-1-p...)Show InChI InChI=1S/C12H17N5O2/c13-12-15-9-7(4-14-10(9)11(19)16-12)5-17-3-1-2-8(17)6-18/h4,8,14,18H,1-3,5-6H2,(H3,13,15,16,19)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human PNP activity |
J Med Chem 49: 6037-45 (2006)
Article DOI: 10.1021/jm060547+
BindingDB Entry DOI: 10.7270/Q2NV9K11 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046248

(2-Amino-8-[(3-methyl-thiophen-2-ylmethyl)-amino]-1...)Show InChI InChI=1S/C11H12N6OS/c1-5-2-3-19-6(5)4-13-11-14-7-8(16-11)15-10(12)17-9(7)18/h2-3H,4H2,1H3,(H5,12,13,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046218

(2-Amino-6-[(3-methyl-thiophen-2-ylmethyl)-amino]-3...)Show InChI InChI=1S/C12H13N5OS/c1-6-2-3-19-8(6)5-14-9-4-7-10(16-9)11(18)17-12(13)15-7/h2-4,14,16H,5H2,1H3,(H3,13,15,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | CHEMBL5290077
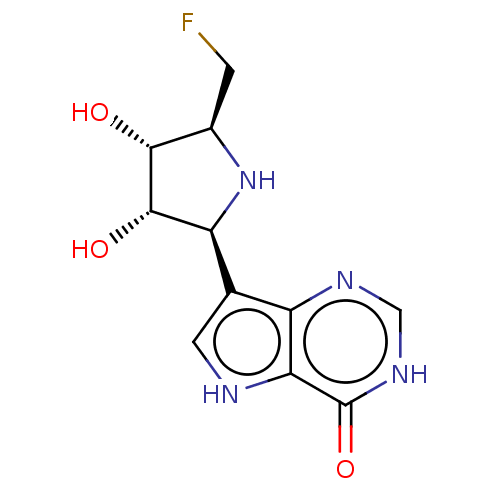
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195597
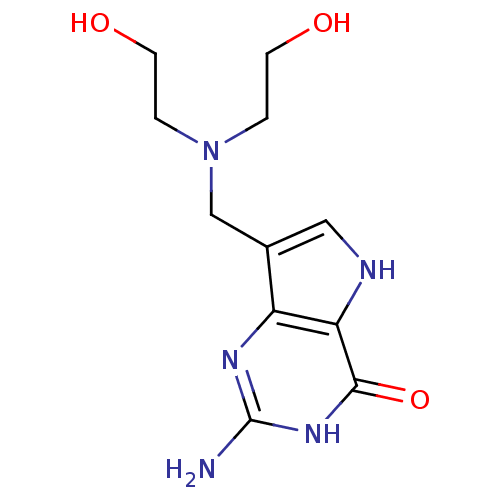
(2-amino-1,5-dihydro-7-[[N,N-bis-[2-(hydroxy)ethyl]...)Show InChI InChI=1S/C11H17N5O3/c12-11-14-8-7(5-13-9(8)10(19)15-11)6-16(1-3-17)2-4-18/h5,13,17-18H,1-4,6H2,(H3,12,14,15,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human PNP activity |
J Med Chem 49: 6037-45 (2006)
Article DOI: 10.1021/jm060547+
BindingDB Entry DOI: 10.7270/Q2NV9K11 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50078452

((S)-3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H](CC(O)=O)c1cccc(Cl)c1 Show InChI InChI=1S/C15H13ClN4O3/c16-8-3-1-2-7(4-8)9(5-11(21)22)10-6-18-13-12(10)19-15(17)20-14(13)23/h1-4,6,9,18H,5H2,(H,21,22)(H3,17,19,20,23)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside Phosphorylase from calf spleen at 1 mM PO4 |
J Med Chem 36: 3771-83 (1994)
BindingDB Entry DOI: 10.7270/Q23J3DKS |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50308521
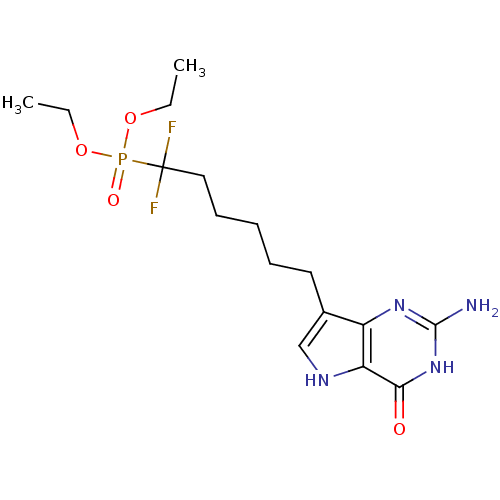
(9-[6',6'-Difluoro-6'-(diethylphosphono)hexyl]-9-de...)Show SMILES CCOP(=O)(OCC)C(F)(F)CCCCCc1c[nH]c2c1nc(N)[nH]c2=O Show InChI InChI=1S/C16H25F2N4O4P/c1-3-25-27(24,26-4-2)16(17,18)9-7-5-6-8-11-10-20-13-12(11)21-15(19)22-14(13)23/h10,20H,3-9H2,1-2H3,(H3,19,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte PNP assessed as inhibition of 25 uM 7-methylguanosine phosphorolysis by spectrophotometry in presence of 1 mM inorgan... |
Bioorg Med Chem 18: 2275-84 (2010)
Article DOI: 10.1016/j.bmc.2010.01.062
BindingDB Entry DOI: 10.7270/Q2N58MG0 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50214708
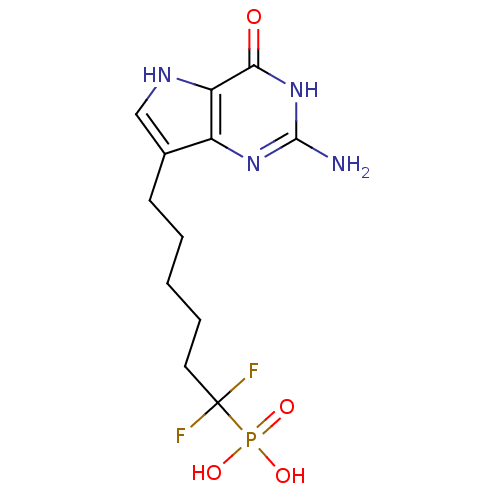
(6-(2-amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyri...)Show SMILES Nc1nc2c(CCCCCC(F)(F)P(O)(O)=O)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C12H17F2N4O4P/c13-12(14,23(20,21)22)5-3-1-2-4-7-6-16-9-8(7)17-11(15)18-10(9)19/h6,16H,1-5H2,(H2,20,21,22)(H3,15,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PNP in presence of 1 mM phosphate |
Bioorg Med Chem Lett 17: 4173-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.054
BindingDB Entry DOI: 10.7270/Q29P31CS |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50382672

(CHEMBL2024005)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CN(C[C@@H]1O)C(=O)P(O)(O)=O |r| Show InChI InChI=1S/C10H13N6O6P/c11-9-13-7-6(8(18)14-9)12-3-16(7)4-1-15(2-5(4)17)10(19)23(20,21)22/h3-5,17H,1-2H2,(H2,20,21,22)(H3,11,13,14,18)/t4-,5+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PNP using MSEG as substrate preincubated with inhibitor within 10 to 20 mins measured after 5 mins by HPLC analysis |
J Med Chem 55: 1612-21 (2012)
Article DOI: 10.1021/jm201409u
BindingDB Entry DOI: 10.7270/Q2VX0HJ2 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046254

(2-Amino-8-thiophen-3-ylmethyl-1,9-dihydro-purin-6-...)Show InChI InChI=1S/C10H9N5OS/c11-10-14-8-7(9(16)15-10)12-6(13-8)3-5-1-2-17-4-5/h1-2,4H,3H2,(H4,11,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50039549
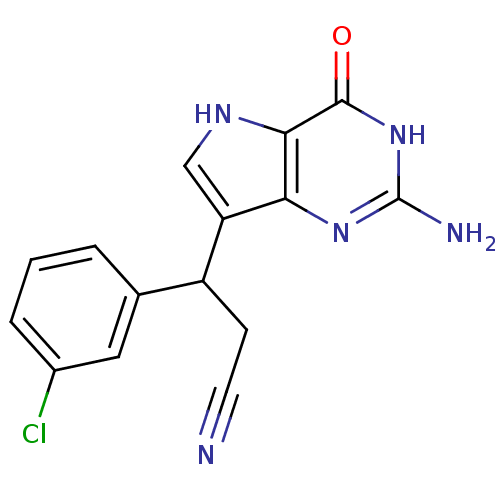
(3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyri...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)C(CC#N)c1cccc(Cl)c1 Show InChI InChI=1S/C15H12ClN5O/c16-9-3-1-2-8(6-9)10(4-5-17)11-7-19-13-12(11)20-15(18)21-14(13)22/h1-3,6-7,10,19H,4H2,(H3,18,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside Phosphorylase from calf spleen at 1 mM PO4 |
J Med Chem 36: 3771-83 (1994)
BindingDB Entry DOI: 10.7270/Q23J3DKS |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046236
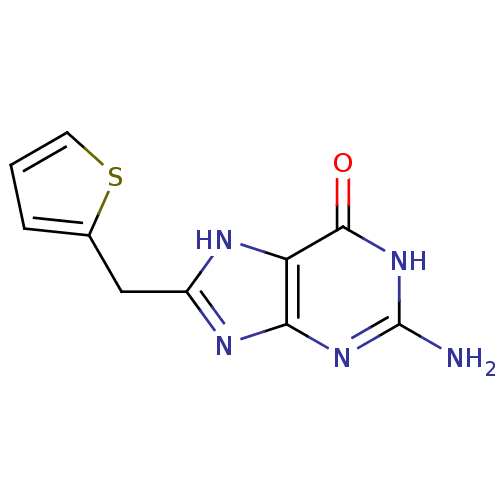
(2-Amino-8-thiophen-2-ylmethyl-1,9-dihydro-purin-6-...)Show InChI InChI=1S/C10H9N5OS/c11-10-14-8-7(9(16)15-10)12-6(13-8)4-5-2-1-3-17-5/h1-3H,4H2,(H4,11,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | CHEMBL5281180
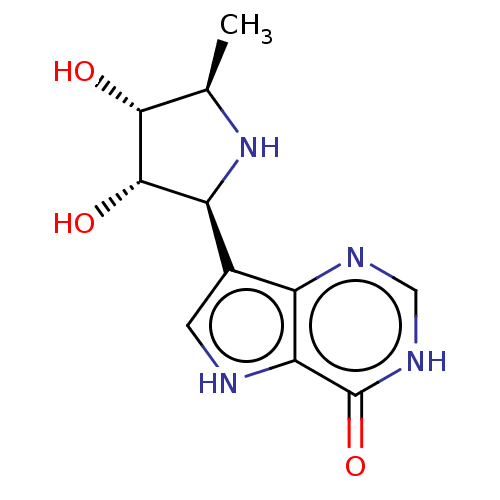
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50042799
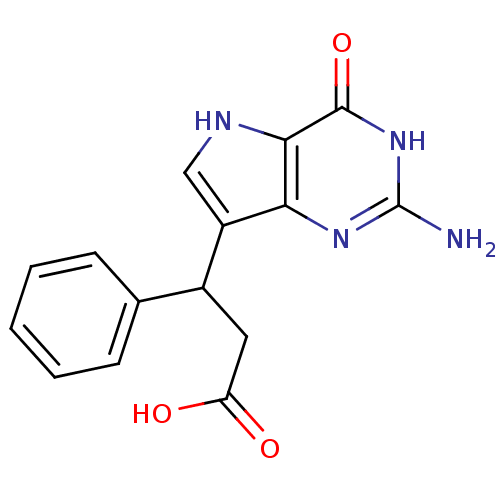
(3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyri...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)C(CC(O)=O)c1ccccc1 Show InChI InChI=1S/C15H14N4O3/c16-15-18-12-10(7-17-13(12)14(22)19-15)9(6-11(20)21)8-4-2-1-3-5-8/h1-5,7,9,17H,6H2,(H,20,21)(H3,16,18,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside Phosphorylase from calf spleen at 1 mM PO4 |
J Med Chem 36: 3771-83 (1994)
BindingDB Entry DOI: 10.7270/Q23J3DKS |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046230
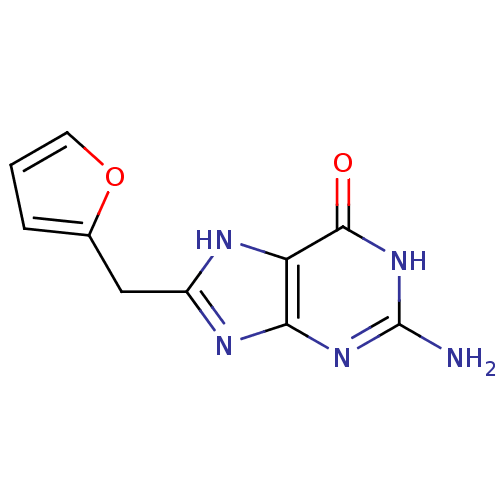
(2-Amino-8-furan-2-ylmethyl-1,9-dihydro-purin-6-one...)Show InChI InChI=1S/C10H9N5O2/c11-10-14-8-7(9(16)15-10)12-6(13-8)4-5-2-1-3-17-5/h1-3H,4H2,(H4,11,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50042804
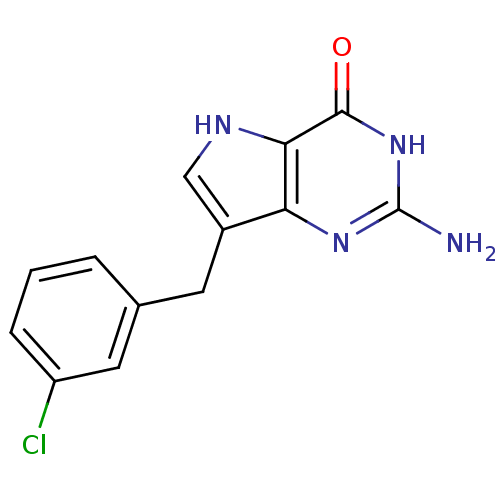
(2-Amino-7-(3-chloro-benzyl)-3,5-dihydro-pyrrolo[3,...)Show InChI InChI=1S/C13H11ClN4O/c14-9-3-1-2-7(5-9)4-8-6-16-11-10(8)17-13(15)18-12(11)19/h1-3,5-6,16H,4H2,(H3,15,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside Phosphorylase from calf spleen at 1 mM PO4 |
J Med Chem 36: 3771-83 (1994)
BindingDB Entry DOI: 10.7270/Q23J3DKS |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50195591

(2-amino-1,5-dihydro-7-[[[2-(hydroxy)ethyl]amino]me...)Show InChI InChI=1S/C9H13N5O2/c10-9-13-6-5(3-11-1-2-15)4-12-7(6)8(16)14-9/h4,11-12,15H,1-3H2,(H3,10,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human PNP activity |
J Med Chem 49: 6037-45 (2006)
Article DOI: 10.1021/jm060547+
BindingDB Entry DOI: 10.7270/Q2NV9K11 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50214705
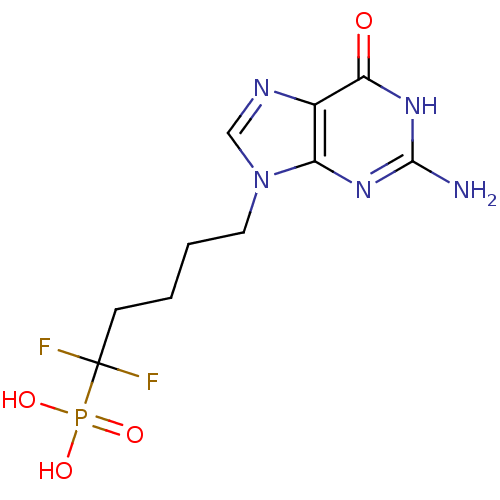
(5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...)Show SMILES Nc1nc2n(CCCCC(F)(F)P(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C10H14F2N5O4P/c11-10(12,22(19,20)21)3-1-2-4-17-5-14-6-7(17)15-9(13)16-8(6)18/h5H,1-4H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PNP in presence of 1 mM phosphate |
Bioorg Med Chem Lett 17: 4173-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.054
BindingDB Entry DOI: 10.7270/Q29P31CS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50214705

(5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...)Show SMILES Nc1nc2n(CCCCC(F)(F)P(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C10H14F2N5O4P/c11-10(12,22(19,20)21)3-1-2-4-17-5-14-6-7(17)15-9(13)16-8(6)18/h5H,1-4H2,(H2,19,20,21)(H3,13,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte PNP assessed as inhibition of 25 uM 7-methylguanosine phosphorolysis by spectrophotometry in presence of 1 mM inorgan... |
Bioorg Med Chem 18: 2275-84 (2010)
Article DOI: 10.1016/j.bmc.2010.01.062
BindingDB Entry DOI: 10.7270/Q2N58MG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50214707

(9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...)Show SMILES Nc1nc2c(CCCCC(F)(F)P(O)(O)=O)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C11H15F2N4O4P/c12-11(13,22(19,20)21)4-2-1-3-6-5-15-8-7(6)16-10(14)17-9(8)18/h5,15H,1-4H2,(H2,19,20,21)(H3,14,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 20.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PNP in presence of 1 mM phosphate |
Bioorg Med Chem Lett 17: 4173-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.054
BindingDB Entry DOI: 10.7270/Q29P31CS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50214707

(9-(5',5'-Difluoro-5'-phosphonopentyl)-9-deazaguani...)Show SMILES Nc1nc2c(CCCCC(F)(F)P(O)(O)=O)c[nH]c2c(=O)[nH]1 Show InChI InChI=1S/C11H15F2N4O4P/c12-11(13,22(19,20)21)4-2-1-3-6-5-15-8-7(6)16-10(14)17-9(8)18/h5,15H,1-4H2,(H2,19,20,21)(H3,14,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 20.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte PNP assessed as inhibition of 25 uM 7-methylguanosine phosphorolysis by spectrophotometry in presence of 1 mM inorgan... |
Bioorg Med Chem 18: 2275-84 (2010)
Article DOI: 10.1016/j.bmc.2010.01.062
BindingDB Entry DOI: 10.7270/Q2N58MG0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50308522
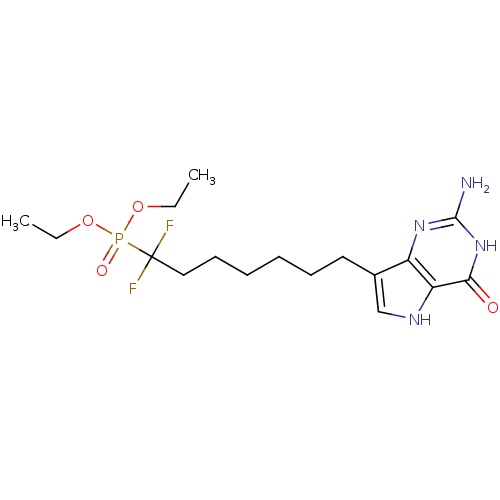
(9-[7',7'-Difluoro-7'-(diethylphosphono)heptyl]-9-d...)Show SMILES CCOP(=O)(OCC)C(F)(F)CCCCCCc1c[nH]c2c1nc(N)[nH]c2=O Show InChI InChI=1S/C17H27F2N4O4P/c1-3-26-28(25,27-4-2)17(18,19)10-8-6-5-7-9-12-11-21-14-13(12)22-16(20)23-15(14)24/h11,21H,3-10H2,1-2H3,(H3,20,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte PNP assessed as inhibition of 25 uM 7-methylguanosine phosphorolysis by spectrophotometry in presence of 1 mM inorgan... |
Bioorg Med Chem 18: 2275-84 (2010)
Article DOI: 10.1016/j.bmc.2010.01.062
BindingDB Entry DOI: 10.7270/Q2N58MG0 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046255
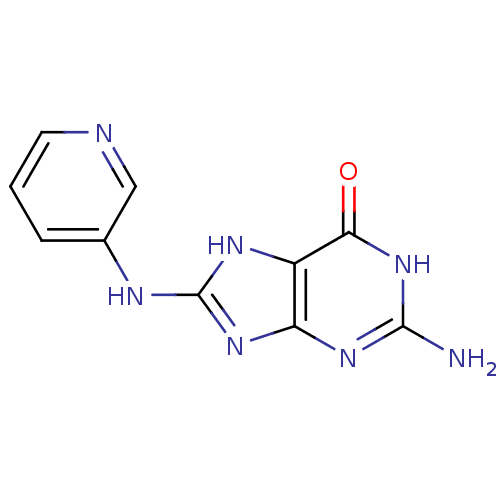
(2-Amino-8-(pyridin-3-ylamino)-1,9-dihydro-purin-6-...)Show InChI InChI=1S/C10H9N7O/c11-9-15-7-6(8(18)17-9)14-10(16-7)13-5-2-1-3-12-4-5/h1-4H,(H5,11,13,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50078454

(2-Amino-7-pyridin-2-ylmethyl-3,5-dihydro-pyrrolo[3...)Show InChI InChI=1S/C12H11N5O/c13-12-16-9-7(5-8-3-1-2-4-14-8)6-15-10(9)11(18)17-12/h1-4,6,15H,5H2,(H3,13,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal da Bahia
Curated by ChEMBL
| Assay Description
Inhibition of human purine nucleoside phosphorylase |
Bioorg Med Chem 18: 1421-7 (2010)
Article DOI: 10.1016/j.bmc.2010.01.022
BindingDB Entry DOI: 10.7270/Q2571C49 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50042803
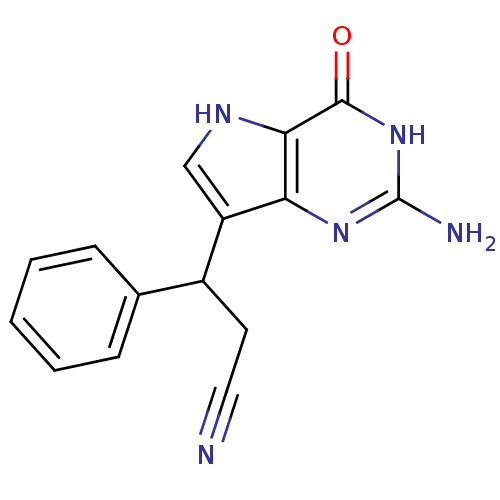
(3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyri...)Show InChI InChI=1S/C15H13N5O/c16-7-6-10(9-4-2-1-3-5-9)11-8-18-13-12(11)19-15(17)20-14(13)21/h1-5,8,10,18H,6H2,(H3,17,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside Phosphorylase from calf spleen at 50 mM PO4 |
J Med Chem 36: 3771-83 (1994)
BindingDB Entry DOI: 10.7270/Q23J3DKS |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50042807

(2-Amino-7-[1-(3-chloro-phenyl)-3-hydroxy-propyl]-3...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)C(CCO)c1cccc(Cl)c1 Show InChI InChI=1S/C15H15ClN4O2/c16-9-3-1-2-8(6-9)10(4-5-21)11-7-18-13-12(11)19-15(17)20-14(13)22/h1-3,6-7,10,18,21H,4-5H2,(H3,17,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside Phosphorylase from calf spleen at 1 mM PO4 |
J Med Chem 36: 3771-83 (1994)
BindingDB Entry DOI: 10.7270/Q23J3DKS |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50046217

(2-Amino-8-benzyl-1,9-dihydro-purin-6-one | CHEMBL1...)Show InChI InChI=1S/C12H11N5O/c13-12-16-10-9(11(18)17-12)14-8(15-10)6-7-4-2-1-3-5-7/h1-5H,6H2,(H4,13,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside phosphorylase was evaluated against the enzyme from Human erythrocytic in 50 mM phosphate |
J Med Chem 36: 55-69 (1993)
BindingDB Entry DOI: 10.7270/Q2B27TCX |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50078452

((S)-3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)[C@@H](CC(O)=O)c1cccc(Cl)c1 Show InChI InChI=1S/C15H13ClN4O3/c16-8-3-1-2-7(4-8)9(5-11(21)22)10-6-18-13-12(10)19-15(17)20-14(13)23/h1-4,6,9,18H,5H2,(H,21,22)(H3,17,19,20,23)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside Phosphorylase from calf spleen at 50 mM PO4 |
J Med Chem 36: 3771-83 (1994)
BindingDB Entry DOI: 10.7270/Q23J3DKS |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50042363
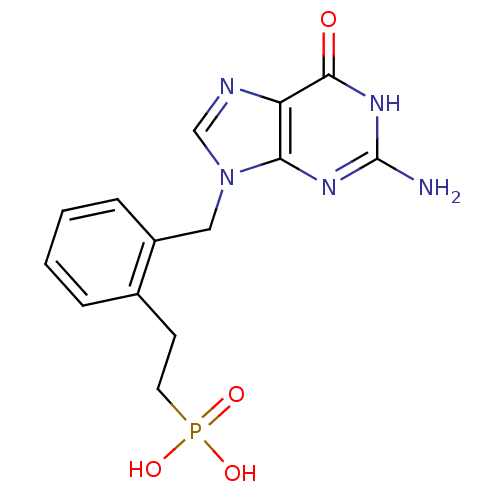
(CHEMBL333946 | {2-[2-(2-Amino-6-oxo-1,6-dihydro-pu...)Show SMILES Nc1nc2n(Cc3ccccc3CCP(O)(O)=O)cnc2c(=O)[nH]1 Show InChI InChI=1S/C14H16N5O4P/c15-14-17-12-11(13(20)18-14)16-8-19(12)7-10-4-2-1-3-9(10)5-6-24(21,22)23/h1-4,8H,5-7H2,(H2,21,22,23)(H3,15,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside Phosphorylase from calf spleen at 1 mM PO4 |
J Med Chem 36: 3771-83 (1994)
BindingDB Entry DOI: 10.7270/Q23J3DKS |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081803
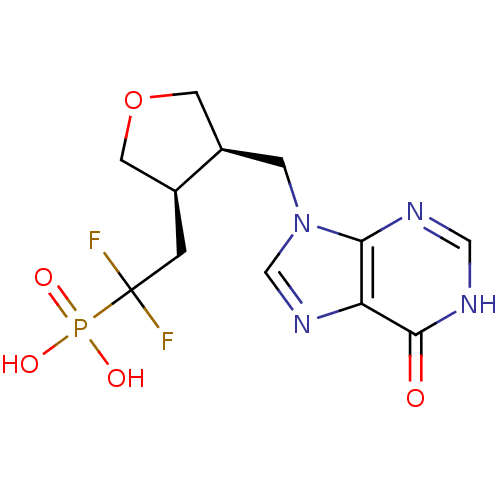
(CHEMBL94840 | {1,1-Difluoro-2-[(3R,4R)-4-(6-oxo-1,...)Show SMILES OP(O)(=O)C(F)(F)C[C@H]1COC[C@H]1Cn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C12H15F2N4O5P/c13-12(14,24(20,21)22)1-7-3-23-4-8(7)2-18-6-17-9-10(18)15-5-16-11(9)19/h5-8H,1-4H2,(H,15,16,19)(H2,20,21,22)/t7-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50081806
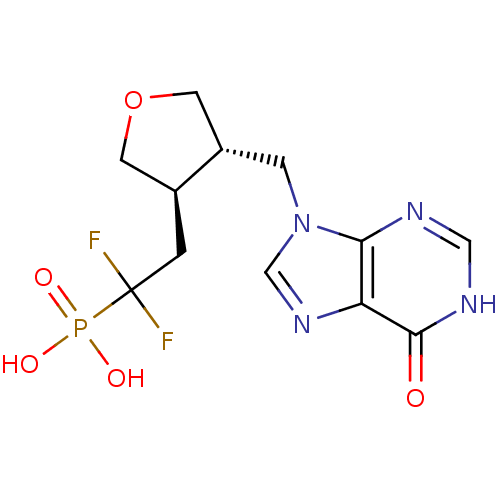
(CHEMBL94920 | {1,1-Difluoro-2-[(3R,4S)-4-(6-oxo-1,...)Show SMILES OP(O)(=O)C(F)(F)C[C@H]1COC[C@@H]1Cn1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C12H15F2N4O5P/c13-12(14,24(20,21)22)1-7-3-23-4-8(7)2-18-6-17-9-10(18)15-5-16-11(9)19/h5-8H,1-4H2,(H,15,16,19)(H2,20,21,22)/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp |
Bioorg Med Chem Lett 9: 2833-6 (1999)
BindingDB Entry DOI: 10.7270/Q2JQ107B |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50042800
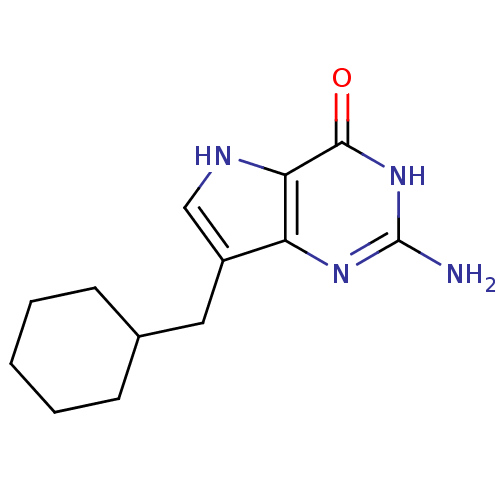
(2-Amino-7-cyclohexylmethyl-3,5-dihydro-pyrrolo[3,2...)Show InChI InChI=1S/C13H18N4O/c14-13-16-10-9(6-8-4-2-1-3-5-8)7-15-11(10)12(18)17-13/h7-8,15H,1-6H2,(H3,14,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Purine nucleoside Phosphorylase from calf spleen at 1 mM PO4 |
J Med Chem 36: 3771-83 (1994)
BindingDB Entry DOI: 10.7270/Q23J3DKS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data