

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22109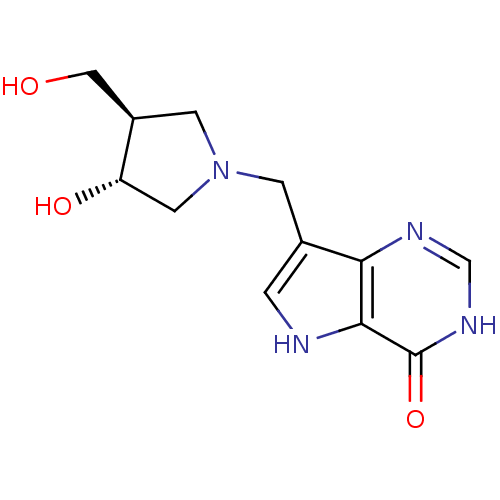 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | -12.6 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22109 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum His-tagged PNP expressed in Escherichia coli assessed as inhibitor constant for enzyme-inhibitor complex formatio... | J Med Chem 62: 8365-8391 (2019) Article DOI: 10.1021/acs.jmedchem.9b00182 BindingDB Entry DOI: 10.7270/Q2T1571N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22109 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation | J Med Chem 62: 8365-8391 (2019) Article DOI: 10.1021/acs.jmedchem.9b00182 BindingDB Entry DOI: 10.7270/Q2T1571N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247158 (7-(((3S,4S)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation | J Med Chem 62: 8365-8391 (2019) Article DOI: 10.1021/acs.jmedchem.9b00182 BindingDB Entry DOI: 10.7270/Q2T1571N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PNP expressed in Escherichia coli BL21(DE3) cells by spectrophotometry | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50293087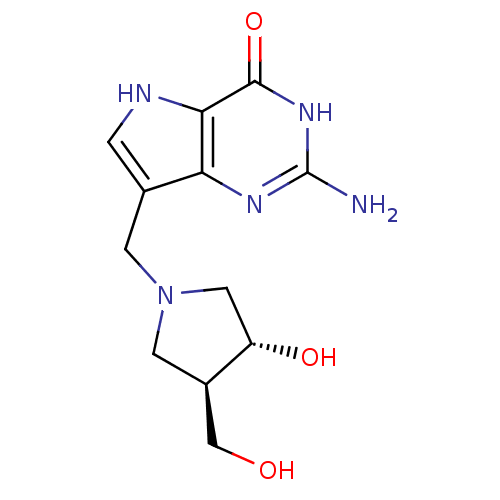 (2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum His-tagged PNP expressed in Escherichia coli assessed as inhibitor constant for enzyme-inhibitor complex formatio... | J Med Chem 62: 8365-8391 (2019) Article DOI: 10.1021/acs.jmedchem.9b00182 BindingDB Entry DOI: 10.7270/Q2T1571N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50293086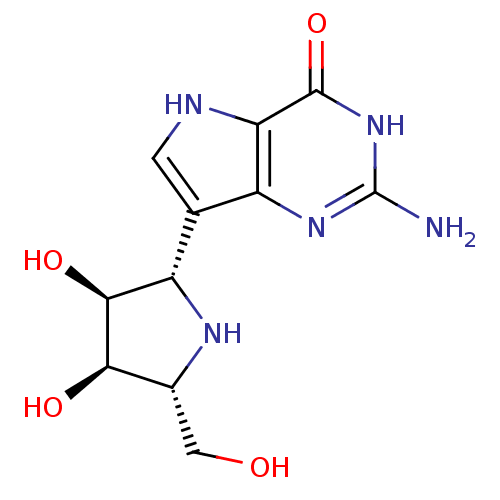 ((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation | J Med Chem 62: 8365-8391 (2019) Article DOI: 10.1021/acs.jmedchem.9b00182 BindingDB Entry DOI: 10.7270/Q2T1571N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50293088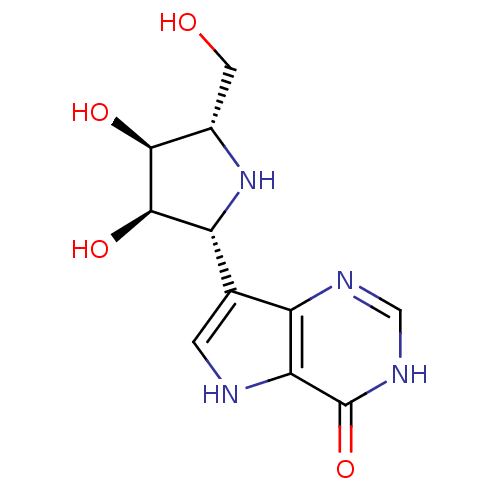 (7-((2R,3R,4S,5S)-3,4-dihydroxy-5-(hydroxymethyl)py...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor-substrate complex formation | J Med Chem 62: 8365-8391 (2019) Article DOI: 10.1021/acs.jmedchem.9b00182 BindingDB Entry DOI: 10.7270/Q2T1571N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247151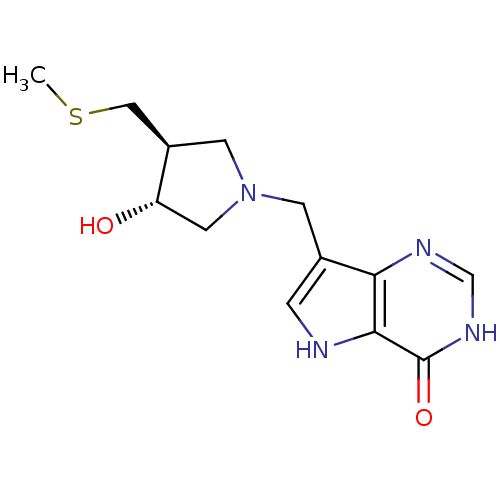 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-in... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50330391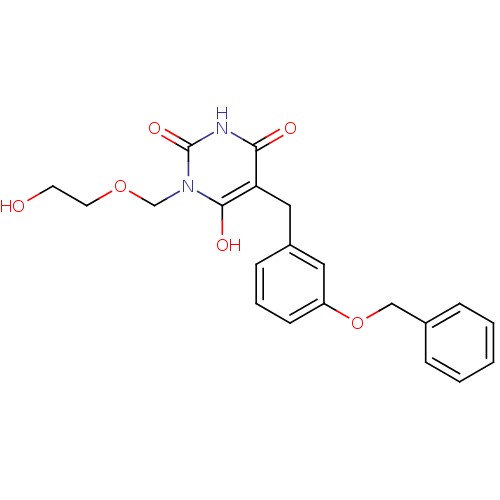 (5-(3-(benzyloxy)benzyl)-1-((2-hydroxyethoxy)methyl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PNP expressed in Escherichia coli BL21(DE3) cells by spectrophotometry | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247158 (7-(((3S,4S)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor complex formation | J Med Chem 62: 8365-8391 (2019) Article DOI: 10.1021/acs.jmedchem.9b00182 BindingDB Entry DOI: 10.7270/Q2T1571N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247149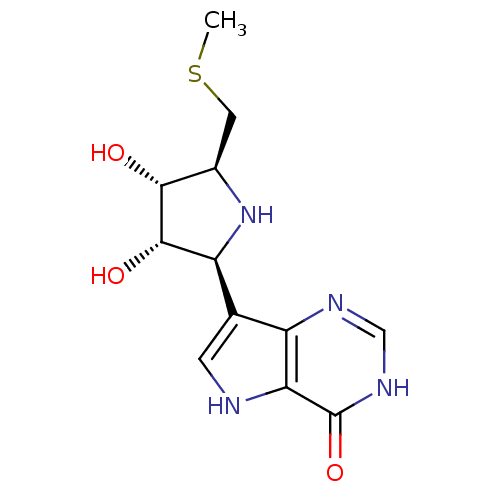 (5'-Methylthio-ImmH | CHEMBL473929 | US9290501, (A)) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum 3D7 PNP expressed in Escherichia coli assessed as reduction in uric acid formation by spectrophotometric method | J Med Chem 62: 8365-8391 (2019) Article DOI: 10.1021/acs.jmedchem.9b00182 BindingDB Entry DOI: 10.7270/Q2T1571N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50330390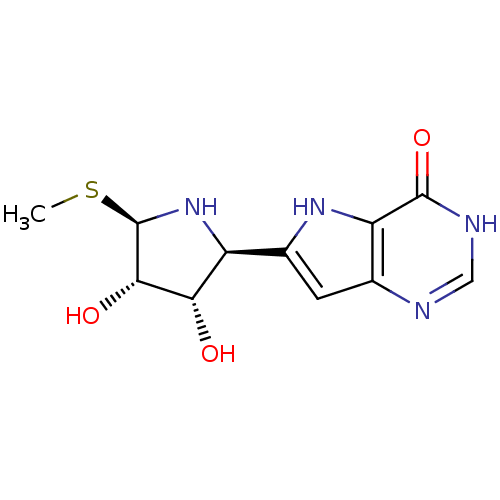 (5'-methylthio-immucillin-H | CHEMBL1275659) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PNP expressed in Escherichia coli BL21(DE3) cells by spectrophotometry | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50252865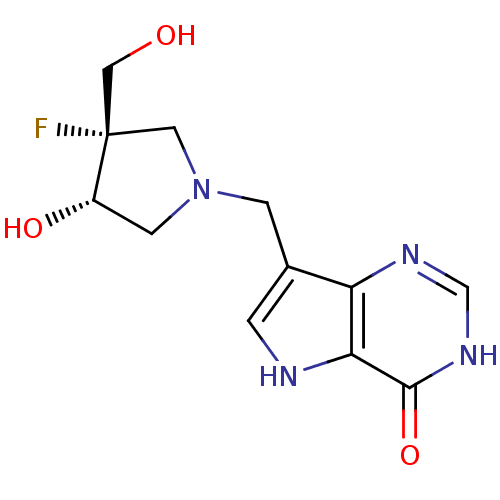 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247151 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50293088 (7-((2R,3R,4S,5S)-3,4-dihydroxy-5-(hydroxymethyl)py...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor complex formation | J Med Chem 62: 8365-8391 (2019) Article DOI: 10.1021/acs.jmedchem.9b00182 BindingDB Entry DOI: 10.7270/Q2T1571N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50252865 ((+/-)-cis-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fl...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Montpellier Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum His-tagged PNP assessed as inhibitor constant for enzyme-inhibitor complex formation | J Med Chem 62: 8365-8391 (2019) Article DOI: 10.1021/acs.jmedchem.9b00182 BindingDB Entry DOI: 10.7270/Q2T1571N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247158 (7-(((3S,4S)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50116358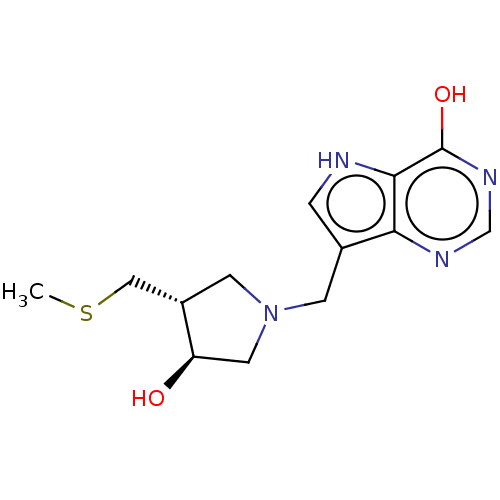 (CHEMBL3604359) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-in... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22108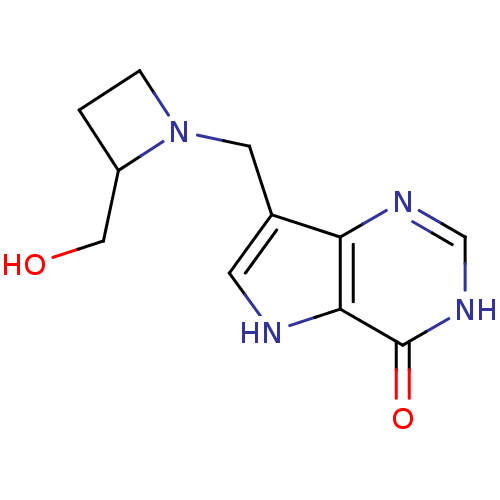 (7-{[2-(hydroxymethyl)azetidin-1-yl]methyl}-3H,4H,5...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 191 | -9.07 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50252909 ((3R,4R)-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fluo...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50116358 (CHEMBL3604359) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 298 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22107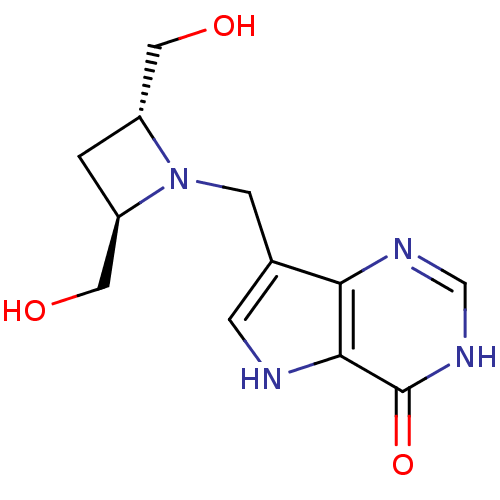 (7-{[(2R,4R)-2,4-bis(hydroxymethyl)azetidin-1-yl]me...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 580 | -8.42 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22106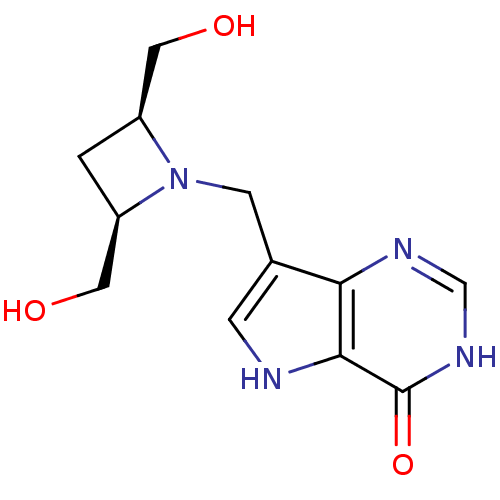 (7-{[(2R,4S)-2,4-bis(hydroxymethyl)azetidin-1-yl]me...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.29E+3 | -7.95 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247158 (7-(((3S,4S)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50252909 ((3R,4R)-1-((9-Deazahypoxanthin-9-yl)methyl)-4-fluo...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum purine nucleoside phosphorylase assessed as slow onset inhibition constant by xanthine-oxidase coupled assay | J Med Chem 51: 5880-4 (2008) Article DOI: 10.1021/jm800792b BindingDB Entry DOI: 10.7270/Q2VQ32H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50330388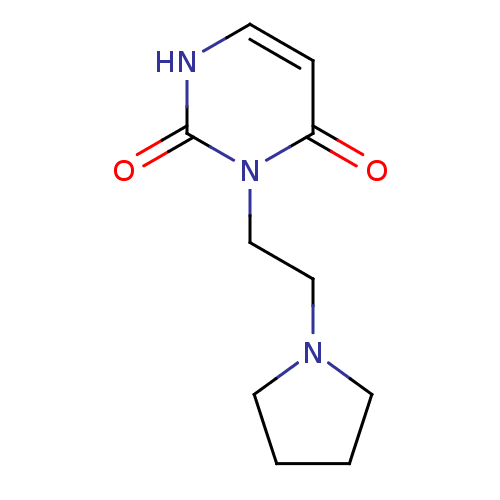 (3-((2-Pyrrolidine-1-yl)-ethyl)uracil | CHEMBL12757...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PNP expressed in Escherichia coli BL21(DE3) cells by spectrophotometry | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22105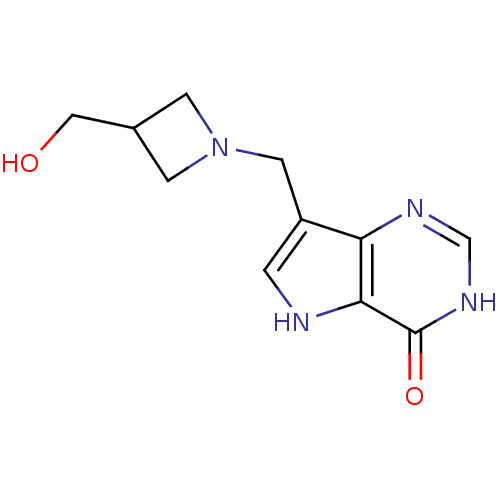 (7-{[3-(hydroxymethyl)azetidin-1-yl]methyl}-3H,4H,5...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-6.75 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM22103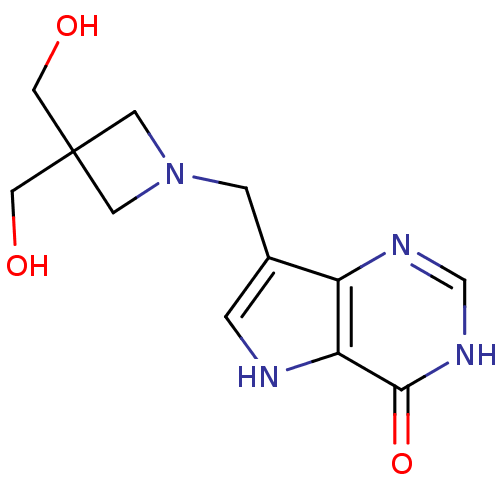 (7-{[3,3-bis(hydroxymethyl)azetidin-1-yl]methyl}-3H...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-6.75 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50330394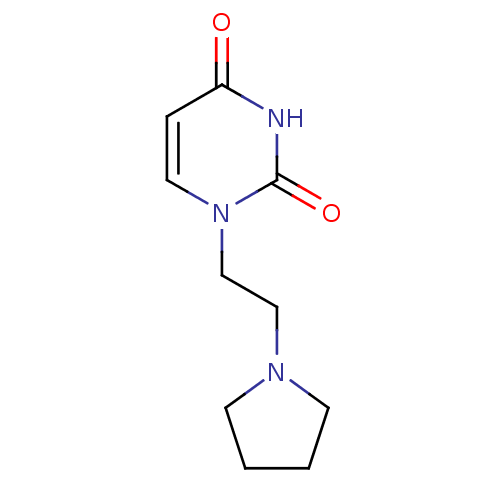 (1-((2-Pyrrolidine-1-yl)-ethyl)uracil | CHEMBL12756...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PNP expressed in Escherichia coli BL21(DE3) cells by spectrophotometry | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50330395 (1-((3-Pyrrolidine-1-yl)-propyl)uracil | CHEMBL1275...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PNP expressed in Escherichia coli BL21(DE3) cells by spectrophotometry | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50330389 (3-((2-(l-Prolinol)-1-yl)-ethyl)uracil | CHEMBL1275...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PNP expressed in Escherichia coli BL21(DE3) cells by spectrophotometry | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50330397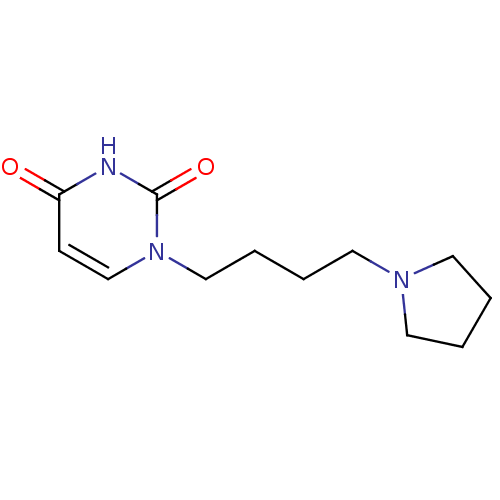 (1-((4-Pyrrolidine-1-yl)-butyl)uracil | CHEMBL12757...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PNP expressed in Escherichia coli BL21(DE3) cells by spectrophotometry | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50330398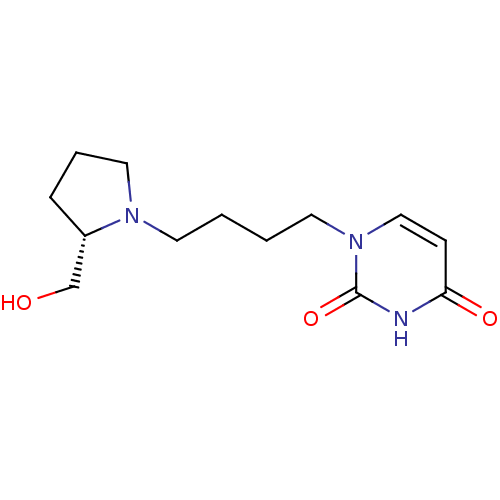 (1-((4-(l-Prolinol)-1-yl)-butyl)uracil | CHEMBL1275...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PNP expressed in Escherichia coli BL21(DE3) cells by spectrophotometry | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50330396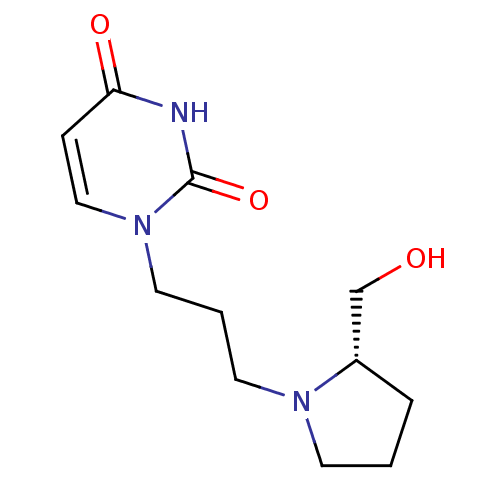 (1-((3-(l-Prolinol)-1-yl)-propyl)uracil | CHEMBL127...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PNP expressed in Escherichia coli BL21(DE3) cells by spectrophotometry | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||