 Found 526 hits of ic50 for Cyclooxygenase-1
Found 526 hits of ic50 for Cyclooxygenase-1
having polymerids = 2261,2299,50000093,50000760,50001408,50002157,50002608,50002633 and
complexids = 50014775,50014921 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247597
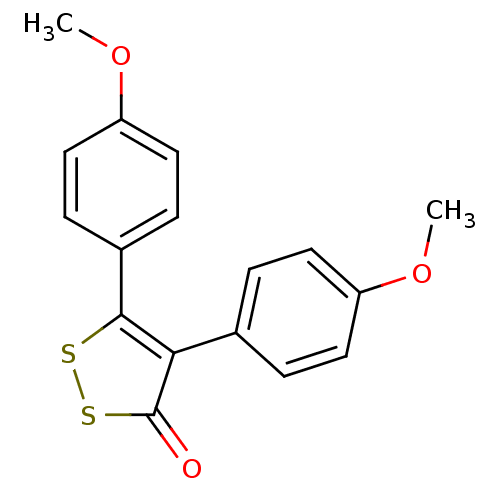
(4,5-Bis(4-methoxyphenyl)-3H-1,2-dithiol-3-one | CH...)Show InChI InChI=1S/C17H14O3S2/c1-19-13-7-3-11(4-8-13)15-16(21-22-17(15)18)12-5-9-14(20-2)10-6-12/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Tested for inhibition against Prostaglandin G/H synthase 2 from mouse resident macrophages |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Università "La Sapienza"
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Prostaglandin G/H synthase 1 in murine J774 cells |
J Med Chem 48: 3428-32 (2005)
Article DOI: 10.1021/jm049121q
BindingDB Entry DOI: 10.7270/Q2N01628 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247826
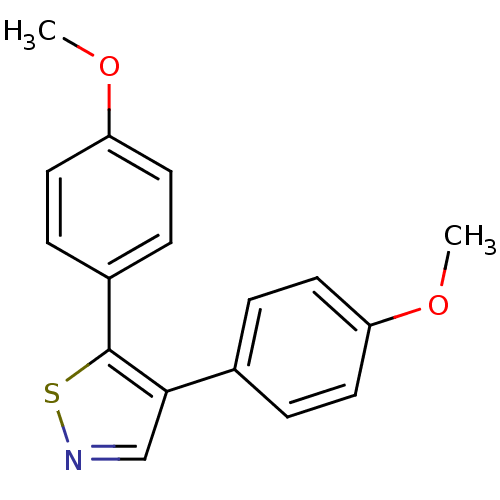
(4,5-bis(4-methoxyphenyl)isothiazole | CHEMBL473946)Show InChI InChI=1S/C17H15NO2S/c1-19-14-7-3-12(4-8-14)16-11-18-21-17(16)13-5-9-15(20-2)10-6-13/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50474760
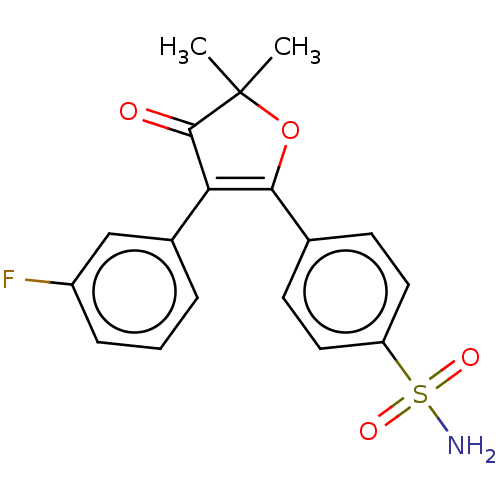
(CG-100649 | CG100649 | Polmacoxib)Show SMILES CC1(C)OC(=C(C1=O)c1cccc(F)c1)c1ccc(cc1)S(N)(=O)=O |c:4| Show InChI InChI=1S/C18H16FNO4S/c1-18(2)17(21)15(12-4-3-5-13(19)10-12)16(24-18)11-6-8-14(9-7-11)25(20,22)23/h3-10H,1-2H3,(H2,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 in LPS-induced mouse peritoneal macrophages |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM13066
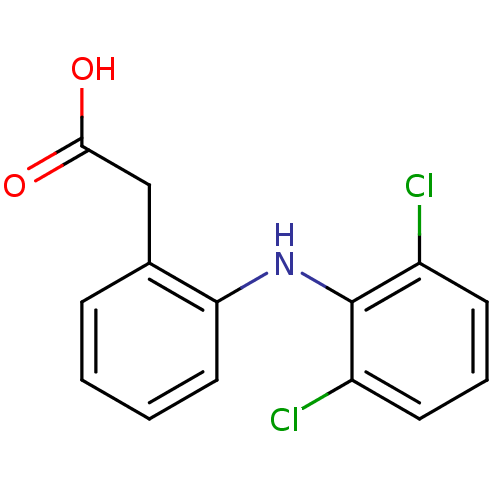
(2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibition of bovine COX1 assessed as inhibition of calcium ionophore A23187-induced 12-hydroxyheptadecatrienoic acid formation by reverse-phase HPLC... |
Bioorg Med Chem Lett 22: 5031-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.012
BindingDB Entry DOI: 10.7270/Q27P90GC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247901
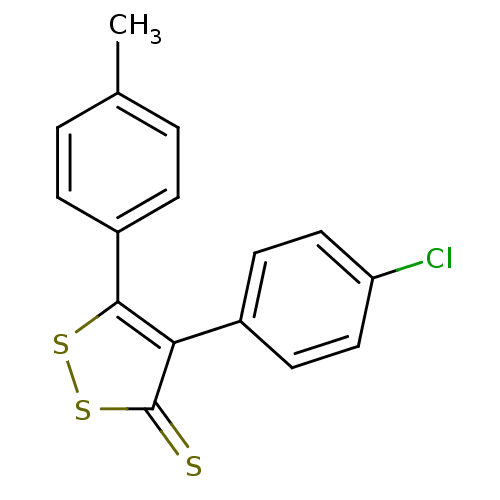
(4-(4-Chlorophenyl)-5-p-tolyl-3H-1,2-dithiole-3-thi...)Show InChI InChI=1S/C16H11ClS3/c1-10-2-4-12(5-3-10)15-14(16(18)20-19-15)11-6-8-13(17)9-7-11/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50245432
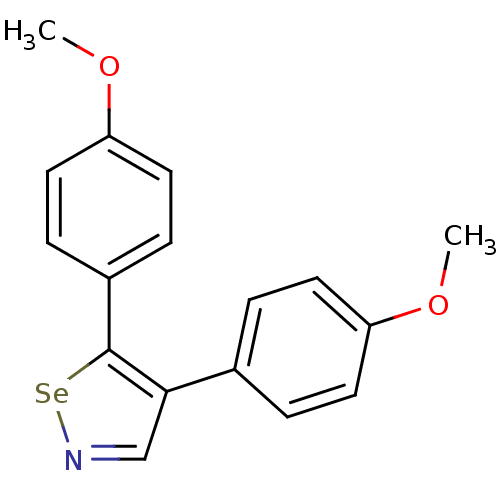
(4,5-Bis(4-methoxyphenyl)-1,2-selenazole | CHEMBL47...)Show InChI InChI=1S/C17H15NO2Se/c1-19-14-7-3-12(4-8-14)16-11-18-21-17(16)13-5-9-15(20-2)10-6-13/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University
Curated by ChEMBL
| Assay Description
Inhibition of bovine COX1 |
Eur J Med Chem 43: 1152-9 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.007
BindingDB Entry DOI: 10.7270/Q2MG7QDR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247827
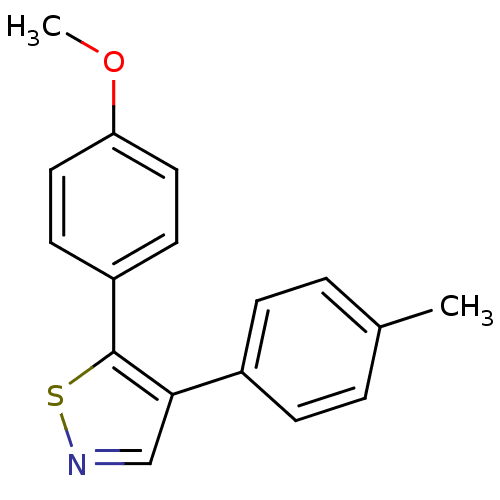
(5-(4-methoxyphenyl)-4-p-tolylisothiazole | CHEMBL5...)Show InChI InChI=1S/C17H15NOS/c1-12-3-5-13(6-4-12)16-11-18-20-17(16)14-7-9-15(19-2)10-8-14/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50245465
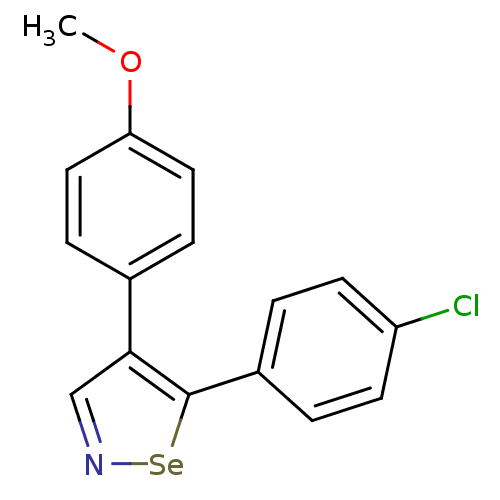
(5-(4-Chlorophenyl)-4-(4-methoxyphenyl)-1,2-selenaz...)Show InChI InChI=1S/C16H12ClNOSe/c1-19-14-8-4-11(5-9-14)15-10-18-20-16(15)12-2-6-13(17)7-3-12/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University
Curated by ChEMBL
| Assay Description
Inhibition of bovine COX1 |
Eur J Med Chem 43: 1152-9 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.007
BindingDB Entry DOI: 10.7270/Q2MG7QDR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247851
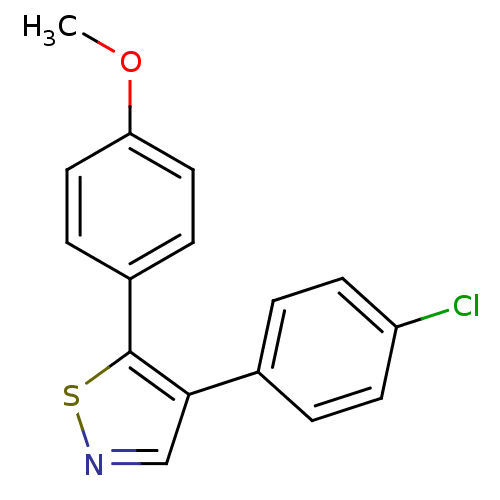
(4-(4-Chlorophenyl)-5-(4-methoxyphenyl)isothiazole ...)Show InChI InChI=1S/C16H12ClNOS/c1-19-14-8-4-12(5-9-14)16-15(10-18-20-16)11-2-6-13(17)7-3-11/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247578
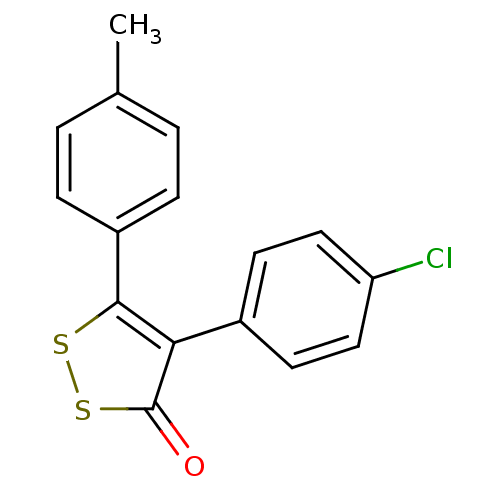
(4-(4-Chlorophenyl)-5-p-tolyl-3H-1,2-dithiol-3-one ...)Show InChI InChI=1S/C16H11ClOS2/c1-10-2-4-12(5-3-10)15-14(16(18)20-19-15)11-6-8-13(17)9-7-11/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50245433
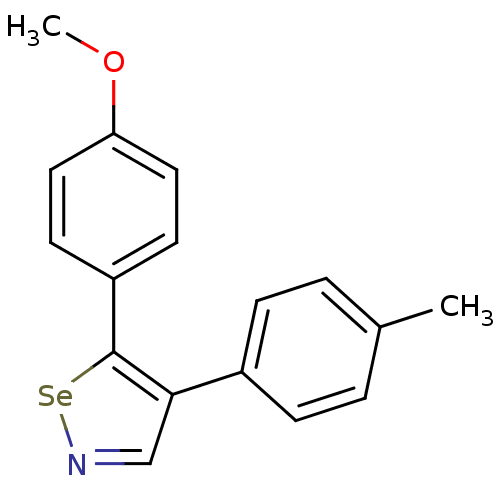
(5-(4-Methoxyphenyl)-4-p-tolyl-1,2-selenazole | CHE...)Show InChI InChI=1S/C17H15NOSe/c1-12-3-5-13(6-4-12)16-11-18-20-17(16)14-7-9-15(19-2)10-8-14/h3-11H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University
Curated by ChEMBL
| Assay Description
Inhibition of bovine COX1 |
Eur J Med Chem 43: 1152-9 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.007
BindingDB Entry DOI: 10.7270/Q2MG7QDR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50245466
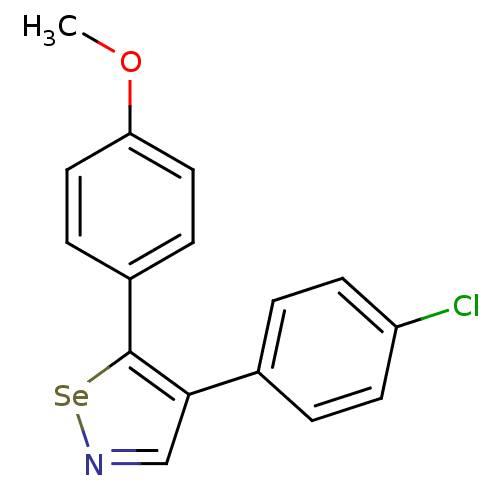
(4-(4-Chlorophenyl)-5-(4-methoxyphenyl)-1,2-selenaz...)Show InChI InChI=1S/C16H12ClNOSe/c1-19-14-8-4-12(5-9-14)16-15(10-18-20-16)11-2-6-13(17)7-3-11/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University
Curated by ChEMBL
| Assay Description
Inhibition of bovine COX1 |
Eur J Med Chem 43: 1152-9 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.007
BindingDB Entry DOI: 10.7270/Q2MG7QDR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247902
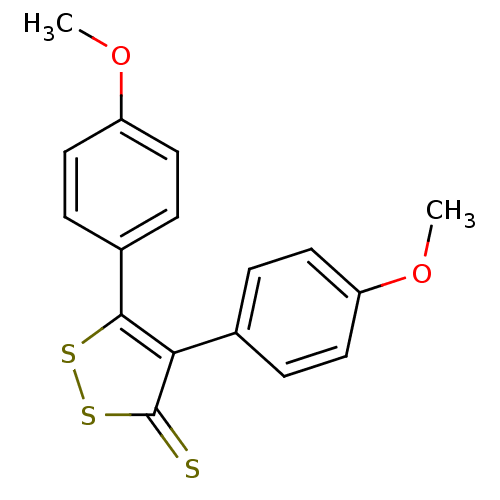
(4,5-bis(4-methoxyphenyl)-3H-1,2-dithiole-3-thione ...)Show InChI InChI=1S/C17H14O2S3/c1-18-13-7-3-11(4-8-13)15-16(21-22-17(15)20)12-5-9-14(19-2)10-6-12/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247847
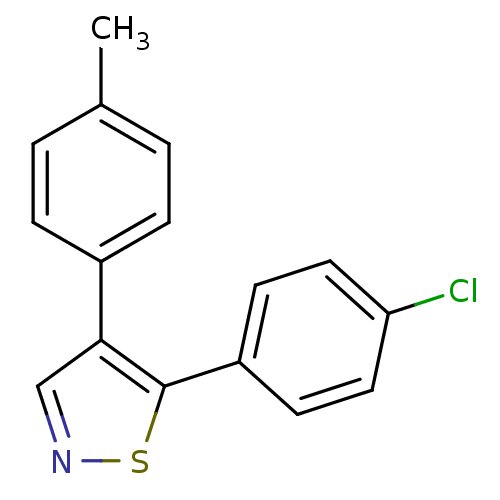
(5-(4-Chlorophenyl)-4-p-tolylisothiazole | CHEMBL52...)Show InChI InChI=1S/C16H12ClNS/c1-11-2-4-12(5-3-11)15-10-18-19-16(15)13-6-8-14(17)9-7-13/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Canis familiaris) | BDBM50137420
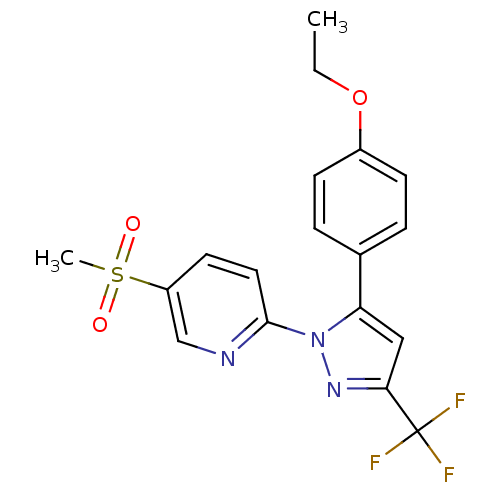
(2-[5-(4-Ethoxy-phenyl)-3-trifluoromethyl-pyrazol-1...)Show SMILES CCOc1ccc(cc1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C18H16F3N3O3S/c1-3-27-13-6-4-12(5-7-13)15-10-16(18(19,20)21)23-24(15)17-9-8-14(11-22-17)28(2,25)26/h4-11H,3H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 1. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50245435
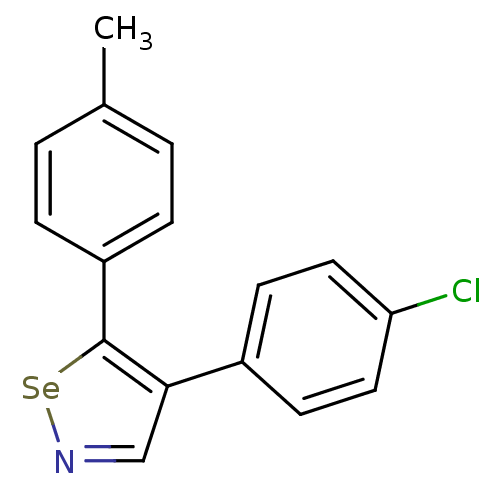
(4-(4-Chlorophenyl)-5-p-tolyl-1,2-selenazole | CHEM...)Show InChI InChI=1S/C16H12ClNSe/c1-11-2-4-13(5-3-11)16-15(10-18-19-16)12-6-8-14(17)9-7-12/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University
Curated by ChEMBL
| Assay Description
Inhibition of bovine COX1 |
Eur J Med Chem 43: 1152-9 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.007
BindingDB Entry DOI: 10.7270/Q2MG7QDR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247850
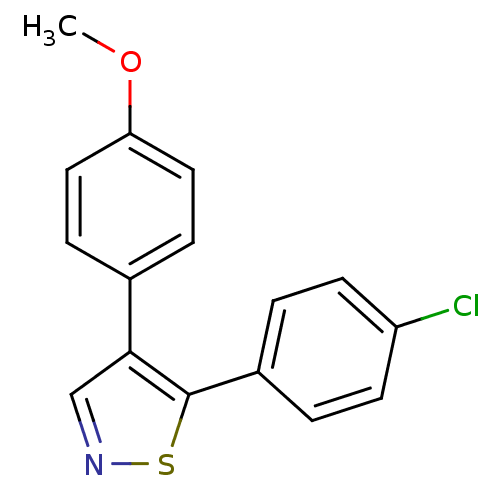
(5-(4-Chlorophenyl)-4-(4-methoxyphenyl)isothiazole ...)Show InChI InChI=1S/C16H12ClNOS/c1-19-14-8-4-11(5-9-14)15-10-18-20-16(15)12-2-6-13(17)7-3-12/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50474774

(CHEMBL352430)Show SMILES CC1(C)OC(=C(C1=O)c1ccc(Br)cc1)c1ccc(cc1)S(C)(=O)=O |c:4| Show InChI InChI=1S/C19H17BrO4S/c1-19(2)18(21)16(12-4-8-14(20)9-5-12)17(24-19)13-6-10-15(11-7-13)25(3,22)23/h4-11H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71.2 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prostaglandin G/H synthase 1 using mouse peritoneal macrophage method |
J Med Chem 47: 792-804 (2004)
Article DOI: 10.1021/jm020545z
BindingDB Entry DOI: 10.7270/Q2QZ2DPG |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50293280
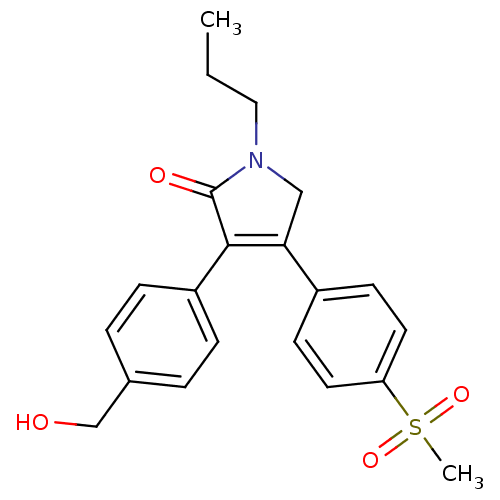
(3-(4-(hydroxymethyl)phenyl)-4-(4-(methylsulfonyl)p...)Show SMILES CCCN1CC(=C(C1=O)c1ccc(CO)cc1)c1ccc(cc1)S(C)(=O)=O |c:5| Show InChI InChI=1S/C21H23NO4S/c1-3-12-22-13-19(16-8-10-18(11-9-16)27(2,25)26)20(21(22)24)17-6-4-15(14-23)5-7-17/h4-11,23H,3,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in mouse peritoneal macrophages |
Bioorg Med Chem Lett 19: 2270-2 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.090
BindingDB Entry DOI: 10.7270/Q2CF9PZR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247849
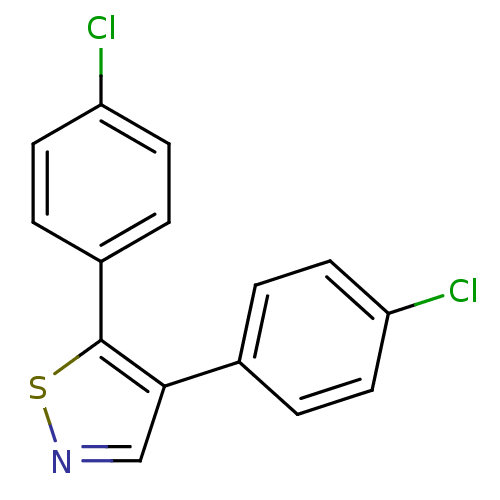
(4,5-Bis(4-chlorophenyl)isothiazole | CHEMBL491088)Show InChI InChI=1S/C15H9Cl2NS/c16-12-5-1-10(2-6-12)14-9-18-19-15(14)11-3-7-13(17)8-4-11/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Canis familiaris) | BDBM50179624
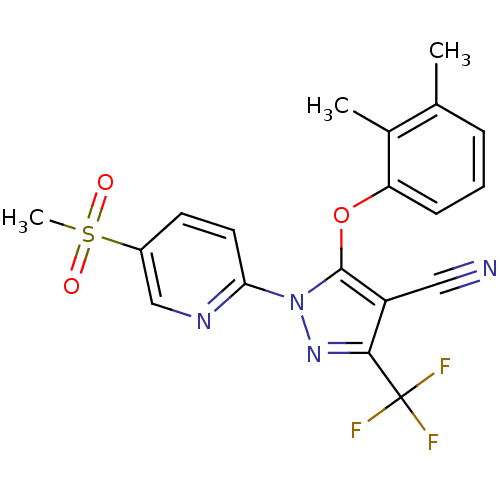
(5-(2,3-dimethylphenoxy)-1-(5-(methylsulfonyl)pyrid...)Show SMILES Cc1cccc(Oc2c(C#N)c(nn2-c2ccc(cn2)S(C)(=O)=O)C(F)(F)F)c1C Show InChI InChI=1S/C19H15F3N4O3S/c1-11-5-4-6-15(12(11)2)29-18-14(9-23)17(19(20,21)22)25-26(18)16-8-7-13(10-24-16)30(3,27)28/h4-8,10H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 by canine whole blood assay |
Bioorg Med Chem Lett 16: 1202-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.110
BindingDB Entry DOI: 10.7270/Q2FT8KKM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Canis familiaris) | BDBM50179602
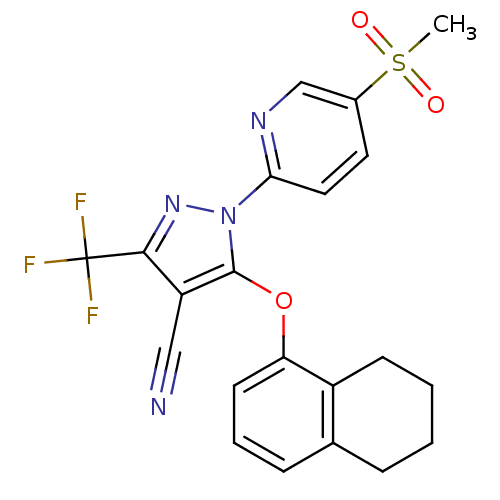
(1-(5-(methylsulfonyl)pyridin-2-yl)-5-(5,6,7,8-tetr...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(c(C#N)c1Oc1cccc2CCCCc12)C(F)(F)F Show InChI InChI=1S/C21H17F3N4O3S/c1-32(29,30)14-9-10-18(26-12-14)28-20(16(11-25)19(27-28)21(22,23)24)31-17-8-4-6-13-5-2-3-7-15(13)17/h4,6,8-10,12H,2-3,5,7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 by canine whole blood assay |
Bioorg Med Chem Lett 16: 1202-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.110
BindingDB Entry DOI: 10.7270/Q2FT8KKM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50094548
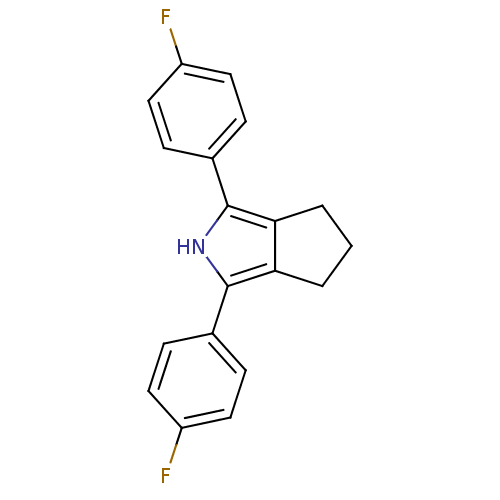
(1,3-Bis-(4-fluoro-phenyl)-2,4,5,6-tetrahydro-cyclo...)Show InChI InChI=1S/C19H15F2N/c20-14-8-4-12(5-9-14)18-16-2-1-3-17(16)19(22-18)13-6-10-15(21)11-7-13/h4-11,22H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibition against prostaglandin G/H synthase 1 from mouse resident macrophages |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50094534
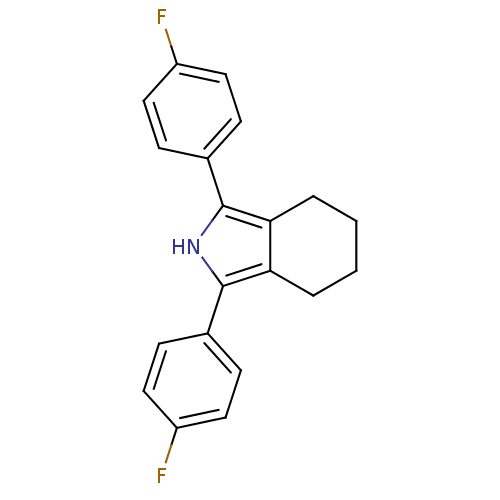
(1,3-Bis-(4-fluoro-phenyl)-4,5,6,7-tetrahydro-2H-is...)Show InChI InChI=1S/C20H17F2N/c21-15-9-5-13(6-10-15)19-17-3-1-2-4-18(17)20(23-19)14-7-11-16(22)12-8-14/h5-12,23H,1-4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibition against prostaglandin G/H synthase 1 from mouse resident macrophages |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50094535
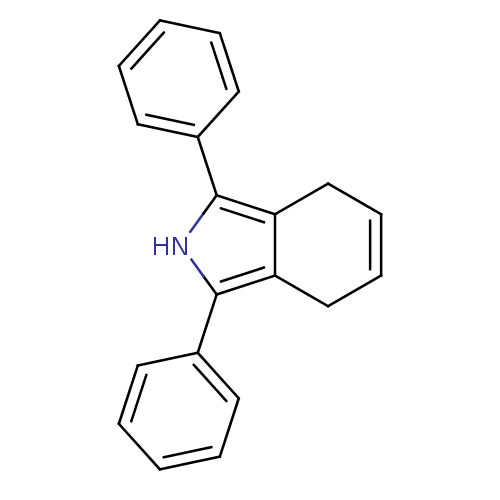
(1,3-diphenyl-4,7-dihydro-2H-isoindole | CHEMBL1408...)Show SMILES C1C=CCc2c1c([nH]c2-c1ccccc1)-c1ccccc1 |c:1| Show InChI InChI=1S/C20H17N/c1-3-9-15(10-4-1)19-17-13-7-8-14-18(17)20(21-19)16-11-5-2-6-12-16/h1-12,21H,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibition against prostaglandin G/H synthase 1 from mouse resident macrophages |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50094558
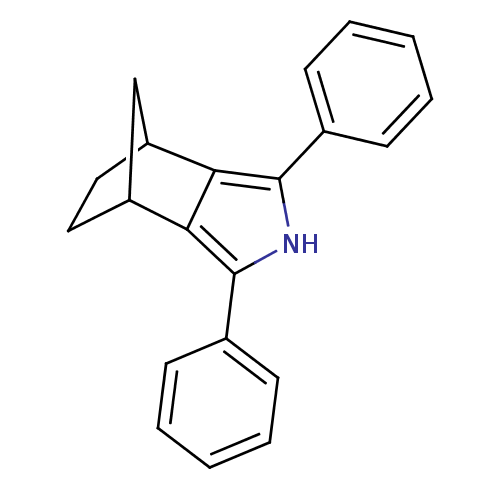
(3,5-Diphenyl-4-aza-tricyclo[5.2.1.0*2,6*]deca-2,5-...)Show InChI InChI=1S/C21H19N/c1-3-7-14(8-4-1)20-18-16-11-12-17(13-16)19(18)21(22-20)15-9-5-2-6-10-15/h1-10,16-17,22H,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibition against prostaglandin G/H synthase 1 from mouse resident macrophages |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50094555
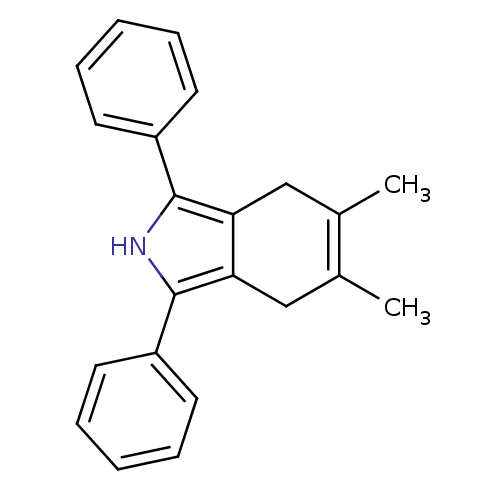
(5,6-Dimethyl-1,3-diphenyl-4,7-dihydro-2H-isoindole...)Show SMILES CC1=C(C)Cc2c(C1)c([nH]c2-c1ccccc1)-c1ccccc1 |c:1| Show InChI InChI=1S/C22H21N/c1-15-13-19-20(14-16(15)2)22(18-11-7-4-8-12-18)23-21(19)17-9-5-3-6-10-17/h3-12,23H,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibition against prostaglandin G/H synthase 1 from mouse resident macrophages |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50094540
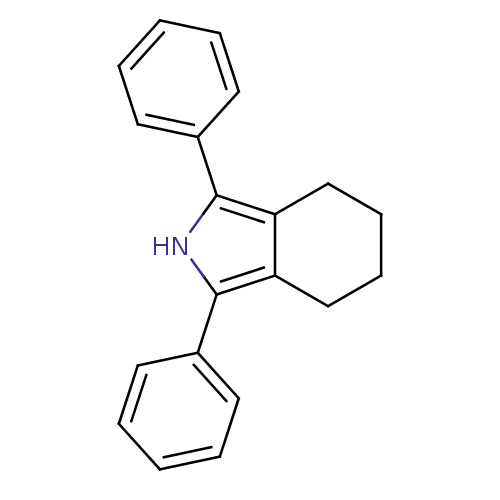
(1,3-diphenyl-4,5,6,7-tetrahydro-2H-isoindole | CHE...)Show InChI InChI=1S/C20H19N/c1-3-9-15(10-4-1)19-17-13-7-8-14-18(17)20(21-19)16-11-5-2-6-12-16/h1-6,9-12,21H,7-8,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibition against prostaglandin G/H synthase 1 from mouse resident macrophages |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50094539
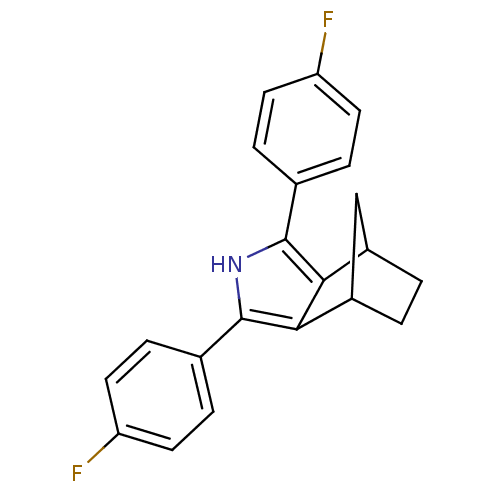
(3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.1.0*2...)Show SMILES Fc1ccc(cc1)-c1[nH]c(c2C3CCC(C3)c12)-c1ccc(F)cc1 Show InChI InChI=1S/C21H17F2N/c22-16-7-3-12(4-8-16)20-18-14-1-2-15(11-14)19(18)21(24-20)13-5-9-17(23)10-6-13/h3-10,14-15,24H,1-2,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibition against prostaglandin G/H synthase 1 from mouse resident macrophages |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50293282
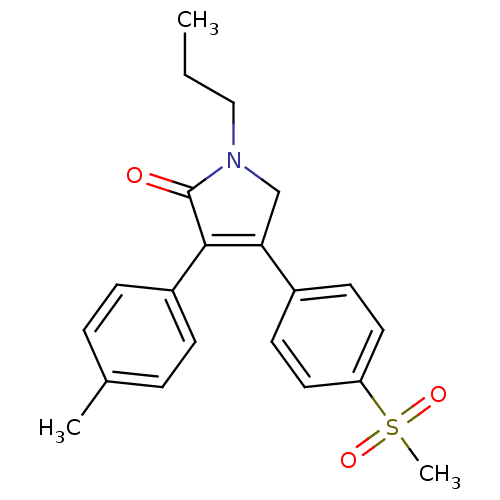
(CHEMBL504535 | Imrecoxib)Show SMILES CCCN1CC(=C(C1=O)c1ccc(C)cc1)c1ccc(cc1)S(C)(=O)=O |c:5| Show InChI InChI=1S/C21H23NO3S/c1-4-13-22-14-19(16-9-11-18(12-10-16)26(3,24)25)20(21(22)23)17-7-5-15(2)6-8-17/h5-12H,4,13-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in mouse peritoneal macrophages |
Bioorg Med Chem Lett 19: 2270-2 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.090
BindingDB Entry DOI: 10.7270/Q2CF9PZR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50293282

(CHEMBL504535 | Imrecoxib)Show SMILES CCCN1CC(=C(C1=O)c1ccc(C)cc1)c1ccc(cc1)S(C)(=O)=O |c:5| Show InChI InChI=1S/C21H23NO3S/c1-4-13-22-14-19(16-9-11-18(12-10-16)26(3,24)25)20(21(22)23)17-7-5-15(2)6-8-17/h5-12H,4,13-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaPhase Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of calcimycin-induced COX-1 in peritoneal macrophage of C57BL/J6 mouse assessed as 6-Keto prostaglandin F1alpha formation preincubated for... |
Bioorg Med Chem 22: 2005-32 (2014)
Article DOI: 10.1016/j.bmc.2014.02.017
BindingDB Entry DOI: 10.7270/Q2WS8VS9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50038649
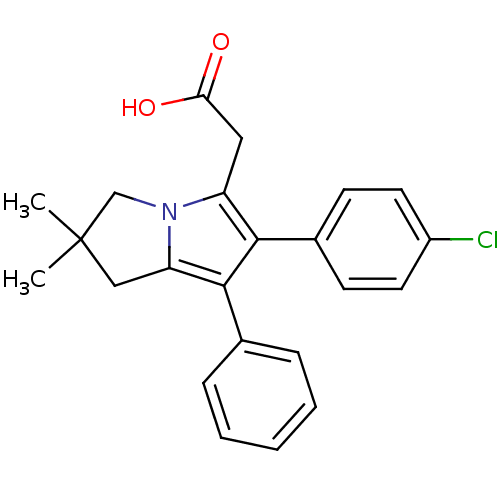
(2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...)Show SMILES CC1(C)Cc2c(c(c(CC(O)=O)n2C1)-c1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C23H22ClNO2/c1-23(2)13-19-22(15-6-4-3-5-7-15)21(16-8-10-17(24)11-9-16)18(12-20(26)27)25(19)14-23/h3-11H,12-14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hamburg
Curated by ChEMBL
| Assay Description
Inhibition of bovine COX1 assessed as inhibition of calcium ionophore A23187-induced 12-hydroxyheptadecatrienoic acid formation by reverse-phase HPLC... |
Bioorg Med Chem Lett 22: 5031-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.012
BindingDB Entry DOI: 10.7270/Q27P90GC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50474742
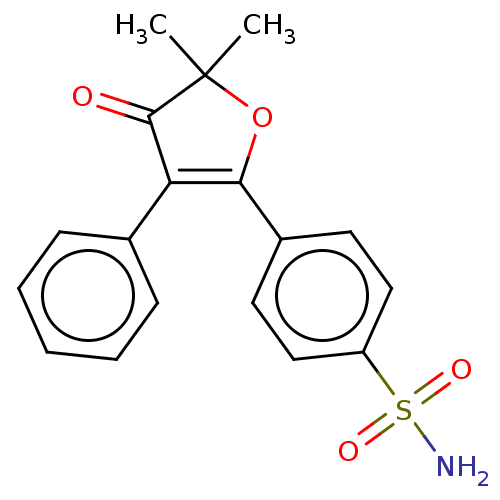
(CHEMBL166295)Show SMILES CC1(C)OC(=C(C1=O)c1ccccc1)c1ccc(cc1)S(N)(=O)=O |c:4| Show InChI InChI=1S/C18H17NO4S/c1-18(2)17(20)15(12-6-4-3-5-7-12)16(23-18)13-8-10-14(11-9-13)24(19,21)22/h3-11H,1-2H3,(H2,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
AmorePacific R&D Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prostaglandin G/H synthase 1 using mouse peritoneal macrophage method |
J Med Chem 47: 792-804 (2004)
Article DOI: 10.1021/jm020545z
BindingDB Entry DOI: 10.7270/Q2QZ2DPG |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50245436
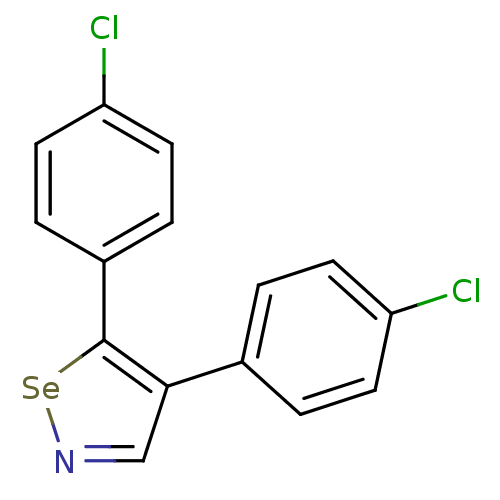
(4,5-Bis(4-chlorophenyl)-1,2-selenazole | CHEMBL501...)Show InChI InChI=1S/C15H9Cl2NSe/c16-12-5-1-10(2-6-12)14-9-18-19-15(14)11-3-7-13(17)8-4-11/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University
Curated by ChEMBL
| Assay Description
Inhibition of bovine COX1 |
Eur J Med Chem 43: 1152-9 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.007
BindingDB Entry DOI: 10.7270/Q2MG7QDR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50038649

(2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...)Show SMILES CC1(C)Cc2c(c(c(CC(O)=O)n2C1)-c1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C23H22ClNO2/c1-23(2)13-19-22(15-6-4-3-5-7-15)21(16-8-10-17(24)11-9-16)18(12-20(26)27)25(19)14-23/h3-11H,12-14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247577
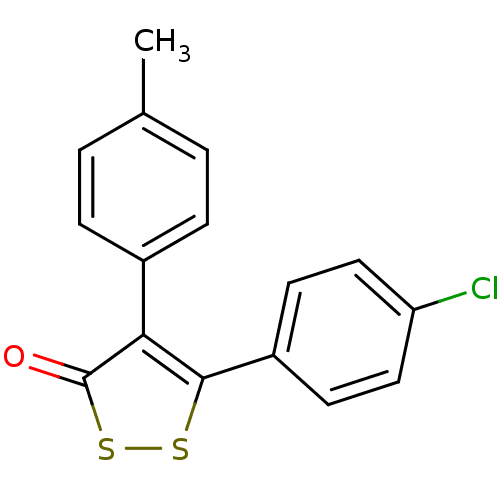
(5-(4-Chlorophenyl)-4-p-tolyl-3H-1,2-dithiol-3-one ...)Show InChI InChI=1S/C16H11ClOS2/c1-10-2-4-11(5-3-10)14-15(19-20-16(14)18)12-6-8-13(17)9-7-12/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50247848
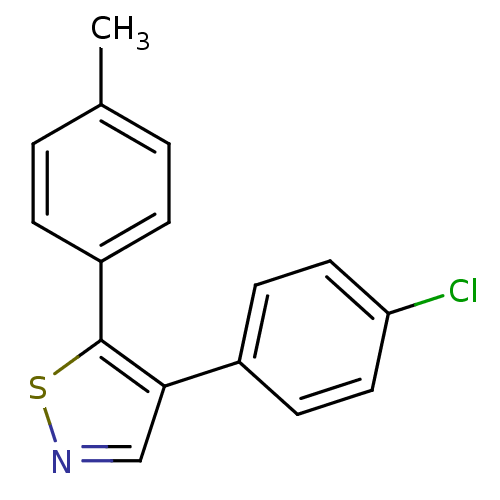
(4-(4-Chlorophenyl)-5-p-tolylisothiazole | CHEMBL49...)Show InChI InChI=1S/C16H12ClNS/c1-11-2-4-13(5-3-11)16-15(10-18-19-16)12-6-8-14(17)9-7-12/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg-University
Curated by ChEMBL
| Assay Description
Inhibition of COX1 in bovine platelets assessed as formation of 12-hydroxyheptadecatrienoic acid by HPLC |
Bioorg Med Chem 17: 558-68 (2009)
Article DOI: 10.1016/j.bmc.2008.11.074
BindingDB Entry DOI: 10.7270/Q2X92B4S |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Canis familiaris) | BDBM50179609
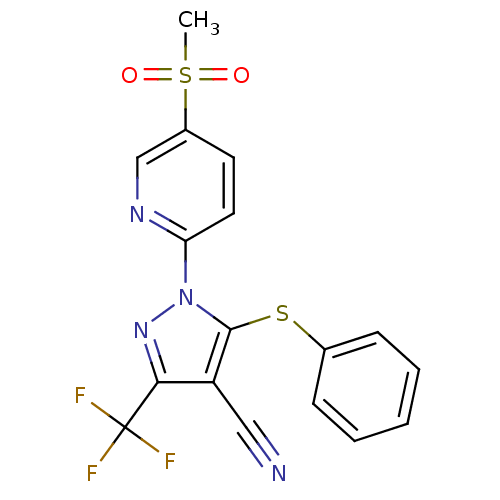
(1-(5-(methylsulfonyl)pyridin-2-yl)-5-(phenylthio)-...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(c(C#N)c1Sc1ccccc1)C(F)(F)F Show InChI InChI=1S/C17H11F3N4O2S2/c1-28(25,26)12-7-8-14(22-10-12)24-16(27-11-5-3-2-4-6-11)13(9-21)15(23-24)17(18,19)20/h2-8,10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 by canine whole blood assay |
Bioorg Med Chem Lett 16: 1202-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.110
BindingDB Entry DOI: 10.7270/Q2FT8KKM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Canis familiaris) | BDBM50179598
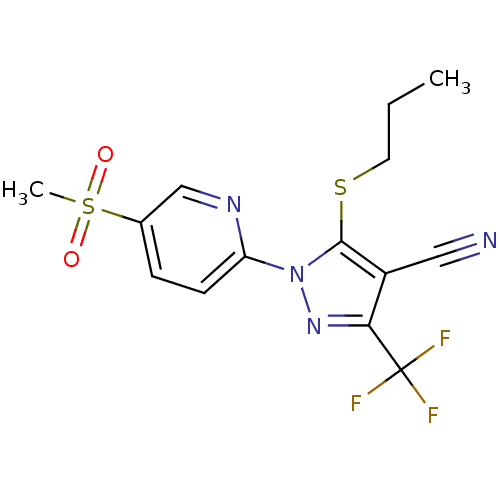
(1-(5-(methylsulfonyl)pyridin-2-yl)-5-(propylthio)-...)Show SMILES CCCSc1c(C#N)c(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C14H13F3N4O2S2/c1-3-6-24-13-10(7-18)12(14(15,16)17)20-21(13)11-5-4-9(8-19-11)25(2,22)23/h4-5,8H,3,6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 by canine whole blood assay |
Bioorg Med Chem Lett 16: 1202-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.110
BindingDB Entry DOI: 10.7270/Q2FT8KKM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Canis familiaris) | BDBM50137411
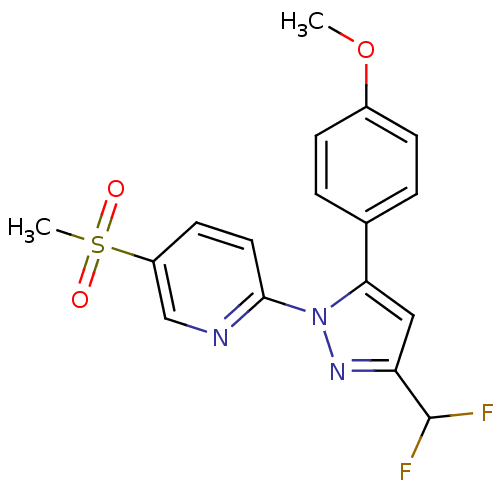
(2-[3-Difluoromethyl-5-(4-methoxy-phenyl)-pyrazol-1...)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1ccc(cn1)S(C)(=O)=O)C(F)F Show InChI InChI=1S/C17H15F2N3O3S/c1-25-12-5-3-11(4-6-12)15-9-14(17(18)19)21-22(15)16-8-7-13(10-20-16)26(2,23)24/h3-10,17H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 1. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM50245434
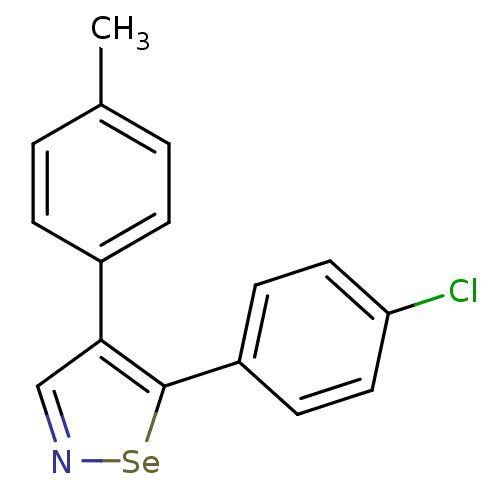
(5-(4-Chlorophenyl)-4-p-tolyl-1,2-selenazole | CHEM...)Show InChI InChI=1S/C16H12ClNSe/c1-11-2-4-12(5-3-11)15-10-18-19-16(15)13-6-8-14(17)9-7-13/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University
Curated by ChEMBL
| Assay Description
Inhibition of bovine COX1 |
Eur J Med Chem 43: 1152-9 (2008)
Article DOI: 10.1016/j.ejmech.2007.09.007
BindingDB Entry DOI: 10.7270/Q2MG7QDR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50094551
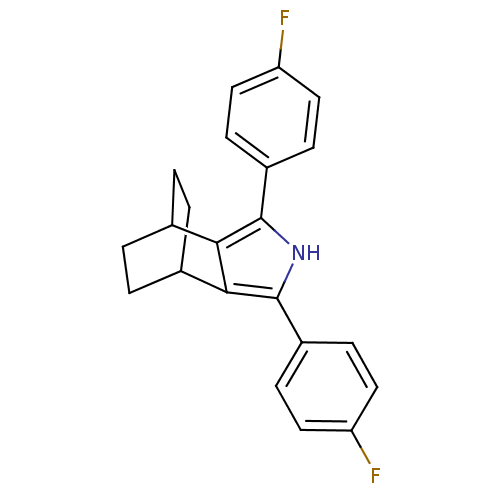
(3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.2.0*2...)Show SMILES Fc1ccc(cc1)-c1[nH]c(c2C3CCC(CC3)c12)-c1ccc(F)cc1 |(21.01,-36.41,;22.48,-35.94,;22.8,-34.43,;24.27,-33.96,;25.41,-35,;25.09,-36.5,;23.63,-36.98,;26.88,-34.53,;28.12,-35.45,;29.38,-34.55,;28.9,-33.08,;29.67,-31.77,;28.93,-30.43,;27.39,-30.42,;26.61,-31.74,;27.93,-30.96,;28.33,-32.53,;27.36,-33.07,;30.84,-35.03,;31.15,-36.54,;32.61,-37.02,;33.76,-36,;35.23,-36.48,;33.44,-34.48,;31.98,-34,)| Show InChI InChI=1S/C22H19F2N/c23-17-9-5-15(6-10-17)21-19-13-1-2-14(4-3-13)20(19)22(25-21)16-7-11-18(24)12-8-16/h5-14,25H,1-4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibition against prostaglandin G/H synthase 1 from mouse resident macrophages |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50094546
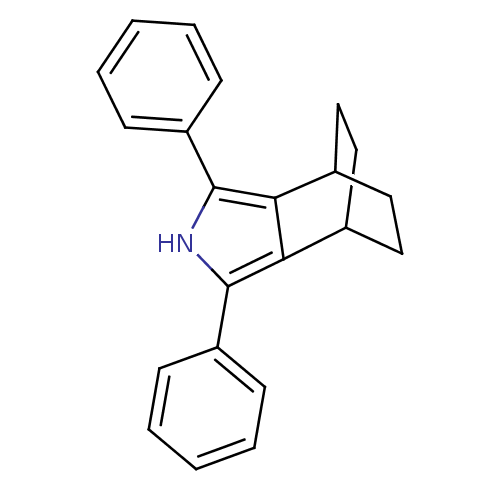
(3,5-Diphenyl-4-aza-tricyclo[5.2.2.0*2,6*]undeca-2,...)Show SMILES C1CC2CCC1c1c([nH]c(c21)-c1ccccc1)-c1ccccc1 |(14.74,-30.53,;13.2,-30.52,;12.42,-31.84,;13.75,-31.06,;14.15,-32.63,;15.49,-31.87,;14.71,-33.19,;15.19,-34.65,;13.94,-35.55,;12.69,-34.64,;13.18,-33.17,;11.23,-35.11,;10.08,-34.06,;8.61,-34.53,;8.28,-36.04,;9.44,-37.08,;10.9,-36.61,;16.65,-35.13,;16.96,-36.64,;18.43,-37.13,;19.58,-36.1,;19.26,-34.58,;17.8,-34.11,)| Show InChI InChI=1S/C22H21N/c1-3-7-17(8-4-1)21-19-15-11-13-16(14-12-15)20(19)22(23-21)18-9-5-2-6-10-18/h1-10,15-16,23H,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibition against prostaglandin G/H synthase 1 from mouse resident macrophages |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50094542
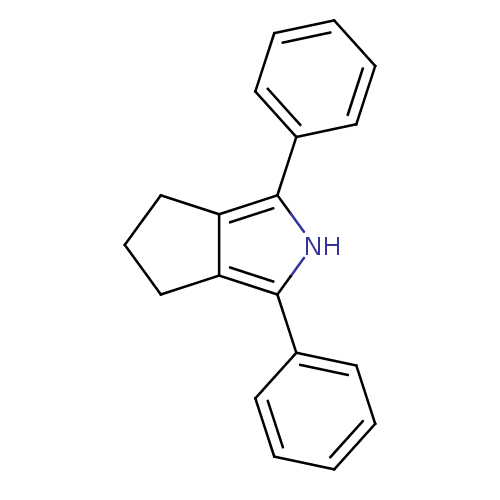
(1,3-Diphenyl-2,4,5,6-tetrahydro-cyclopenta[c]pyrro...)Show InChI InChI=1S/C19H17N/c1-3-8-14(9-4-1)18-16-12-7-13-17(16)19(20-18)15-10-5-2-6-11-15/h1-6,8-11,20H,7,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier
Curated by ChEMBL
| Assay Description
Inhibition against prostaglandin G/H synthase 1 from mouse resident macrophages |
J Med Chem 43: 4582-93 (2001)
BindingDB Entry DOI: 10.7270/Q23B60T1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Canis familiaris) | BDBM50137423
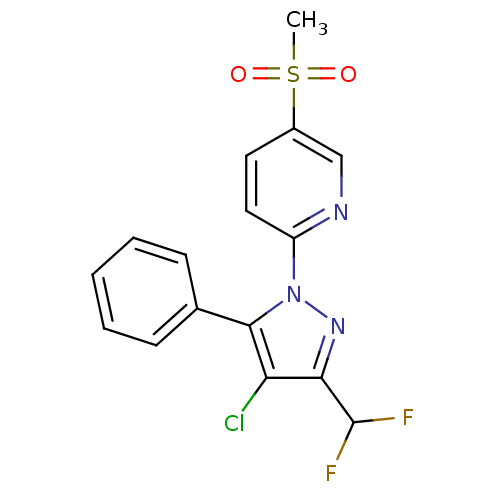
(2-(4-Chloro-3-difluoromethyl-5-phenyl-pyrazol-1-yl...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(C(F)F)c(Cl)c1-c1ccccc1 Show InChI InChI=1S/C16H12ClF2N3O2S/c1-25(23,24)11-7-8-12(20-9-11)22-15(10-5-3-2-4-6-10)13(17)14(21-22)16(18)19/h2-9,16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against cannine prostaglandin G/H synthase 1. |
Bioorg Med Chem Lett 14: 95-8 (2003)
BindingDB Entry DOI: 10.7270/Q25B01WP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Bos taurus) | BDBM22360
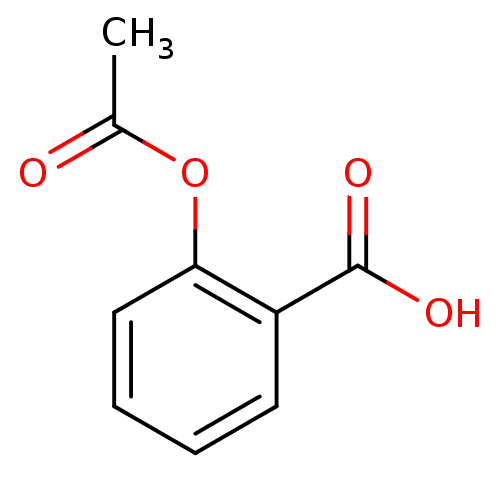
(2-(acetyloxy)benzoate | 2-(acetyloxy)benzoic acid ...)Show InChI InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by ChEMBL
| Assay Description
Inhibition of bovine COX-1 by enzyme immuno assay |
Bioorg Med Chem Lett 19: 6855-61 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.083
BindingDB Entry DOI: 10.7270/Q2D79CB6 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Canis familiaris) | BDBM50179596
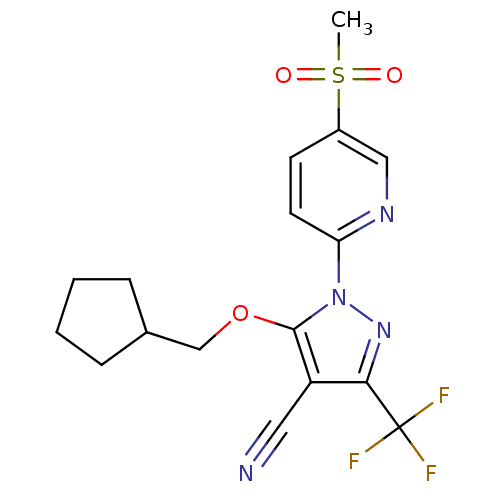
(5-(cyclopentylmethoxy)-1-(5-(methylsulfonyl)pyridi...)Show SMILES CS(=O)(=O)c1ccc(nc1)-n1nc(c(C#N)c1OCC1CCCC1)C(F)(F)F Show InChI InChI=1S/C17H17F3N4O3S/c1-28(25,26)12-6-7-14(22-9-12)24-16(27-10-11-4-2-3-5-11)13(8-21)15(23-24)17(18,19)20/h6-7,9,11H,2-5,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX1 by canine whole blood assay |
Bioorg Med Chem Lett 16: 1202-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.110
BindingDB Entry DOI: 10.7270/Q2FT8KKM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data