

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50127604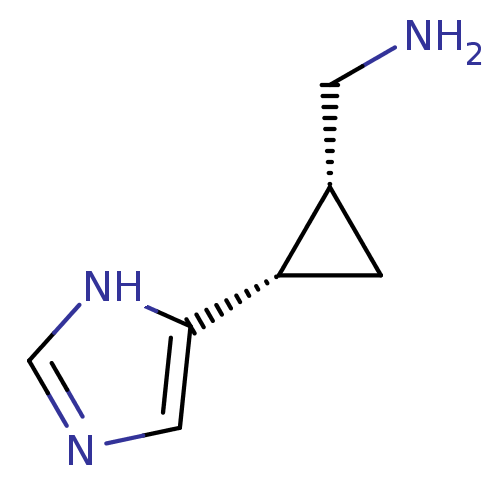 (C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Histamine H4 receptor | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50127608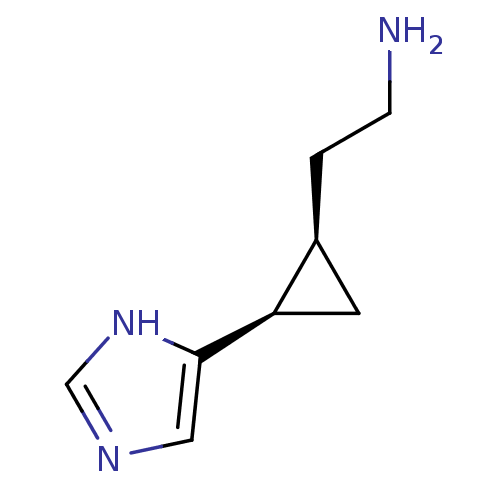 (2-[2-(1H-Imidazol-4-yl)-cyclopropyl]-ethylamine | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Histamine H4 receptor | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50127605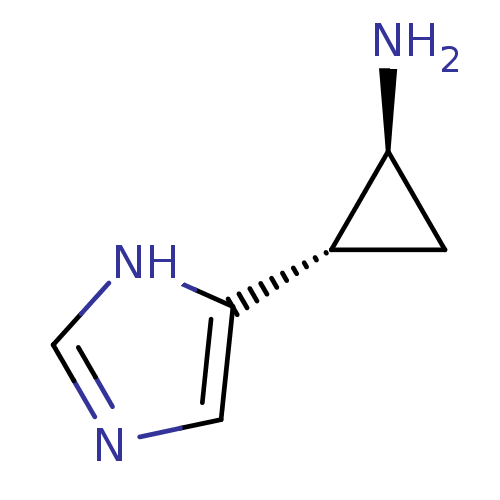 ((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Histamine H4 receptor | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50127607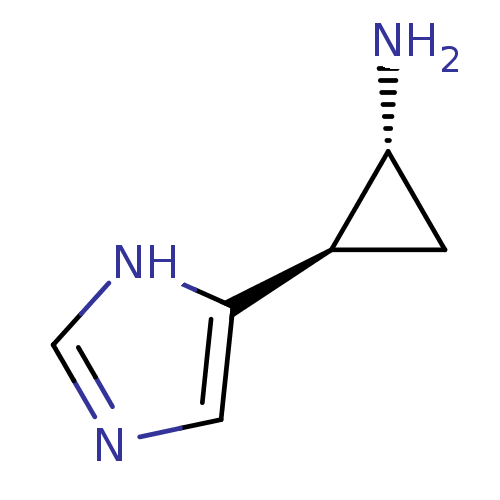 ((1R,2R)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Histamine H4 receptor | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50127606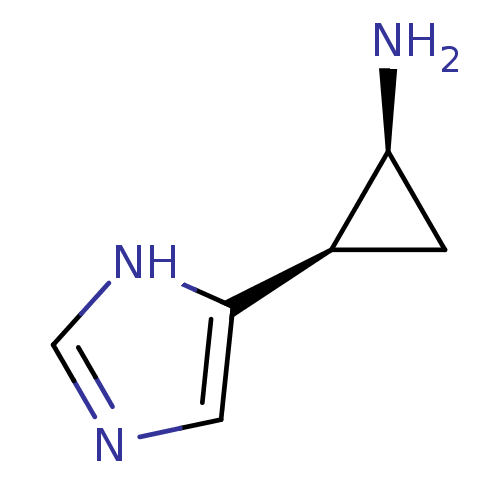 (2-(1H-Imidazol-4-yl)-cyclopropylamine | CHEMBL2940...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Histamine H4 receptor | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50127602 (C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Histamine H4 receptor | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50127609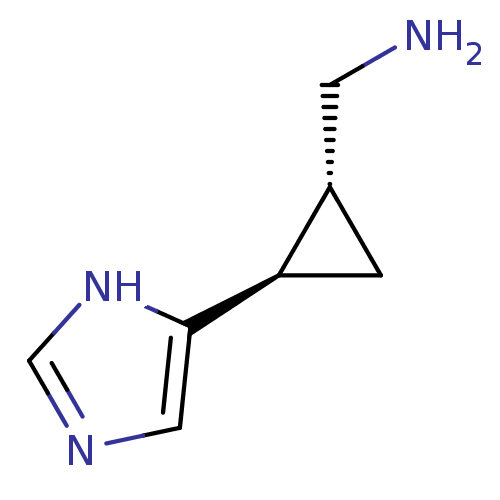 (C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Histamine H4 receptor | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50127603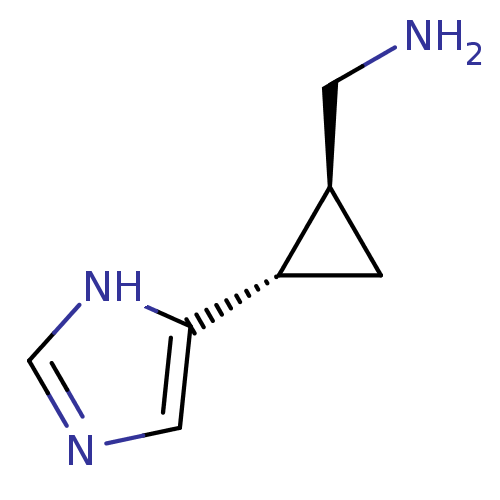 (C-[2-(1H-Imidazol-4-yl)-cyclopropyl]-methylamine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Histamine H4 receptor | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50127610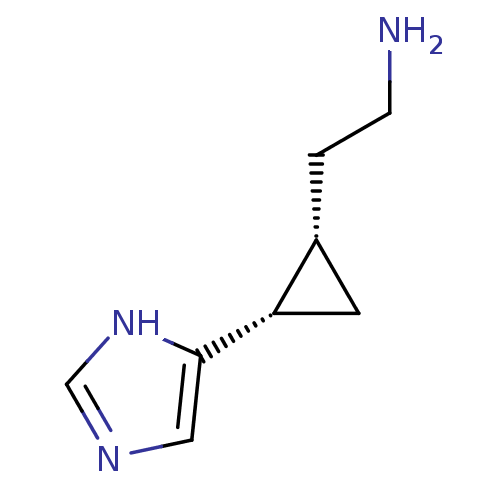 ((1S,2S)-2-(2-aminoethyl)-1-(1H-imidazol-4-yl)cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Histamine H4 receptor | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50127601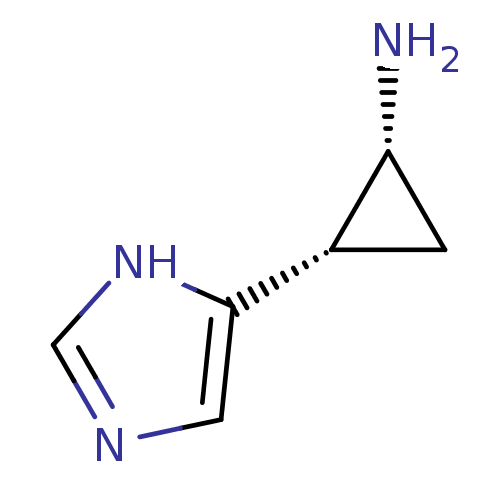 (2-(1H-Imidazol-4-yl)-cyclopropylamine | CHEMBL5938...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >0.000100 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Histamine H4 receptor | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50509072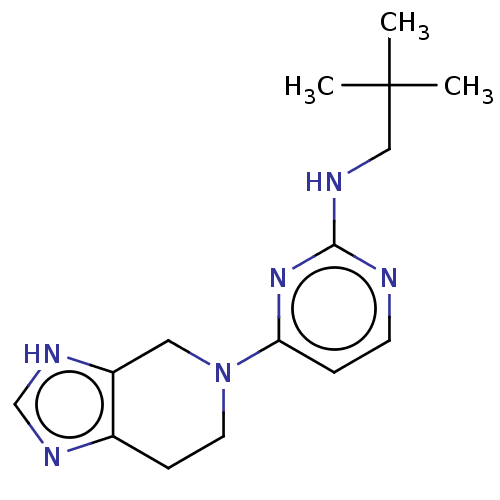 (CHEMBL4454158) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.123 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R expressed in HEK293-SF-hH4R-His6-CRE-Luc cells incubated for 5 hrs by luciferase reporter gene assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319300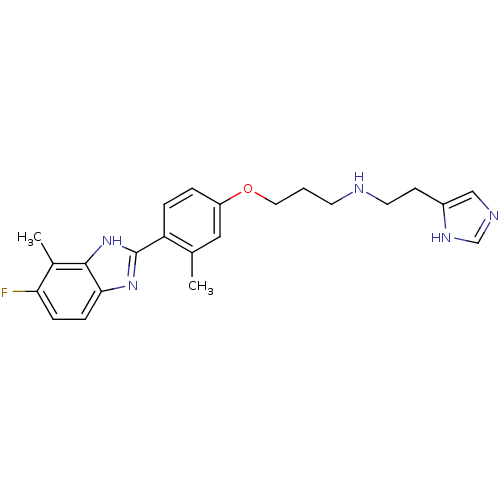 (CHEMBL1083162 | N-(2-(1H-imidazol-4-yl)ethyl)-3-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko... | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50509078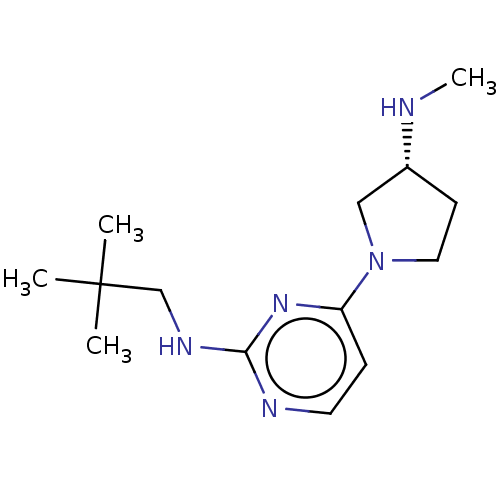 (CHEMBL4443126) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R expressed in HEK293-SF-hH4R-His6-CRE-Luc cells incubated for 5 hrs by luciferase reporter gene assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50418058 (CHEMBL1688946) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
VU University Amsterdam Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in HEK293 cells assessed by forskolin induced cAMP response element activation by luciferas... | J Med Chem 54: 1693-703 (2011) Article DOI: 10.1021/jm1013488 BindingDB Entry DOI: 10.7270/Q20G3MDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50509065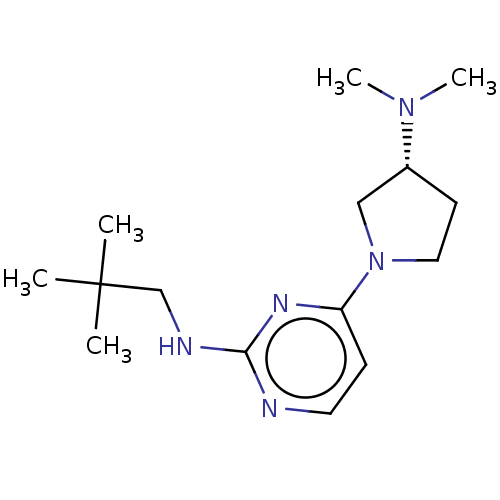 (CHEMBL4439142) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R expressed in HEK293-SF-hH4R-His6-CRE-Luc cells incubated for 5 hrs by luciferase reporter gene assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50413499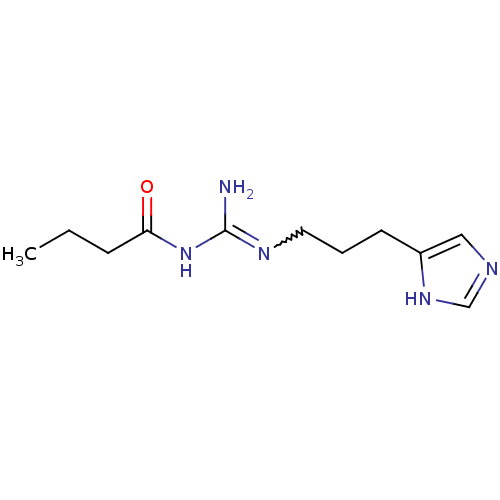 (CHEMBL465170 | UR-PI295) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4 receptor expressed in insect Sf9 cells co-expressing RGS19 fusion protein and Gialpha2, Gbeta1gamma2 assessed as gamma[3... | J Med Chem 52: 2623-7 (2009) Article DOI: 10.1021/jm9000693 BindingDB Entry DOI: 10.7270/Q2P270BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50415917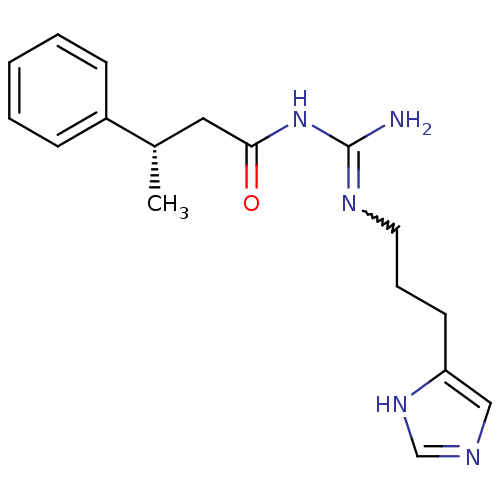 (CHEMBL1096429) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.63 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Activity at human recombinant GAIP-fused histamine H4 receptor expressed in Sf9 cells coexpressing Galphai, Gbeta1gamma2 by steady-state GTPase activ... | Bioorg Med Chem Lett 20: 3173-6 (2010) Article DOI: 10.1016/j.bmcl.2010.03.082 BindingDB Entry DOI: 10.7270/Q2RF5W9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50413498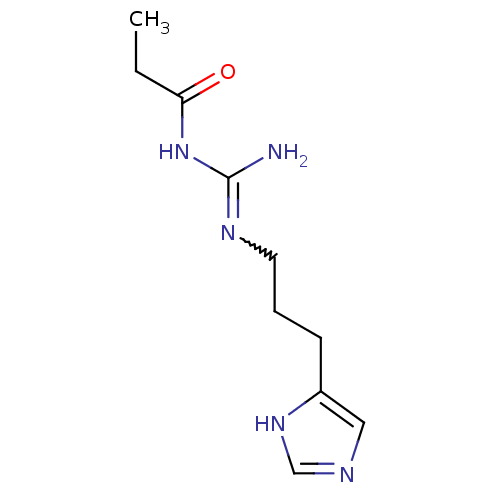 (CHEMBL475621 | UR-PI294) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4 receptor expressed in insect Sf9 cells co-expressing RGS19 fusion protein and Gialpha2, Gbeta1gamma2 assessed as gamma[3... | J Med Chem 52: 2623-7 (2009) Article DOI: 10.1021/jm9000693 BindingDB Entry DOI: 10.7270/Q2P270BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50514105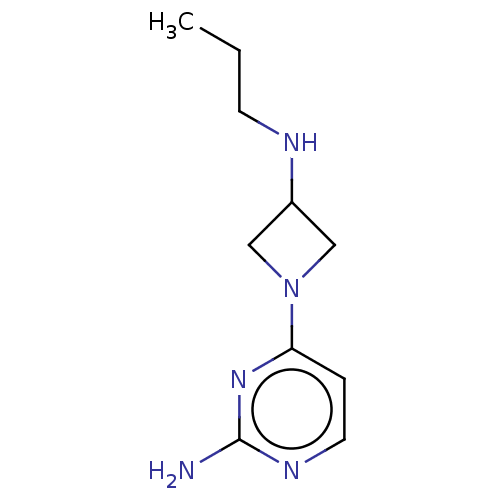 (CHEMBL4441731) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Agonist activity at human H4R expressed on HEK293T cells assessed as inhibition of forskolin-induced CRE-driven luciferase activity co-incubated with... | J Med Chem 62: 10848-10866 (2019) Article DOI: 10.1021/acs.jmedchem.9b01462 BindingDB Entry DOI: 10.7270/Q2M61PKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319302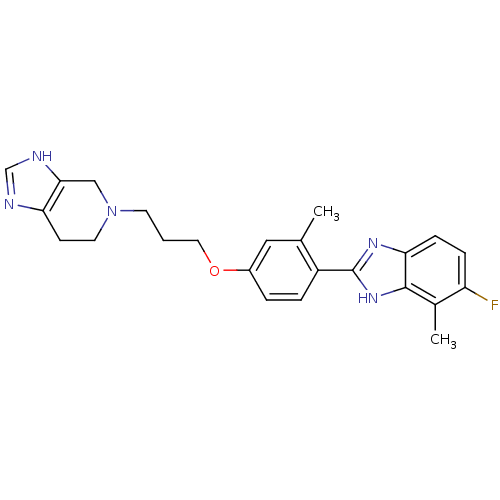 (5-(3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko... | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319295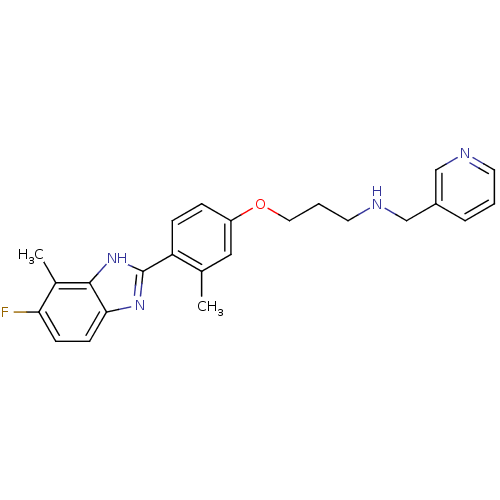 (3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-2-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko... | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50509071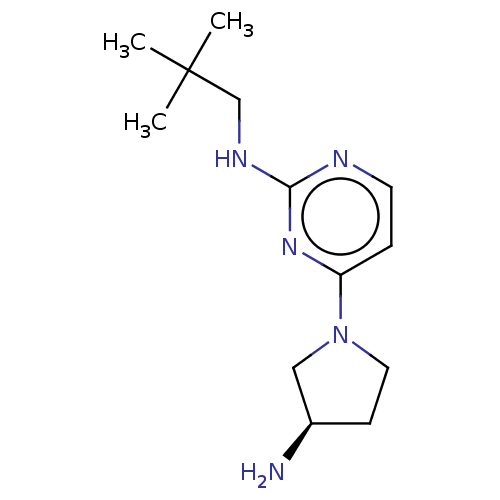 (CHEMBL4455324) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R expressed in HEK293-SF-hH4R-His6-CRE-Luc cells incubated for 5 hrs by luciferase reporter gene assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50413500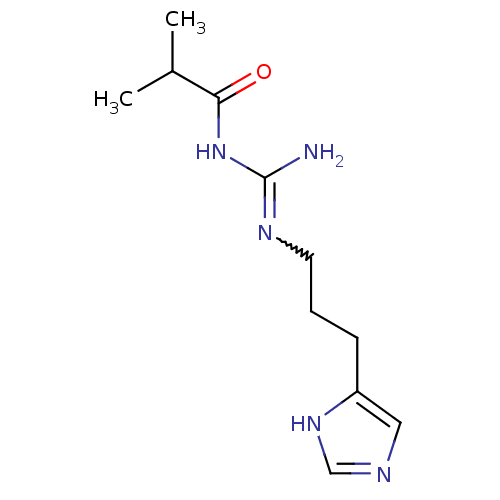 (CHEMBL471724 | UR-PI287) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4 receptor expressed in insect Sf9 cells co-expressing RGS19 fusion protein and Gialpha2, Gbeta1gamma2 assessed as gamma[3... | J Med Chem 52: 2623-7 (2009) Article DOI: 10.1021/jm9000693 BindingDB Entry DOI: 10.7270/Q2P270BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50061056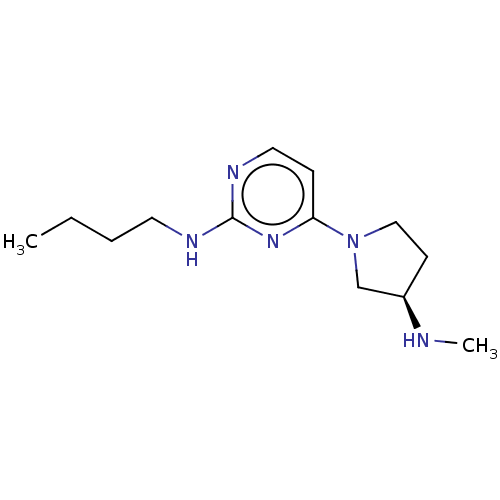 (CHEMBL3393542 | US9732087, 73) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research& Development, LLC Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H4 receptor expressed in SK-N-MC cells assessed as effect on forskolin-stimulated cAMP-mediated repor... | Bioorg Med Chem Lett 25: 956-9 (2015) Article DOI: 10.1016/j.bmcl.2014.12.027 BindingDB Entry DOI: 10.7270/Q2639RDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50509072 (CHEMBL4454158) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R expressed in HEK293T-beta-arr2-hH4R cells by beta-arrestin2 recruitment assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50413498 (CHEMBL475621 | UR-PI294) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R by [35S]-GTPgammaS-binding assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50415915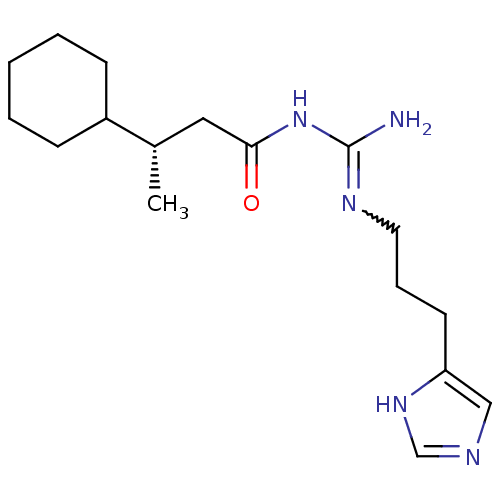 (CHEMBL1096431) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.57 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Activity at human recombinant GAIP-fused histamine H4 receptor expressed in Sf9 cells coexpressing Galphai, Gbeta1gamma2 by steady-state GTPase activ... | Bioorg Med Chem Lett 20: 3173-6 (2010) Article DOI: 10.1016/j.bmcl.2010.03.082 BindingDB Entry DOI: 10.7270/Q2RF5W9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50413497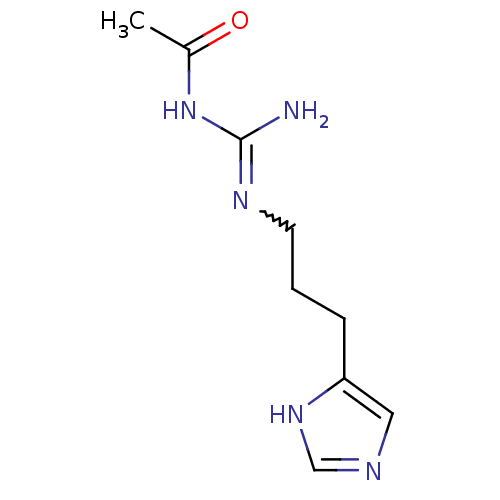 (CHEMBL443896 | UR-PI288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4 receptor expressed in insect Sf9 cells co-expressing RGS19 fusion protein and Gialpha2, Gbeta1gamma2 assessed as gamma[3... | J Med Chem 52: 2623-7 (2009) Article DOI: 10.1021/jm9000693 BindingDB Entry DOI: 10.7270/Q2P270BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50276073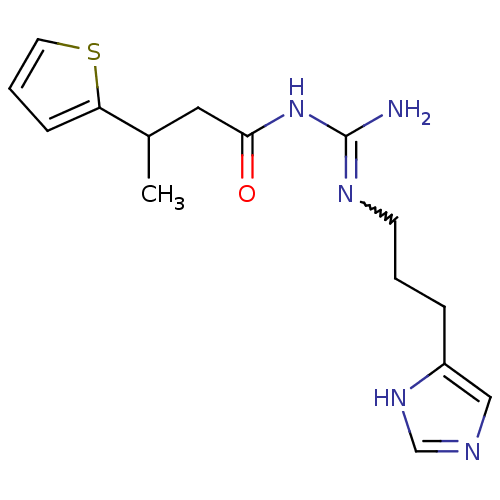 (CHEMBL502339 | N1-[3-(1H-Imidazol-4-yl)propyl]-N2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Activity at human recombinant histamine H4 receptor-RGS4 fusion protein expressed in Sf9 cells coexpressing Galphai2 and G-beta-1-gamma-2 by steady-s... | J Med Chem 51: 7193-204 (2009) Article DOI: 10.1021/jm800841w BindingDB Entry DOI: 10.7270/Q2RN38T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50518052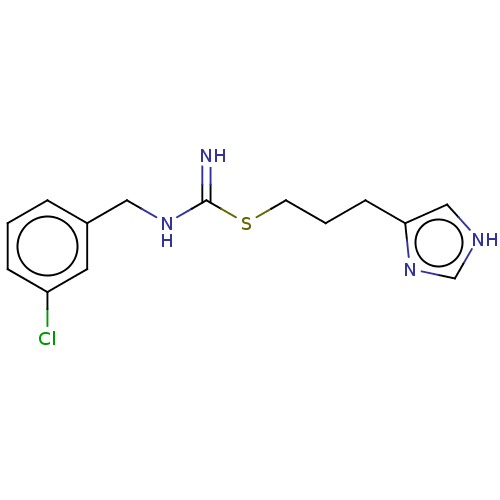 (CHEMBL551790) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at histamine H4 receptor (unknown origin) expressed in HEK293T cells harboring CRE-luciferase after 6 hrs by luciferase reporter gen... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50319307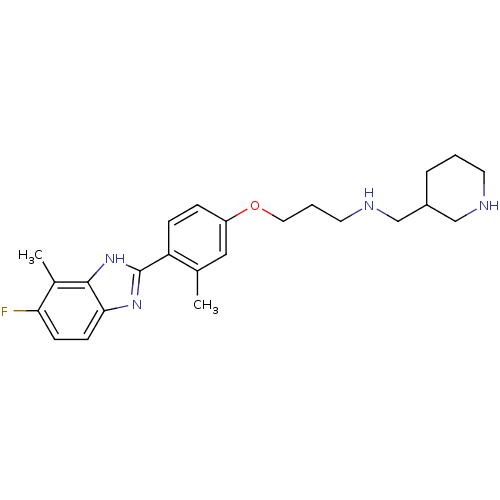 ((+/-)-3-(4-(5-fluoro-4-methyl-1H-benzo[d]imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor in SK-N-MC cells assessed as forskolin-stimulated cAMP release preincubated for 10 mins before forsko... | Bioorg Med Chem Lett 20: 3367-71 (2010) Article DOI: 10.1016/j.bmcl.2010.04.017 BindingDB Entry DOI: 10.7270/Q23J3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50509078 (CHEMBL4443126) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R expressed in HEK293T-beta-arr2-hH4R cells by beta-arrestin2 recruitment assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50413503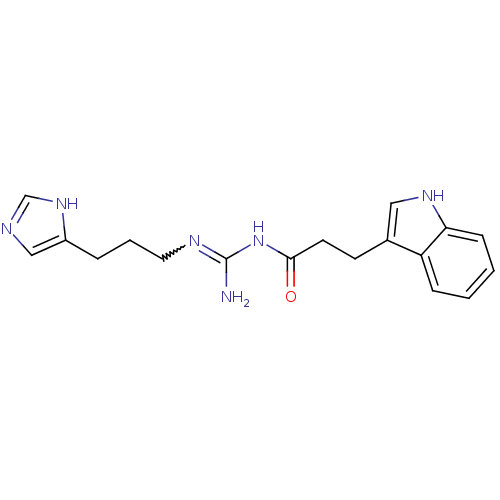 (CHEMBL514641 | UR-PI141) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4 receptor expressed in insect Sf9 cells co-expressing RGS19 fusion protein and Gialpha2, Gbeta1gamma2 assessed as gamma[3... | J Med Chem 52: 2623-7 (2009) Article DOI: 10.1021/jm9000693 BindingDB Entry DOI: 10.7270/Q2P270BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R by [35S]-GTPgammaS-binding assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50275884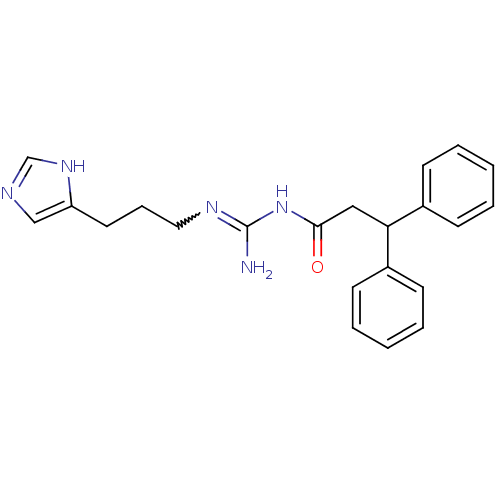 (CHEMBL471413 | N1-(3,3-Diphenylpropanoyl)-N2-[3-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H4 receptor expressed in Sf9 cells coexpressing RGS19, Galphai2, Gbeta1gamma2 by steady-state GTPase ... | Bioorg Med Chem Lett 20: 7191-9 (2010) Article DOI: 10.1016/j.bmcl.2010.10.041 BindingDB Entry DOI: 10.7270/Q2Q81GX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at human histamine 4 receptor expressed in Sf9 cell membranes co-expressing Galphai2 and Gbeta1gamma2 assessed as [35S]GTPgammaS ... | Bioorg Med Chem Lett 26: 292-300 (2016) Article DOI: 10.1016/j.bmcl.2015.12.035 BindingDB Entry DOI: 10.7270/Q2Z03B0K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50413501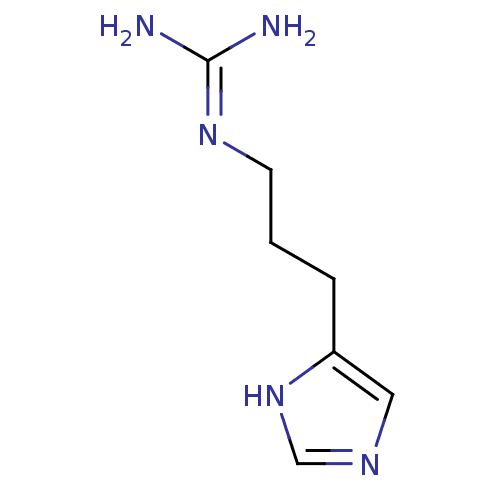 (CHEMBL325327 | SK&F-91486 | SK-91486) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4 receptor expressed in insect Sf9 cells co-expressing RGS19 fusion protein and Gialpha2, Gbeta1gamma2 assessed as gamma[3... | J Med Chem 52: 2623-7 (2009) Article DOI: 10.1021/jm9000693 BindingDB Entry DOI: 10.7270/Q2P270BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50415916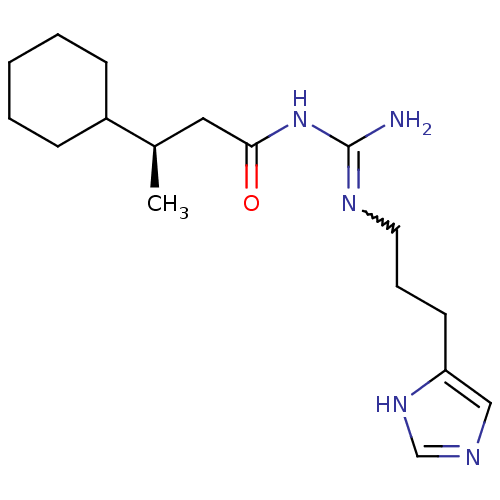 (CHEMBL1096430) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.32 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Activity at human recombinant GAIP-fused histamine H4 receptor expressed in Sf9 cells coexpressing Galphai, Gbeta1gamma2 by steady-state GTPase activ... | Bioorg Med Chem Lett 20: 3173-6 (2010) Article DOI: 10.1016/j.bmcl.2010.03.082 BindingDB Entry DOI: 10.7270/Q2RF5W9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50394703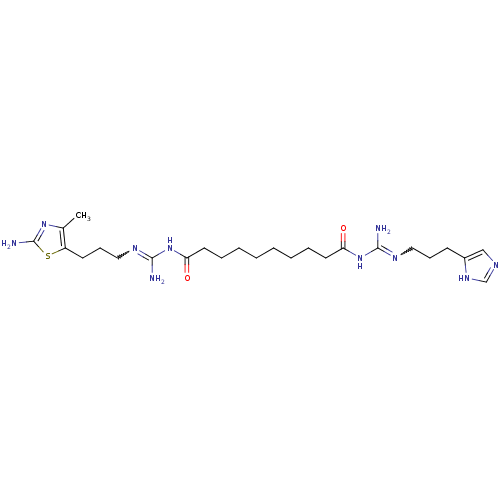 (CHEMBL2165628) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.51 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R-RGS19 Galphai2 Gbeta1gamma2 expressed in Sf9 cells at 0.1 nM to 1 mM by steady state GTPase activity assay | J Med Chem 55: 1147-60 (2012) Article DOI: 10.1021/jm201128q BindingDB Entry DOI: 10.7270/Q2HQ411W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50394694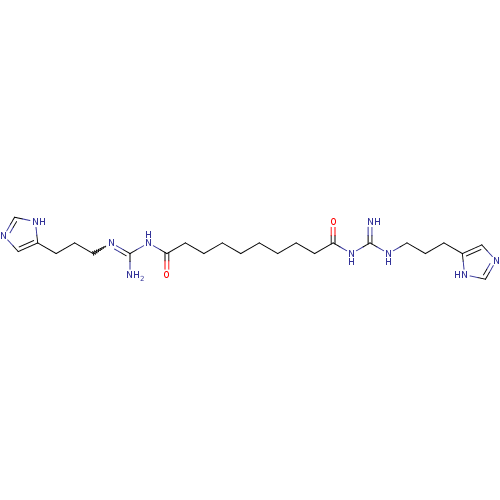 (CHEMBL2165642) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.51 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R-RGS19 Galphai2 Gbeta1gamma2 expressed in Sf9 cells at 0.1 nM to 1 mM by steady state GTPase activity assay | J Med Chem 55: 1147-60 (2012) Article DOI: 10.1021/jm201128q BindingDB Entry DOI: 10.7270/Q2HQ411W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50275884 (CHEMBL471413 | N1-(3,3-Diphenylpropanoyl)-N2-[3-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Activity at human recombinant histamine H4 receptor-RGS4 fusion protein expressed in Sf9 cells coexpressing Galphai2 and G-beta-1-gamma-2 by steady-s... | J Med Chem 51: 7193-204 (2009) Article DOI: 10.1021/jm800841w BindingDB Entry DOI: 10.7270/Q2RN38T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM7966 (2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 9.12 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Activity at human recombinant GAIP-fused histamine H4 receptor expressed in Sf9 cells coexpressing Galphai, Gbeta1gamma2 by steady-state GTPase activ... | Bioorg Med Chem Lett 20: 3173-6 (2010) Article DOI: 10.1016/j.bmcl.2010.03.082 BindingDB Entry DOI: 10.7270/Q2RF5W9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50343016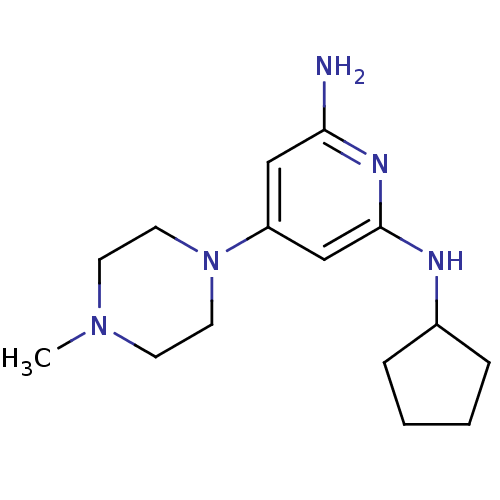 (CHEMBL1770966 | N2-cyclopentyl-4-(4-methylpiperazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in sf9 cell membrane co-expressing mammalian Galphai2 and Gbeta1gamma2 incubated for 90 min... | J Med Chem 59: 3452-70 (2016) Article DOI: 10.1021/acs.jmedchem.6b00120 BindingDB Entry DOI: 10.7270/Q23T9K42 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM7966 (2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 11.6 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Activity at human recombinant histamine H4 receptor-RGS4 fusion protein expressed in Sf9 cells coexpressing Galphai2 and G-beta-1-gamma-2 by steady-s... | J Med Chem 51: 7193-204 (2009) Article DOI: 10.1021/jm800841w BindingDB Entry DOI: 10.7270/Q2RN38T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50342987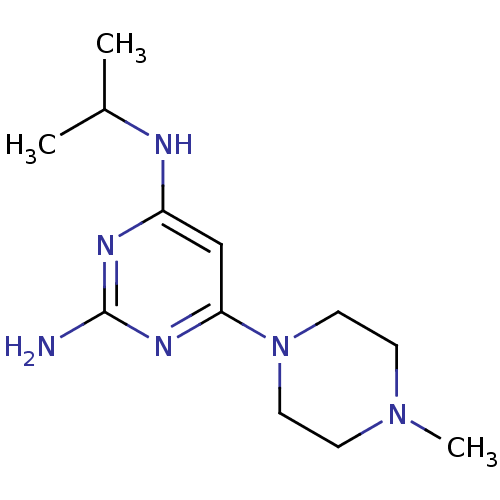 (CHEMBL1770998 | N4-isopropyl-6-(4-methylpiperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as effect on forskolin-induced cAMP accumulation after 6 hr... | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM7966 (2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4 receptor expressed in insect Sf9 cells co-expressing RGS19 fusion protein and Gialpha2, Gbeta1gamma2 assessed as gamma[3... | J Med Chem 52: 2623-7 (2009) Article DOI: 10.1021/jm9000693 BindingDB Entry DOI: 10.7270/Q2P270BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM7966 (2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 12.0 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in Sf9 cells coexpressing RGS19 protein by steady-state GTPase assay | J Med Chem 52: 6297-313 (2009) Article DOI: 10.1021/jm900526h BindingDB Entry DOI: 10.7270/Q2PN96VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor | J Med Chem 54: 26-53 (2011) Article DOI: 10.1021/jm100064d BindingDB Entry DOI: 10.7270/Q2VQ33RV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50483134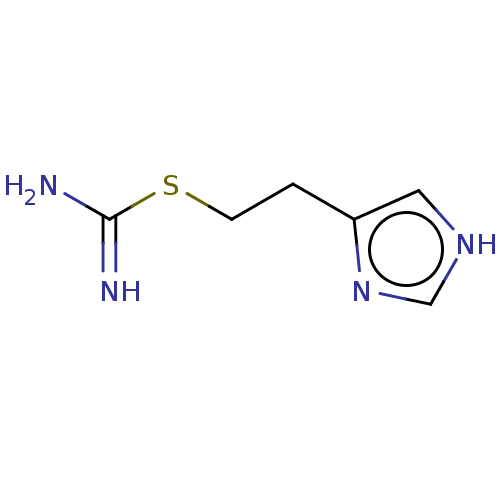 (CHEBI:64156 | Imetit) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human histamine H4 receptor expressed in human SK-N-MC cells by CRE-beta galactosidase reporter gene assay | Bioorg Med Chem Lett 20: 7191-9 (2010) Article DOI: 10.1016/j.bmcl.2010.10.041 BindingDB Entry DOI: 10.7270/Q2Q81GX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 330 total ) | Next | Last >> |