

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356884 (CHEMBL1915540) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at H4R in human eosinophils assessed as inhibition of histamine-induced shape change by GAFS assay | Bioorg Med Chem Lett 21: 6596-602 (2011) Article DOI: 10.1016/j.bmcl.2011.07.125 BindingDB Entry DOI: 10.7270/Q20C4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315348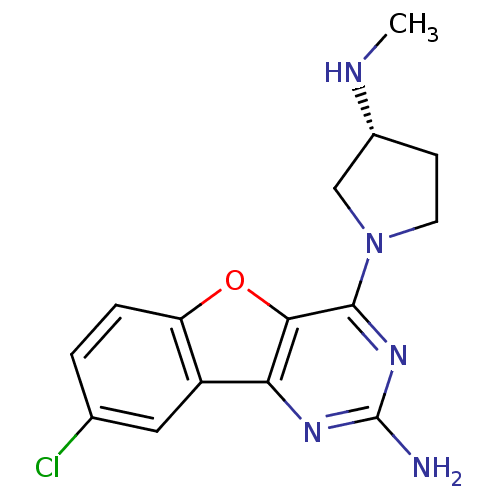 ((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356884 (CHEMBL1915540) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at H4R in human eosinophils assessed as inhibition of histamine-induced actin polymerisation | Bioorg Med Chem Lett 21: 6596-602 (2011) Article DOI: 10.1016/j.bmcl.2011.07.125 BindingDB Entry DOI: 10.7270/Q20C4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50538677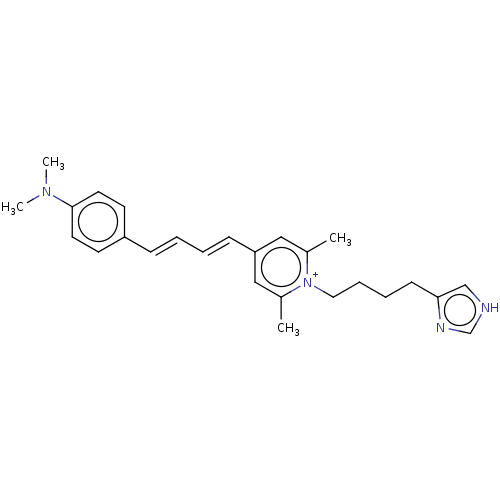 (CHEMBL4635634) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inverse agonist activity at human H4R expressed in HEK293-SF-hH4R-His6-CRE-Luc cells assessed as reduction in histamine-induced inhibition of forskol... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315348 ((R)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inverse agonist activity at human histamine H4 receptor assessed as inhibition of [35S]GTPgammaS binding after 15 mins by scintillation proximity ass... | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315349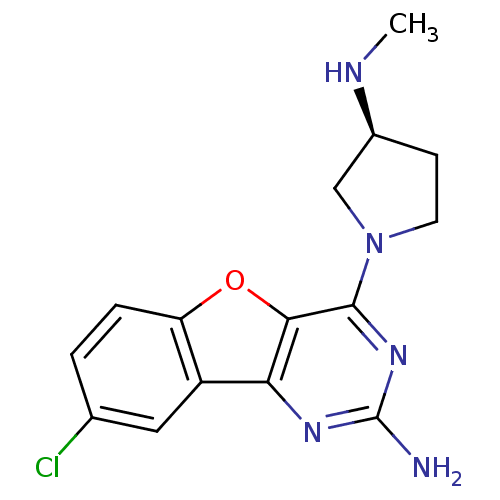 ((S)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inverse agonist activity at human histamine H4 receptor assessed as inhibition of [35S]GTPgammaS binding after 15 mins by scintillation proximity ass... | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356884 (CHEMBL1915540) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at H4R in human eosinophils assessed as inhibition of histamine-induced CD11b upregulation | Bioorg Med Chem Lett 21: 6596-602 (2011) Article DOI: 10.1016/j.bmcl.2011.07.125 BindingDB Entry DOI: 10.7270/Q20C4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at H4R in human eosinophils assessed as inhibition of histamine-induced actin polymerisation | Bioorg Med Chem Lett 21: 6596-602 (2011) Article DOI: 10.1016/j.bmcl.2011.07.125 BindingDB Entry DOI: 10.7270/Q20C4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50415489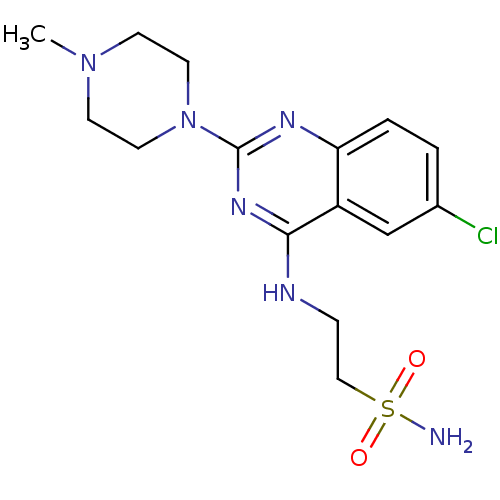 (CHEMBL591969) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV Curated by ChEMBL | Assay Description Inverse agonist activity at human histamine H4 receptor expressed in HEK293T cells by CRE-beta-galactosidase assay | J Med Chem 53: 2390-400 (2010) Article DOI: 10.1021/jm901379s BindingDB Entry DOI: 10.7270/Q2G44RJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294419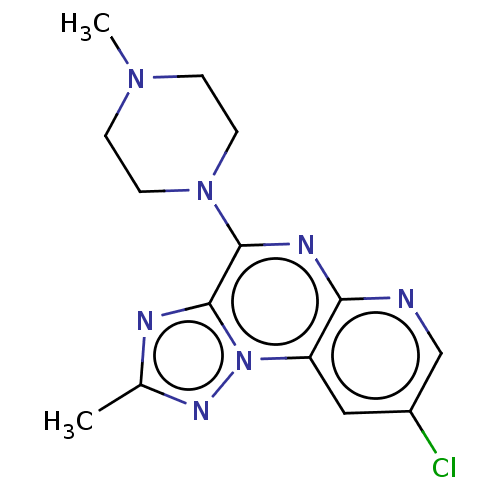 (US9586959, Compound 98) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Antagonist activity at recombinant human H4 receptor expressed in CHOK1 cells co-expressing Galpha16/aequorin assessed as inhibition of histamine-ind... | Bioorg Med Chem 27: 1254-1262 (2019) Article DOI: 10.1016/j.bmc.2019.02.020 BindingDB Entry DOI: 10.7270/Q2C250Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315349 ((S)-8-chloro-4-(3-(methylamino)pyrrolidin-1-yl)ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50538677 (CHEMBL4635634) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inverse agonist activity at human H4R expressed in HEK293 cells co-expressing ELucC/ELucN-beta-arrestin2 assessed as inhibition of histamine-induced ... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294378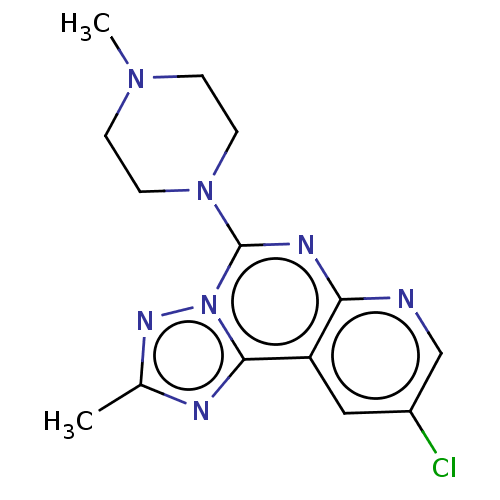 (US9586959, Compound 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315314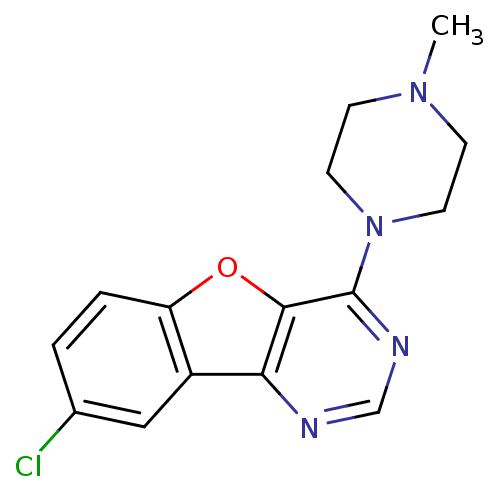 (8-chloro-4-(4-methylpiperazin-1-yl)benzofuro[3,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£ Curated by ChEMBL | Assay Description Antagonist activity at human H4 receptor expressed in CHO-K1 cells co-expressing G protein alpha16 assessed as inhibition of histamine-induced calciu... | J Med Chem 62: 11416-11422 (2019) Article DOI: 10.1021/acs.jmedchem.9b00937 BindingDB Entry DOI: 10.7270/Q2736V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294427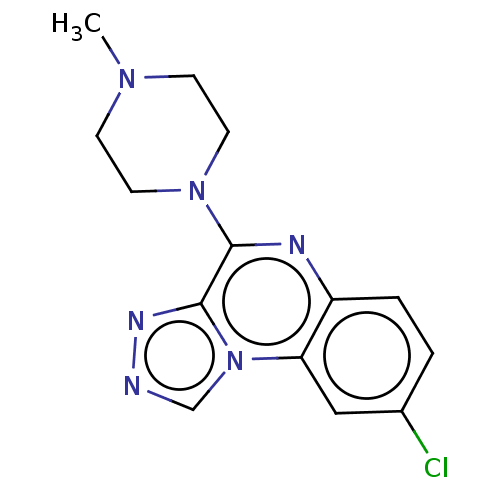 (US9586959, Compound disclosed in WO 2010030785, Ex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294427 (US9586959, Compound disclosed in WO 2010030785, Ex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294407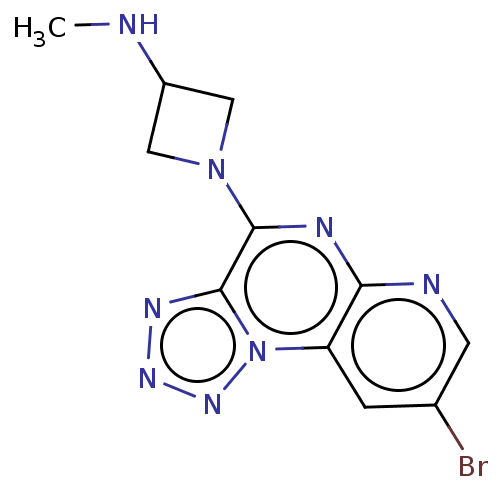 (US9586959, Compound 69 | US9586959, Compound 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294407 (US9586959, Compound 69 | US9586959, Compound 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at recombinant human histamine H4 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine induced st... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294418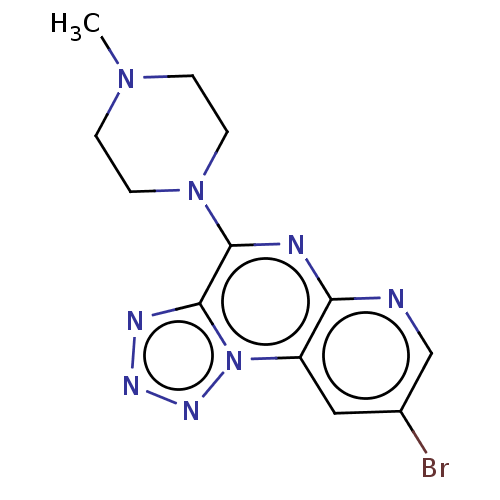 (US9586959, Compound 96) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294418 (US9586959, Compound 96) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at H4R in human eosinophils assessed as inhibition of imetit-induced shape change by GAFS assay | Bioorg Med Chem Lett 21: 6596-602 (2011) Article DOI: 10.1016/j.bmcl.2011.07.125 BindingDB Entry DOI: 10.7270/Q20C4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294373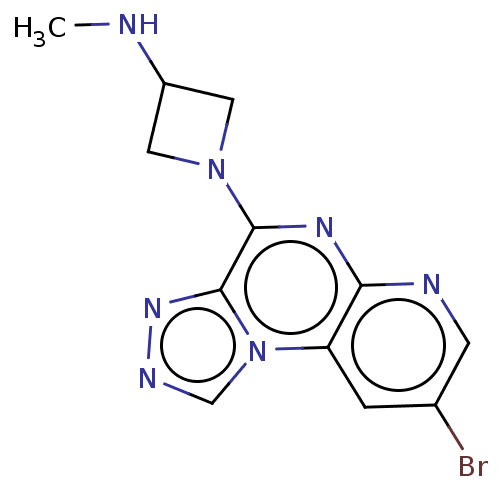 (US9586959, Compound 104 | US9586959, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315333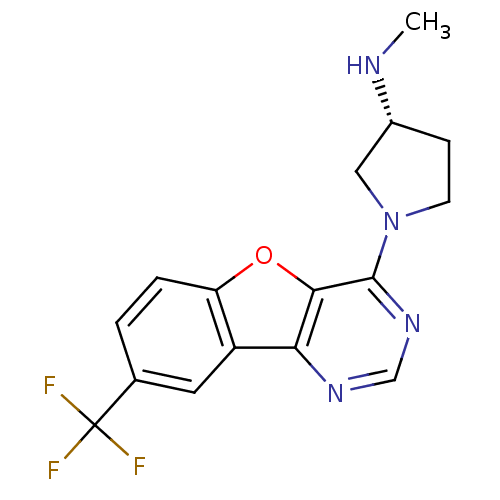 ((R)-N-methyl-1-(8-(trifluoromethyl)benzofuro[3,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50537206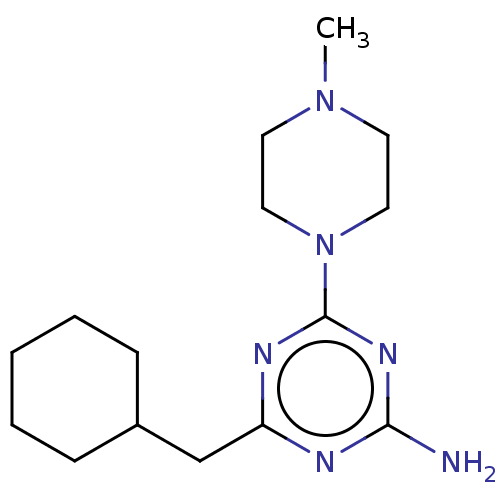 (CHEMBL4483783) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Antagonist activity at recombinant human H4 receptor expressed in CHOK1 cells co-expressing Galpha16/aequorin assessed as inhibition of histamine-ind... | Bioorg Med Chem 27: 1254-1262 (2019) Article DOI: 10.1016/j.bmc.2019.02.020 BindingDB Entry DOI: 10.7270/Q2C250Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315323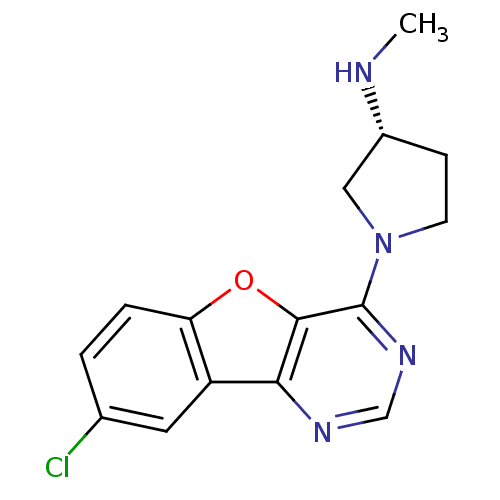 ((R)-1-(8-chlorobenzofuro[3,2-d]pyrimidin-4-yl)-N-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50361015 (CHEMBL592379) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV Curated by ChEMBL | Assay Description Inverse agonist activity at human histamine H4 receptor expressed in HEK293T cells by CRE-beta-galactosidase assay | J Med Chem 53: 2390-400 (2010) Article DOI: 10.1021/jm901379s BindingDB Entry DOI: 10.7270/Q2G44RJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294386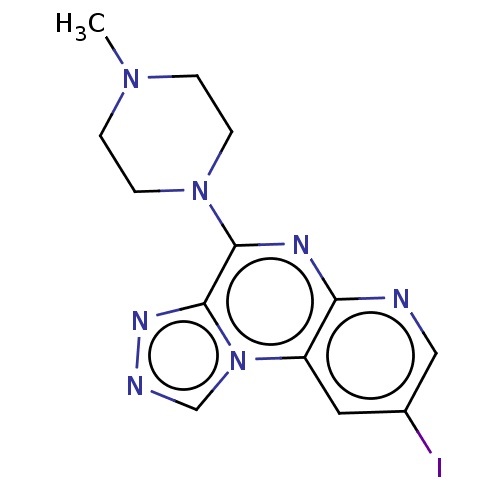 (US9586959, Compound 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294386 (US9586959, Compound 49) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294407 (US9586959, Compound 69 | US9586959, Compound 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294373 (US9586959, Compound 104 | US9586959, Compound 24 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315339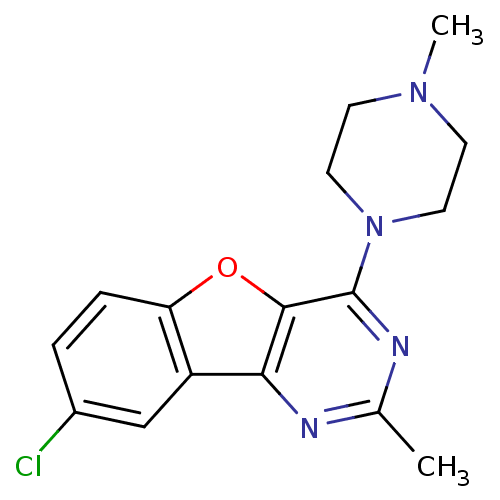 (8-chloro-2-methyl-4-(4-methylpiperazin-1-yl)benzof...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]histamine dihydrochloride from human histamine H4 receptor after 2.5 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315342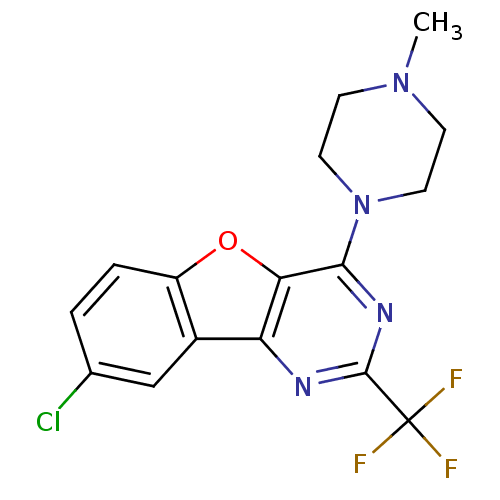 (8-chloro-4-(4-methylpiperazin-1-yl)-2-(trifluorome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inverse agonist activity at human histamine H4 receptor assessed as inhibition of [35S]GTPgammaS binding after 15 mins by scintillation proximity ass... | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294361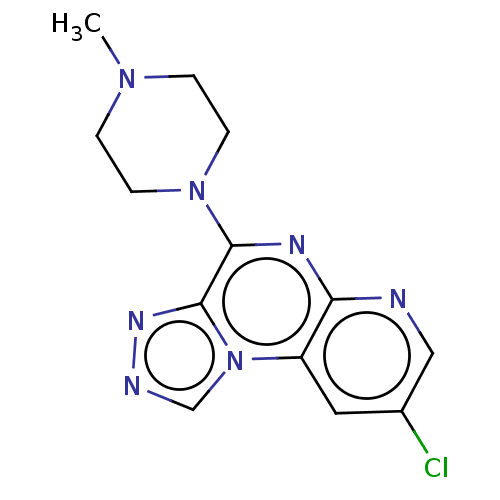 (US9586959, Compound 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50315323 ((R)-1-(8-chlorobenzofuro[3,2-d]pyrimidin-4-yl)-N-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inverse agonist activity at human histamine H4 receptor assessed as inhibition of [35S]GTPgammaS binding after 15 mins by scintillation proximity ass... | Bioorg Med Chem Lett 20: 2516-9 (2010) Article DOI: 10.1016/j.bmcl.2010.02.097 BindingDB Entry DOI: 10.7270/Q2CN74V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294414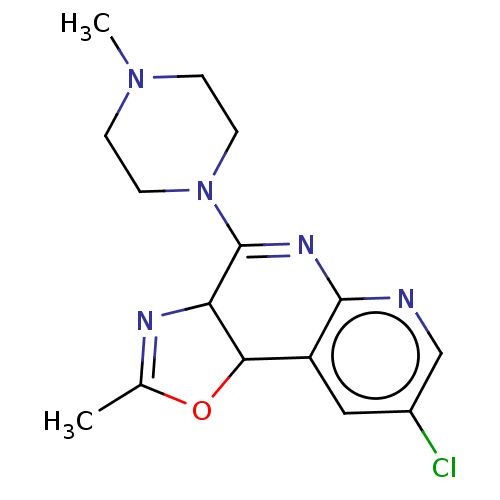 (US9586959, Compound 92) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294407 (US9586959, Compound 69 | US9586959, Compound 95) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50361010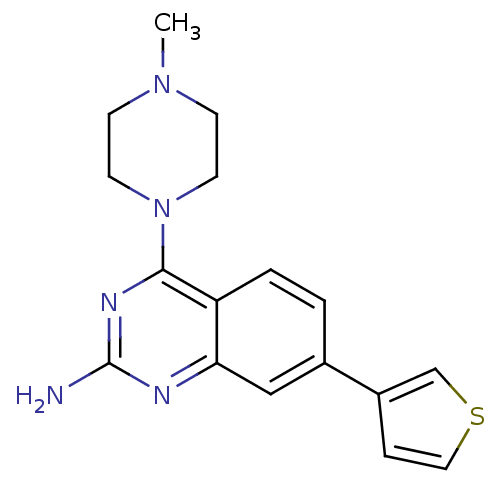 (CHEMBL1935572) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV Curated by ChEMBL | Assay Description Antagonist activity at human H4 receptor expressed in HEK293T cells assessed as inhibition of histamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 22: 461-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.104 BindingDB Entry DOI: 10.7270/Q2D79BT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50361035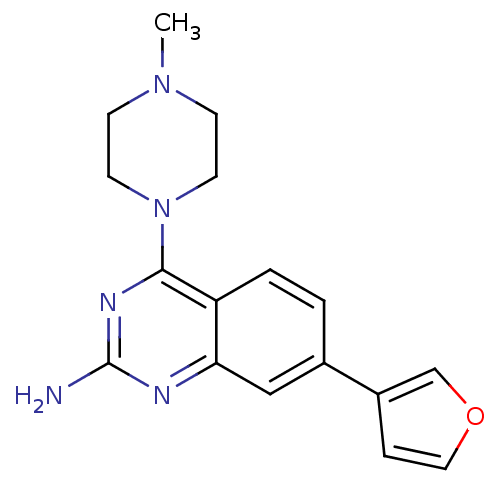 (CHEMBL1935571) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV Curated by ChEMBL | Assay Description Antagonist activity at human H4 receptor expressed in HEK293T cells assessed as inhibition of histamine-induced [35S]GTPgammaS binding | Bioorg Med Chem Lett 22: 461-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.104 BindingDB Entry DOI: 10.7270/Q2D79BT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294377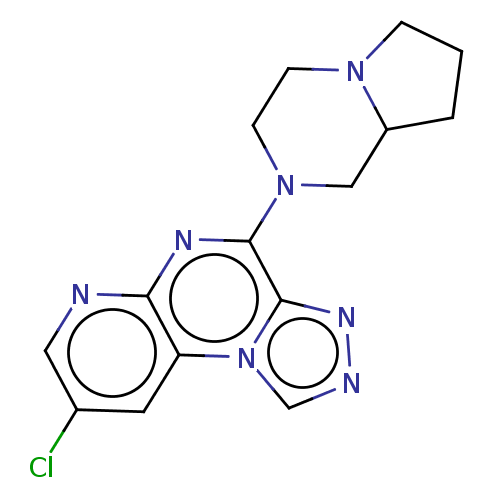 (US9586959, Compound 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294377 (US9586959, Compound 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294415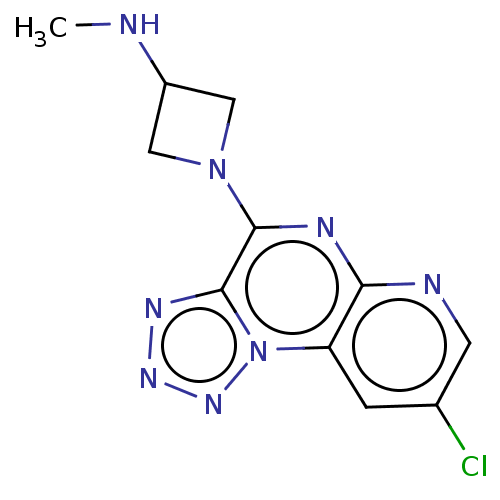 (US9586959, Compound 93) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294368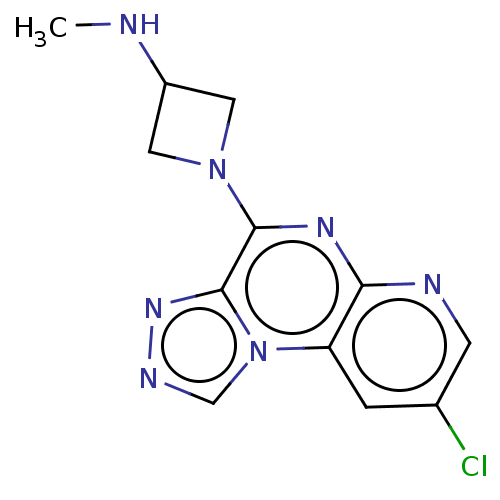 (US9586959, Compound 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294408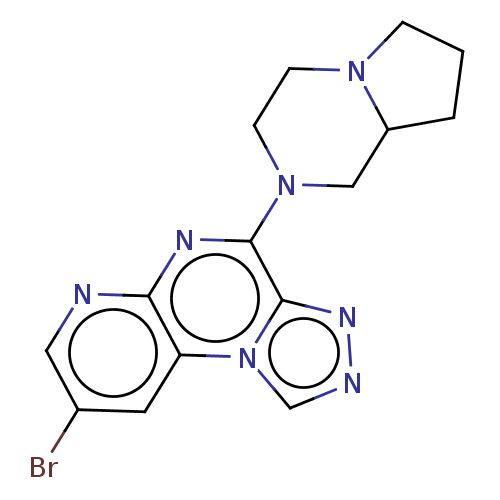 (US9586959, Compound 74) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294416 (US9586959, Compound 94) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294416 (US9586959, Compound 94) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-histamine from recombinant human histamine H4 receptor expressed in CHO-K1 cell membranes measured after 30 mins by microbeta sc... | Citation and Details Article DOI: 10.1021/acs.jmedchem.7b01855 BindingDB Entry DOI: 10.7270/Q2ZG6WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294409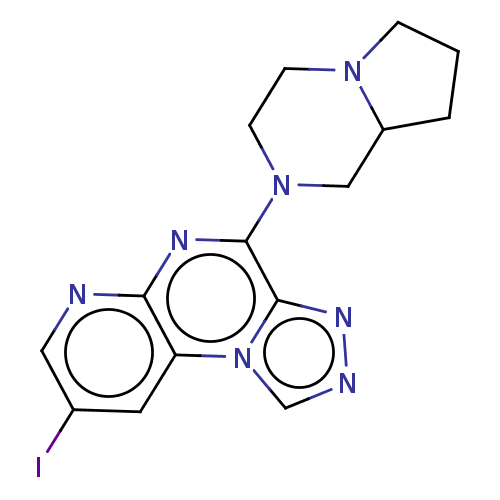 (US9586959, Compound 75) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM294366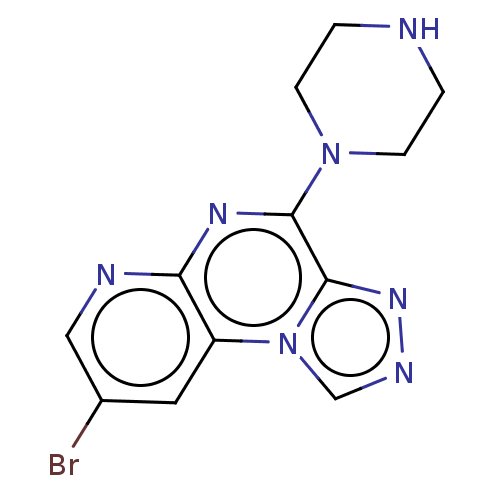 (US9586959, Compound 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | 25 |
C&C RESEARCH LABORATORIES US Patent | Assay Description In vitro profiling protein kinases was performed using the HotSpot assay platform. Briefly, specific kinase/substrate pairs along with required cofac... | US Patent US9586959 (2017) BindingDB Entry DOI: 10.7270/Q2C53NWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 208 total ) | Next | Last >> |