

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM168608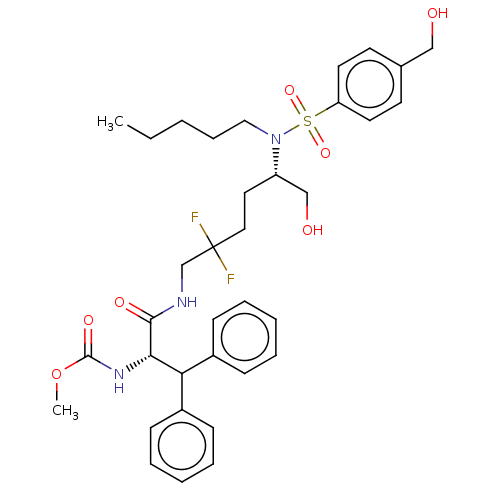 (US9079834, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Canada Inc. US Patent | Assay Description Assays for the inhibition of acute HIV infection of T-lymphoid cells were conducted in accordance with Vacca, J.P. et al, Proc. Natl. Acad. Sci. USA ... | US Patent US9079834 (2015) BindingDB Entry DOI: 10.7270/Q2PV6J44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM60467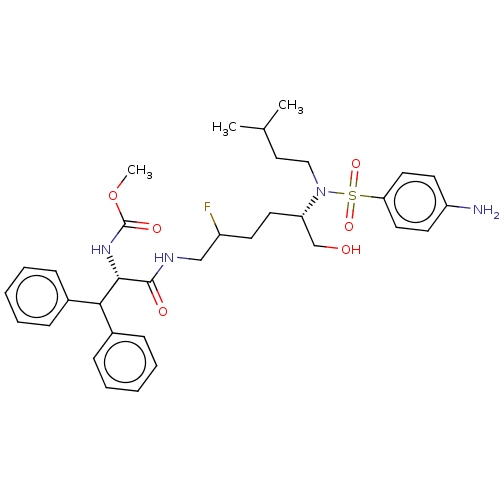 (BDBM168607 | US9079834, 1-1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Canada Inc. US Patent | Assay Description Assays for the inhibition of acute HIV infection of T-lymphoid cells were conducted in accordance with Vacca, J.P. et al, Proc. Natl. Acad. Sci. USA ... | US Patent US9079834 (2015) BindingDB Entry DOI: 10.7270/Q2PV6J44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180212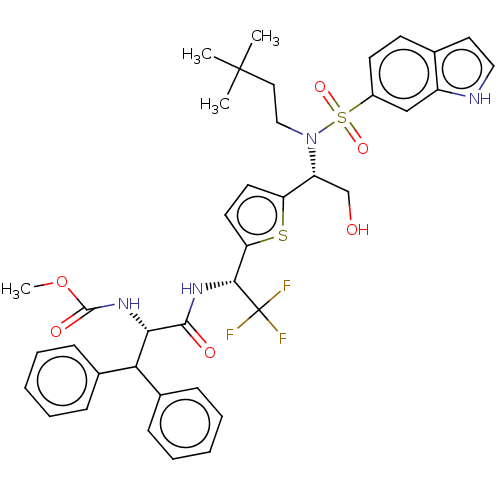 (US9133157, 94) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM60623 (BDBM180165 | US9133157, 103) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180171 (US9133157, 53) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.156 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180183 (US9133157, 65) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180163 (US9133157, 44) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.196 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180201 (US9133157, 83) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180164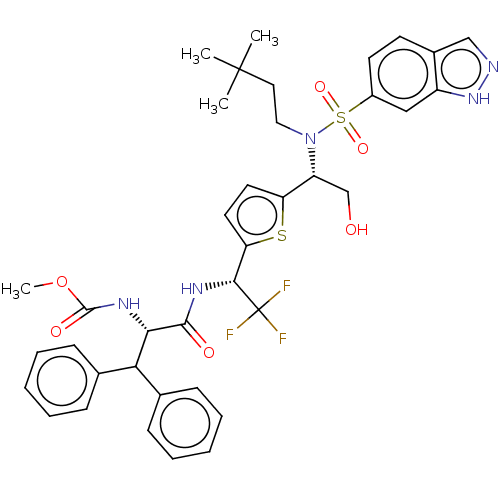 (US9133157, 102 | US9133157, 45) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.308 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180211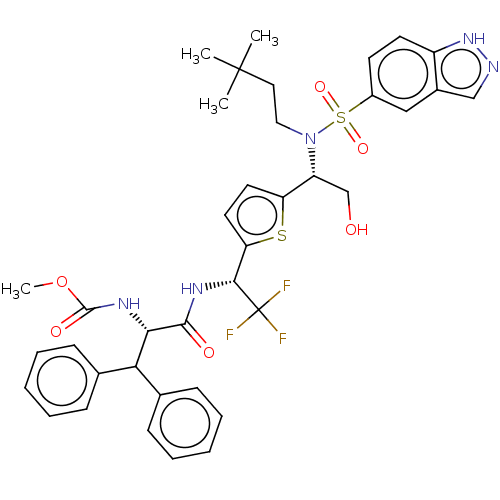 (US9133157, 93) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180119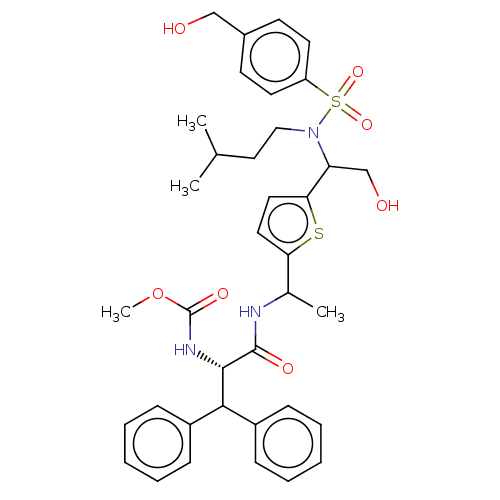 (US9133157, 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180206 (US9133157, 88) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180203 (US9133157, 85) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180204 (US9133157, 86) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM168610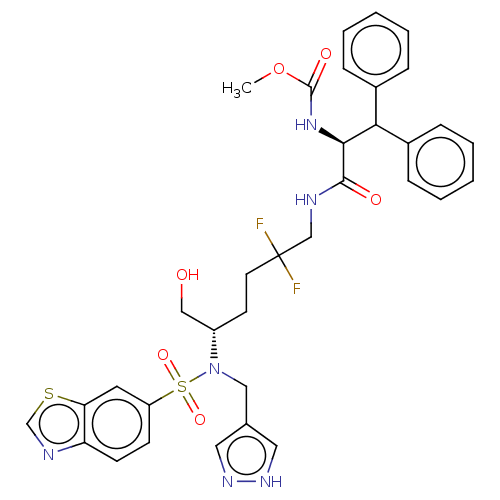 (US9079834, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Canada Inc. US Patent | Assay Description Assays for the inhibition of acute HIV infection of T-lymphoid cells were conducted in accordance with Vacca, J.P. et al, Proc. Natl. Acad. Sci. USA ... | US Patent US9079834 (2015) BindingDB Entry DOI: 10.7270/Q2PV6J44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461442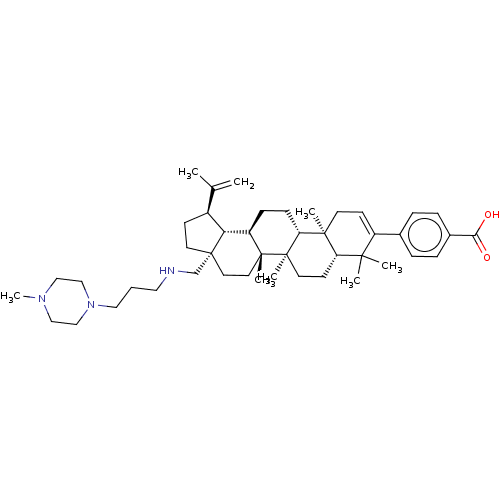 (CHEMBL4225363) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S/V370A double mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461431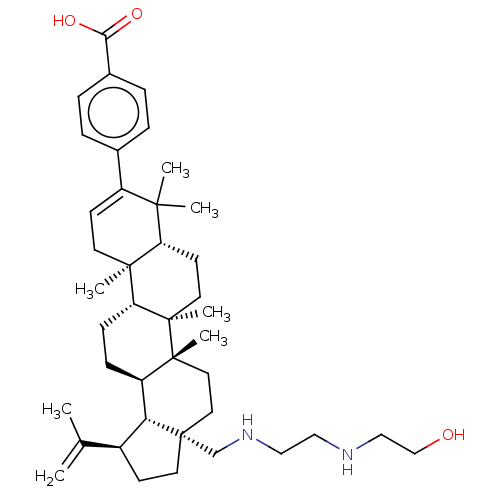 (CHEMBL4228517) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S/V370A double mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM168612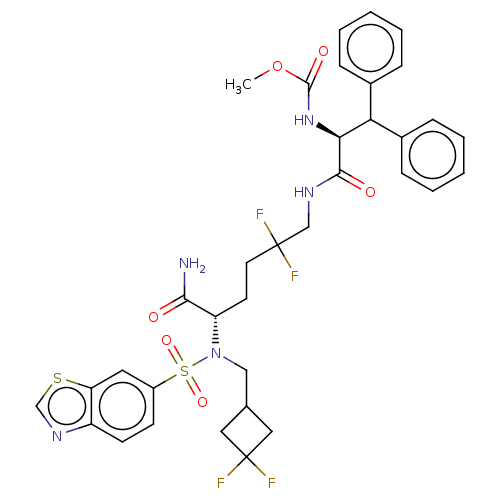 (US9079834, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Canada Inc. US Patent | Assay Description Assays for the inhibition of acute HIV infection of T-lymphoid cells were conducted in accordance with Vacca, J.P. et al, Proc. Natl. Acad. Sci. USA ... | US Patent US9079834 (2015) BindingDB Entry DOI: 10.7270/Q2PV6J44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180216 (US9133157, 98) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180135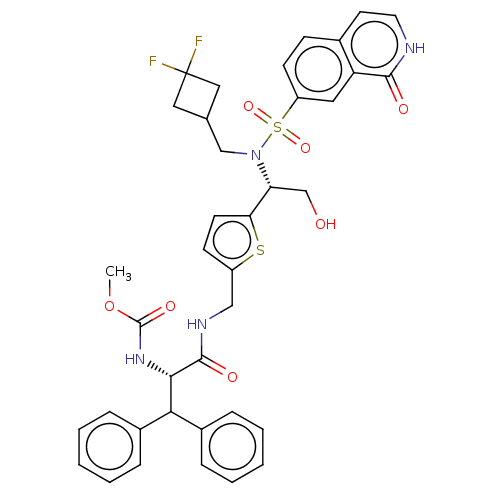 (US9133157, 16) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180192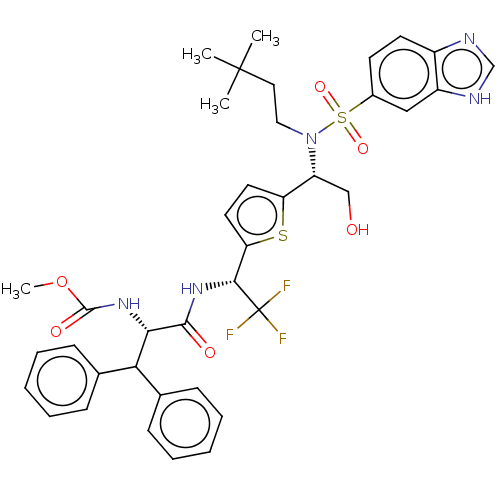 (US9133157, 74) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180232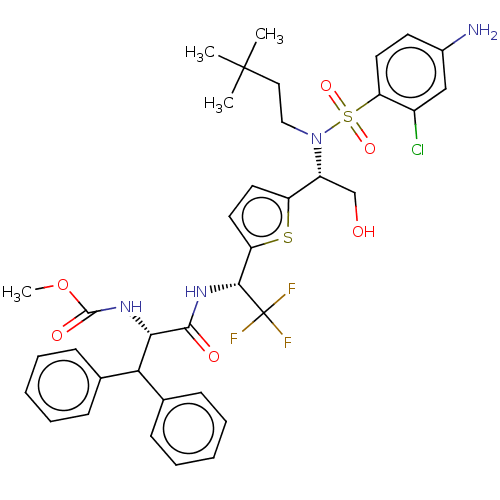 (US9133157, 117) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180116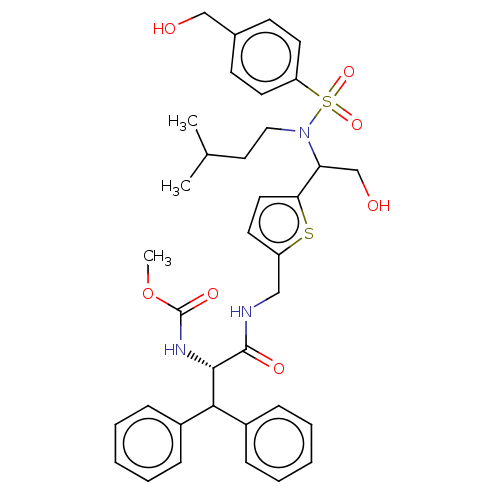 (US9133157, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM168609 (US9079834, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Canada Inc. US Patent | Assay Description Assays for the inhibition of acute HIV infection of T-lymphoid cells were conducted in accordance with Vacca, J.P. et al, Proc. Natl. Acad. Sci. USA ... | US Patent US9079834 (2015) BindingDB Entry DOI: 10.7270/Q2PV6J44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180164 (US9133157, 102 | US9133157, 45) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180120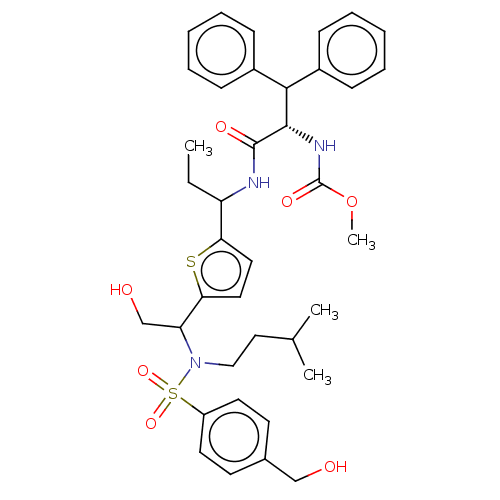 (US9133157, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180202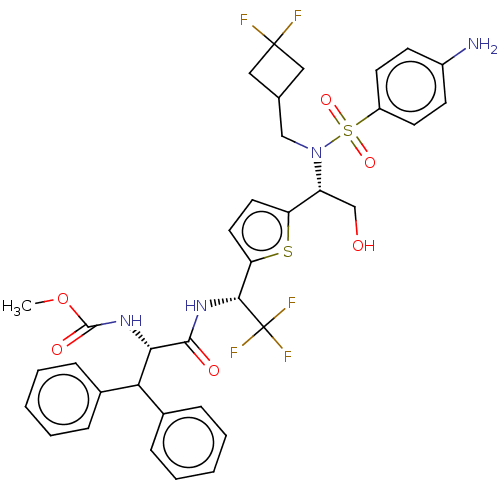 (US9133157, 84) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180229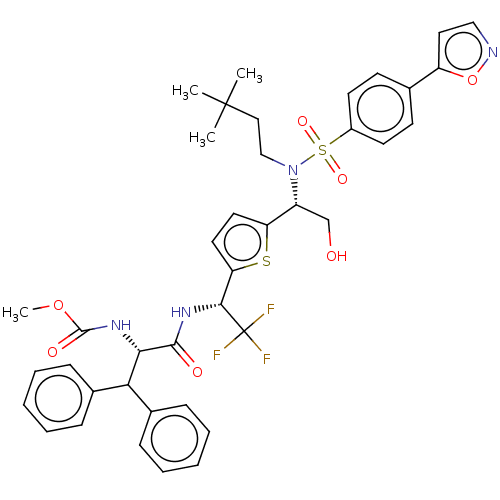 (US9133157, 114) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180219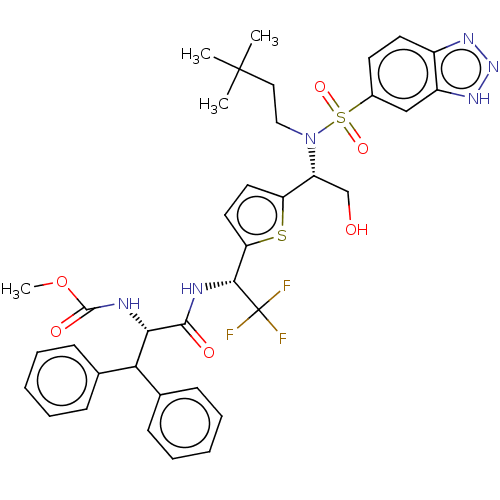 (US9133157, 101) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180222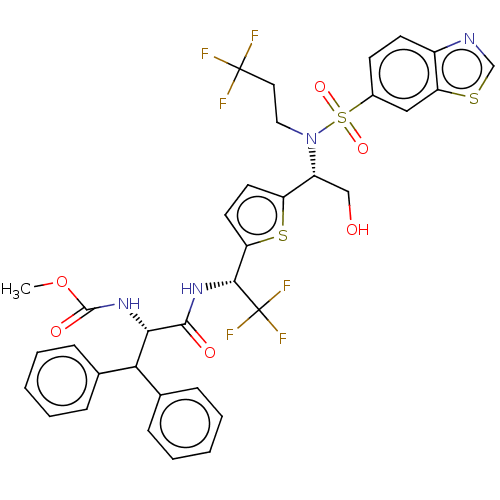 (US9133157, 104) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180218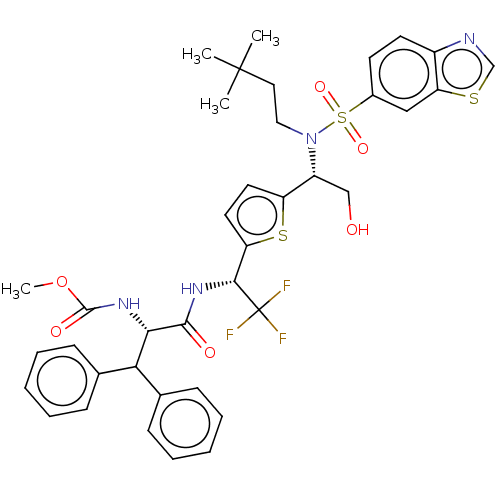 (US9133157, 100) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461430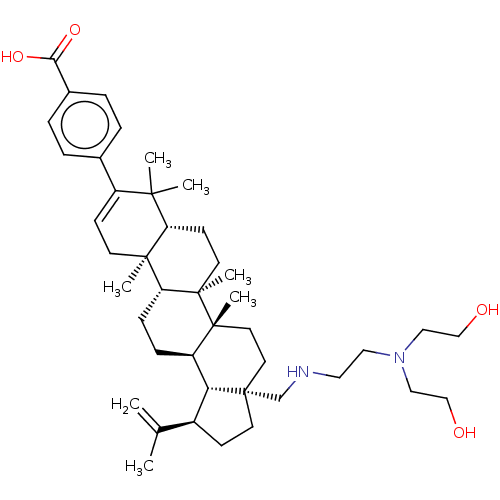 (CHEMBL4226862) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S/V370A double mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180215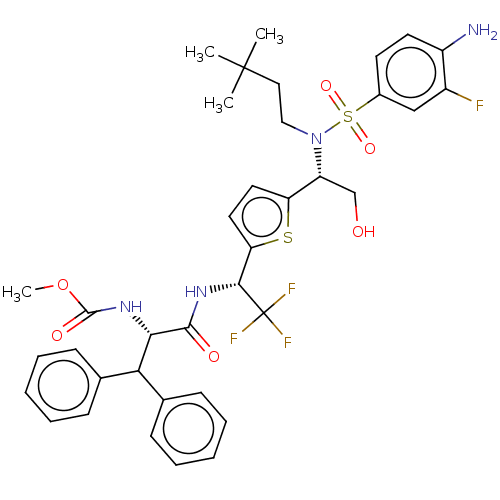 (US9133157, 97) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.23 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461417 (CHEMBL4225028) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S/V370A double mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180175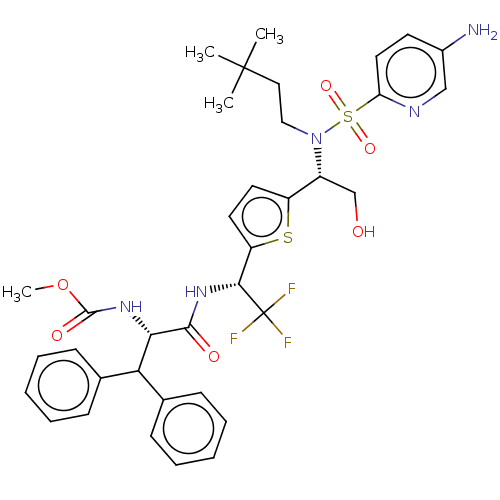 (US9133157, 57) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180147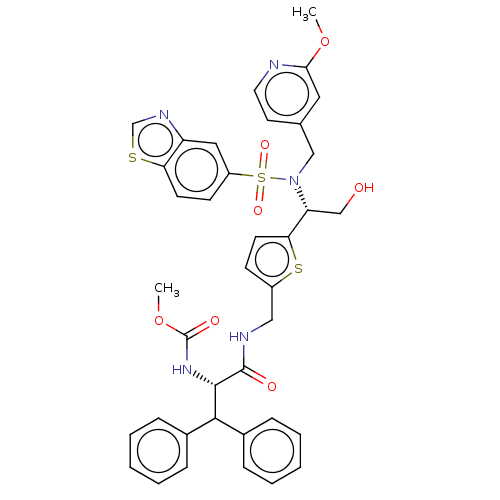 (US9133157, 28) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461438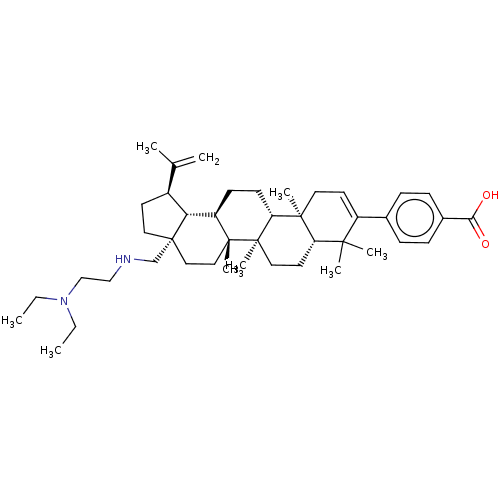 (CHEMBL4226921) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S/V370A double mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180130 (US9133157, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.61 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461421 (CHEMBL3828596) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S/V370A double mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180223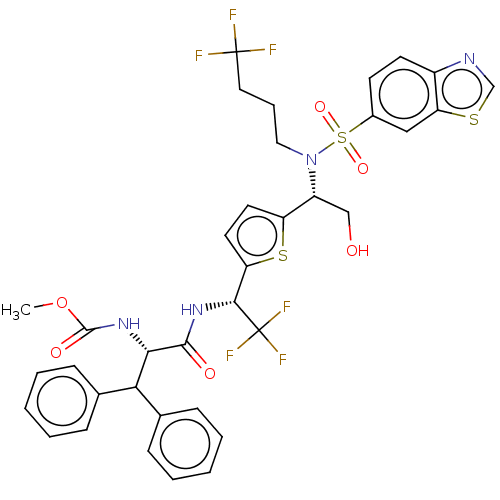 (US9133157, 105) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.77 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27595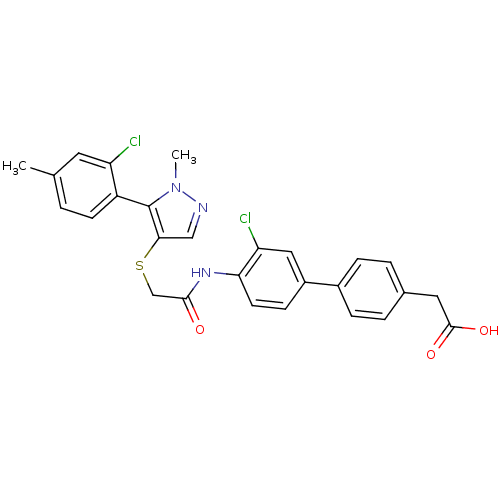 (2-{4-[3-chloro-4-(2-{[5-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27583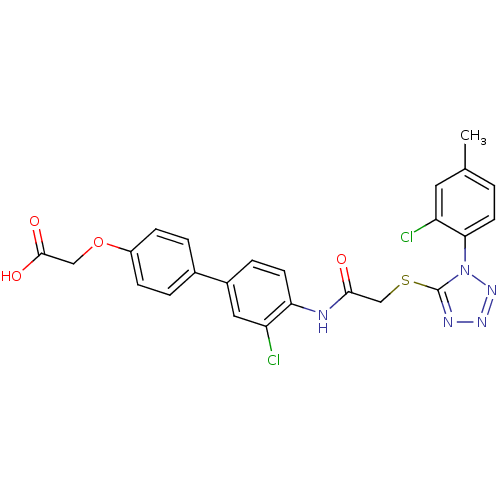 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180214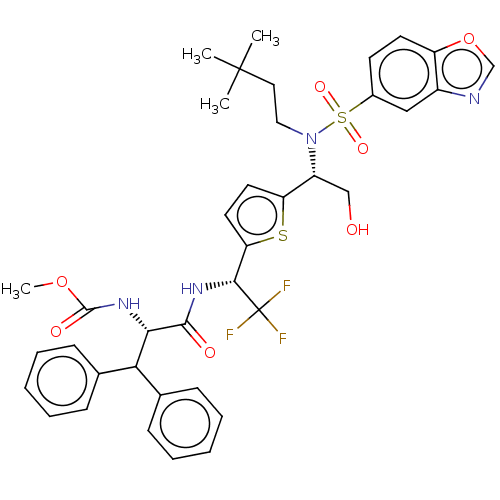 (US9133157, 96) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM25330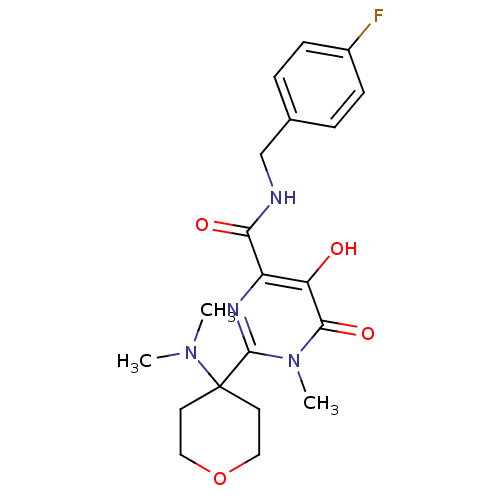 (2-[4-(dimethylamino)oxan-4-yl]-N-[(4-fluorophenyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Merck Research Laboratories | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 5843-55 (2008) Article DOI: 10.1021/jm800245z BindingDB Entry DOI: 10.7270/Q2QJ7FMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461435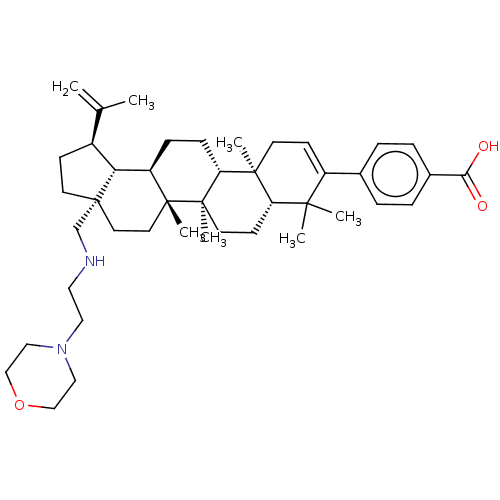 (CHEMBL4224769) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S/V370A double mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27599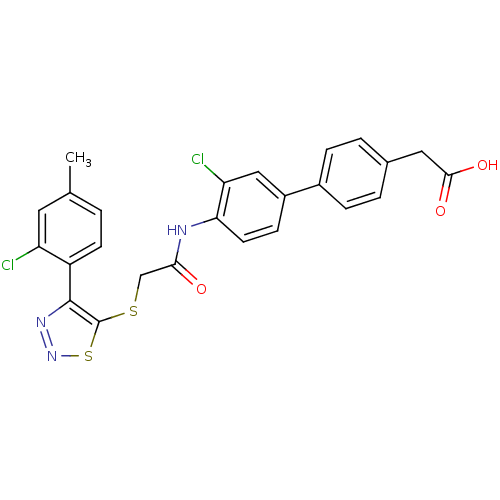 (2-{4-[3-chloro-4-(2-{[4-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180170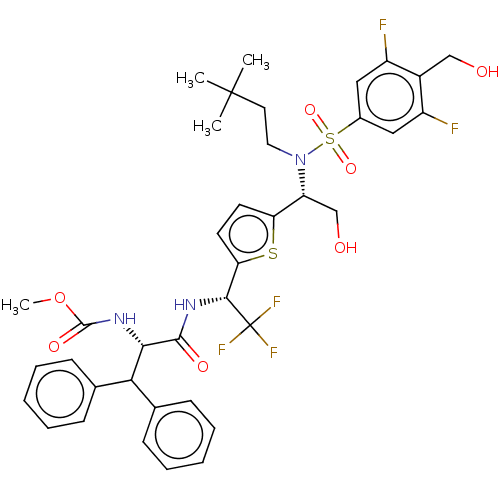 (US9133157, 52) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180178 (US9133157, 60) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.01 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180191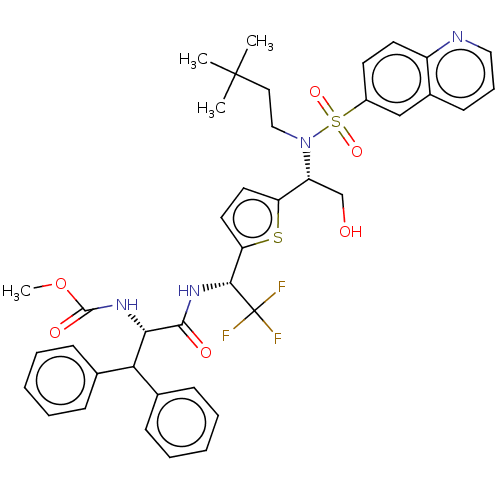 (US9133157, 73) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.03 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM180151 (US9133157, 32) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.27 | n/a | n/a | n/a | n/a | 5.5 | 25 |
Merck Canada Inc. US Patent | Assay Description The inhibition of WT HIV-1 protease was studied using the reaction of the protease (expressed in Eschericia coli) with a peptide substrate [Val-Ser-G... | US Patent US9133157 (2015) BindingDB Entry DOI: 10.7270/Q2Q52NDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1374 total ) | Next | Last >> |