 Found 199 hits of ic50 for UniProtKB: P43119
Found 199 hits of ic50 for UniProtKB: P43119 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50095207

(CHEMBL430154 | [6-(4-Benzhydryl-pyrazol-1-ylmethyl...)Show SMILES OC(=O)COc1cccc2C=C(Cn3cc(cn3)C(c3ccccc3)c3ccccc3)CCc12 |t:10| Show InChI InChI=1S/C29H26N2O3/c32-28(33)20-34-27-13-7-12-24-16-21(14-15-26(24)27)18-31-19-25(17-30-31)29(22-8-3-1-4-9-22)23-10-5-2-6-11-23/h1-13,16-17,19,29H,14-15,18,20H2,(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against Prostaglandin I2 receptor using conventional ligand assay by the displacement of [3H]-iloprost f... |
Bioorg Med Chem Lett 5: 1083-1086 (1995)
Article DOI: 10.1016/0960-894X(95)00170-X
BindingDB Entry DOI: 10.7270/Q22807KH |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human PGI2 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human recombinant IP receptor expressed in HEK293 cells measured after 60 mins by scintillation counting method |
Bioorg Med Chem 25: 471-482 (2017)
Article DOI: 10.1016/j.bmc.2016.11.014
BindingDB Entry DOI: 10.7270/Q2CF9S3S |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]iloprost from human recombinant Prostanoid IP receptor expressed in HEK293 cells |
Bioorg Med Chem 24: 1793-810 (2016)
Article DOI: 10.1016/j.bmc.2016.03.006
BindingDB Entry DOI: 10.7270/Q2J67JS7 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50284372
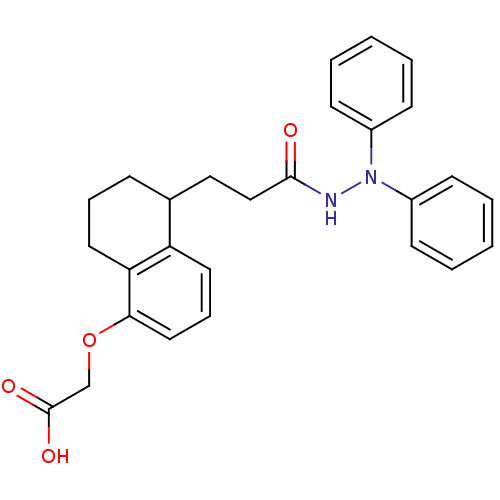
(CHEMBL11211 | {5-[2-(N',N'-Diphenyl-hydrazinocarbo...)Show SMILES OC(=O)COc1cccc2C(CCC(=O)NN(c3ccccc3)c3ccccc3)CCCc12 Show InChI InChI=1S/C27H28N2O4/c30-26(28-29(21-10-3-1-4-11-21)22-12-5-2-6-13-22)18-17-20-9-7-15-24-23(20)14-8-16-25(24)33-19-27(31)32/h1-6,8,10-14,16,20H,7,9,15,17-19H2,(H,28,30)(H,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for prostacyclin (PGI-2) binding by displacement of [3H]-iloprost from human platelets using conventional ligand binding assay |
Bioorg Med Chem Lett 5: 1077-1082 (1995)
Article DOI: 10.1016/0960-894X(95)00169-T
BindingDB Entry DOI: 10.7270/Q261108S |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235375
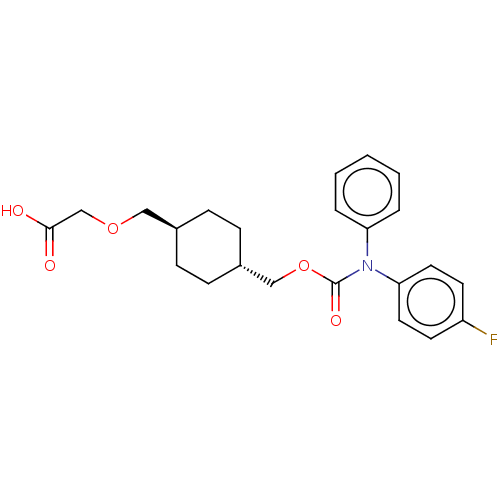
(CHEMBL3975122)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(F)cc2)CC1 |r,wU:6.5,wD:9.9,(31.35,-33.64,;30.02,-34.41,;30.02,-35.95,;28.69,-33.64,;28.69,-32.1,;27.35,-31.33,;26.02,-32.1,;26.02,-33.64,;24.69,-34.41,;23.35,-33.64,;22.02,-34.41,;20.68,-33.64,;19.35,-34.41,;19.35,-35.95,;18.02,-33.64,;18.02,-32.1,;16.68,-31.33,;16.68,-29.79,;18.02,-29.02,;19.35,-29.79,;19.35,-31.33,;16.68,-34.41,;16.68,-35.95,;15.35,-36.72,;14.02,-35.95,;12.68,-36.72,;14.02,-34.41,;15.35,-33.64,;23.35,-32.1,;24.69,-31.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50163291
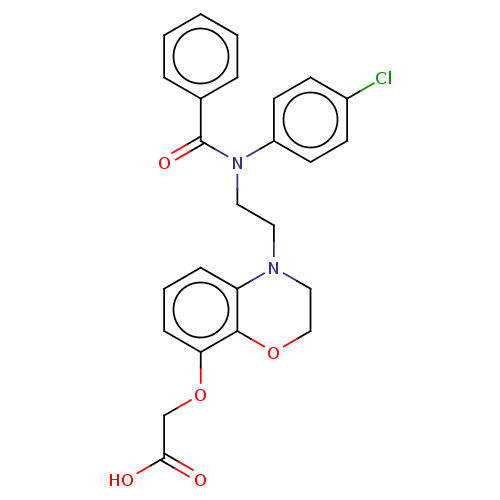
(CHEMBL3793903)Show SMILES OC(=O)COc1cccc2N(CCN(C(=O)c3ccccc3)c3ccc(Cl)cc3)CCOc12 Show InChI InChI=1S/C25H23ClN2O5/c26-19-9-11-20(12-10-19)28(25(31)18-5-2-1-3-6-18)14-13-27-15-16-32-24-21(27)7-4-8-22(24)33-17-23(29)30/h1-12H,13-17H2,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in platelet rich plasma assessed as inhibition of ADP-induced platelet aggregation preincubated for 1 min follo... |
Bioorg Med Chem Lett 26: 2360-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.009
BindingDB Entry DOI: 10.7270/Q2Q52RJW |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Binding affinity to PGI2 receptor (unknown origin) by radioligand displacement assay |
Bioorg Med Chem 21: 2764-71 (2013)
Article DOI: 10.1016/j.bmc.2013.03.016
BindingDB Entry DOI: 10.7270/Q2N87C5Q |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235370
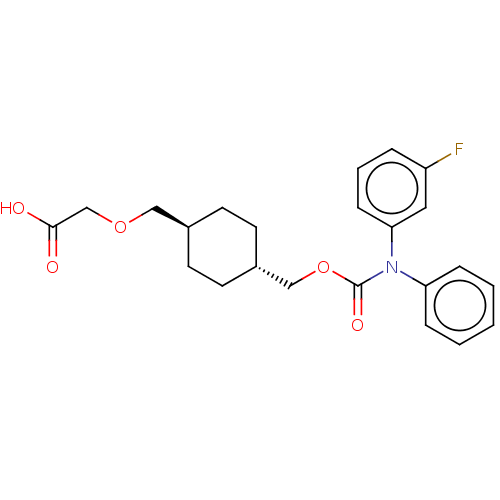
(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50284377
(CHEMBL11746 | {5-[2-(4-Benzhydryl-pyrazol-1-yl)-et...)Show SMILES OC(=O)COc1cccc2C(CCn3cc(cn3)C(c3ccccc3)c3ccccc3)CCCc12 Show InChI InChI=1S/C30H30N2O3/c33-29(34)21-35-28-16-8-14-26-22(13-7-15-27(26)28)17-18-32-20-25(19-31-32)30(23-9-3-1-4-10-23)24-11-5-2-6-12-24/h1-6,8-12,14,16,19-20,22,30H,7,13,15,17-18,21H2,(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against Prostaglandin I2 receptor using conventional ligand assay by the displacement of [3H]-iloprost f... |
Bioorg Med Chem Lett 5: 1083-1086 (1995)
Article DOI: 10.1016/0960-894X(95)00170-X
BindingDB Entry DOI: 10.7270/Q22807KH |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235378
(CHEMBL3981509)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(F)c(F)c2)CC1 |r,wU:6.5,wD:9.9,(31.35,-32.87,;30.02,-33.64,;30.02,-35.18,;28.69,-32.87,;28.69,-31.33,;27.35,-30.56,;26.02,-31.33,;26.02,-32.87,;24.69,-33.64,;23.35,-32.87,;22.02,-33.64,;20.68,-32.87,;19.35,-33.64,;19.35,-35.18,;18.02,-32.87,;18.02,-31.33,;16.68,-30.56,;16.68,-29.02,;18.02,-28.25,;19.35,-29.02,;19.35,-30.56,;16.68,-33.64,;15.35,-32.87,;14.02,-33.64,;14.02,-35.18,;12.68,-35.95,;15.35,-35.95,;15.35,-37.49,;16.68,-35.18,;23.35,-31.33,;24.69,-30.56,)| Show InChI InChI=1S/C23H25F2NO5/c24-20-11-10-19(12-21(20)25)26(18-4-2-1-3-5-18)23(29)31-14-17-8-6-16(7-9-17)13-30-15-22(27)28/h1-5,10-12,16-17H,6-9,13-15H2,(H,27,28)/t16-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50284370

(CHEMBL10836 | [6-(N',N'-Diphenyl-hydrazinocarbonyl...)Show SMILES OC(=O)COc1cccc2CC(CC(=O)NN(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C26H26N2O4/c29-25(27-28(21-9-3-1-4-10-21)22-11-5-2-6-12-22)17-19-14-15-23-20(16-19)8-7-13-24(23)32-18-26(30)31/h1-13,19H,14-18H2,(H,27,29)(H,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for prostacyclin (PGI-2) binding by displacement of [3H]-iloprost from human platelets using conventional ligand binding assay |
Bioorg Med Chem Lett 5: 1077-1082 (1995)
Article DOI: 10.1016/0960-894X(95)00169-T
BindingDB Entry DOI: 10.7270/Q261108S |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235368
(CHEMBL3893346)Show SMILES COc1ccc(cc1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1ccccc1 |r,wU:16.17,wD:13.13,(12.53,-35.95,;13.86,-36.72,;15.19,-35.95,;16.53,-36.72,;17.86,-35.95,;17.86,-34.41,;16.53,-33.64,;15.19,-34.41,;19.19,-33.64,;20.53,-34.41,;20.53,-35.95,;21.86,-33.64,;23.2,-34.41,;24.53,-33.64,;25.86,-34.41,;27.2,-33.64,;27.2,-32.1,;28.53,-31.33,;29.86,-32.1,;29.86,-33.64,;31.2,-34.41,;32.53,-33.64,;31.2,-35.95,;25.86,-31.33,;24.53,-32.1,;19.19,-32.1,;17.86,-31.33,;17.86,-29.79,;19.19,-29.02,;20.53,-29.79,;20.53,-31.33,)| Show InChI InChI=1S/C24H29NO6/c1-29-22-13-11-21(12-14-22)25(20-5-3-2-4-6-20)24(28)31-16-19-9-7-18(8-10-19)15-30-17-23(26)27/h2-6,11-14,18-19H,7-10,15-17H2,1H3,(H,26,27)/t18-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235373
(CHEMBL3928729)Show SMILES COc1cccc(c1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1ccccc1 |r,wU:16.17,wD:13.13,(30.85,-28.01,;29.51,-28.78,;29.51,-30.32,;30.85,-31.09,;30.85,-32.63,;29.51,-33.4,;28.18,-32.63,;28.18,-31.09,;26.85,-33.4,;25.51,-32.63,;25.51,-31.09,;24.18,-33.4,;22.85,-32.63,;21.51,-33.4,;20.18,-32.63,;18.84,-33.4,;18.84,-34.94,;17.51,-35.71,;16.18,-34.94,;16.18,-33.4,;14.84,-32.63,;13.51,-33.4,;14.84,-31.09,;20.18,-35.71,;21.51,-34.94,;26.85,-34.94,;28.18,-35.71,;28.18,-37.25,;26.85,-38.02,;25.51,-37.25,;25.51,-35.71,)| Show InChI InChI=1S/C24H29NO6/c1-29-22-9-5-8-21(14-22)25(20-6-3-2-4-7-20)24(28)31-16-19-12-10-18(11-13-19)15-30-17-23(26)27/h2-9,14,18-19H,10-13,15-17H2,1H3,(H,26,27)/t18-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against cathepsin B |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235376
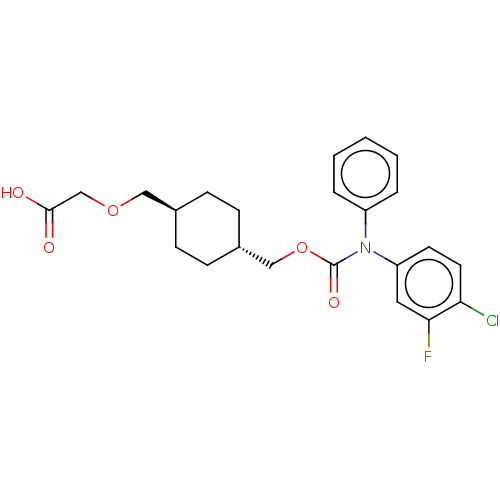
(CHEMBL3926078)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)c(F)c2)CC1 |r,wU:6.5,wD:9.9,(31.59,-32.87,;30.26,-33.64,;30.26,-35.18,;28.93,-32.87,;28.93,-31.33,;27.59,-30.56,;26.26,-31.33,;26.26,-32.87,;24.92,-33.64,;23.59,-32.87,;22.26,-33.64,;20.92,-32.87,;19.59,-33.64,;19.59,-35.18,;18.26,-32.87,;18.26,-31.33,;16.92,-30.56,;16.92,-29.02,;18.26,-28.25,;19.59,-29.02,;19.59,-30.56,;16.92,-33.64,;15.59,-32.87,;14.26,-33.64,;14.26,-35.18,;12.92,-35.95,;15.59,-35.95,;15.59,-37.49,;16.92,-35.18,;23.59,-31.33,;24.92,-30.56,)| Show InChI InChI=1S/C23H25ClFNO5/c24-20-11-10-19(12-21(20)25)26(18-4-2-1-3-5-18)23(29)31-14-17-8-6-16(7-9-17)13-30-15-22(27)28/h1-5,10-12,16-17H,6-9,13-15H2,(H,27,28)/t16-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50016954
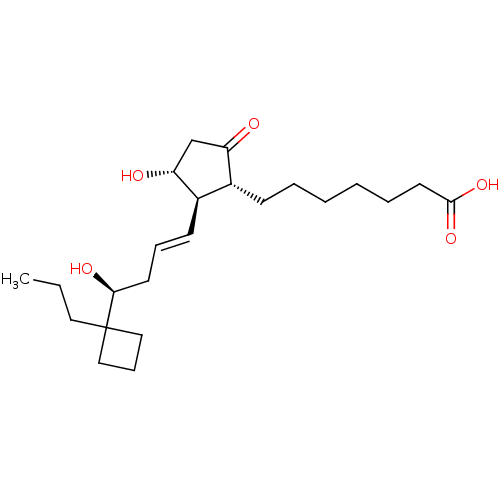
(CHEMBL1628262)Show SMILES CCCC1(CCC1)[C@@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O Show InChI InChI=1S/C23H38O5/c1-2-13-23(14-8-15-23)21(26)11-7-10-18-17(19(24)16-20(18)25)9-5-3-4-6-12-22(27)28/h7,10,17-18,20-21,25-26H,2-6,8-9,11-16H2,1H3,(H,27,28)/b10-7+/t17-,18-,20-,21+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Agonist activity at prostanoid IP receptor (unknown origin) by functional assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235367
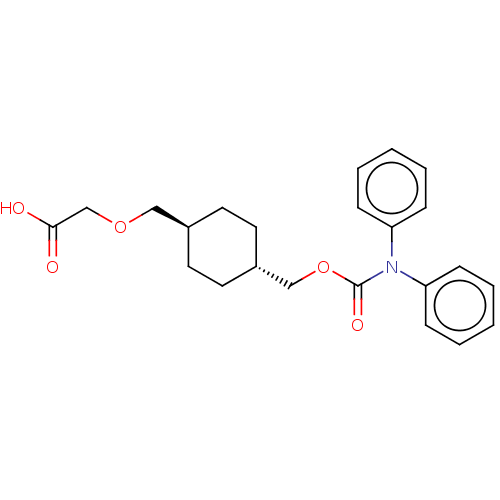
(CHEMBL3952237)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccccc2)CC1 |r,wU:6.5,wD:9.9,(30.68,-33.76,;29.35,-34.53,;29.35,-36.07,;28.02,-33.76,;28.02,-32.22,;26.68,-31.45,;25.35,-32.22,;25.35,-33.76,;24.02,-34.53,;22.68,-33.76,;21.35,-34.53,;20.01,-33.76,;18.68,-34.53,;18.68,-36.07,;17.35,-33.76,;16.01,-34.53,;16.01,-36.07,;14.68,-36.84,;13.35,-36.07,;13.35,-34.53,;14.68,-33.76,;17.35,-32.22,;16.01,-31.45,;16.01,-29.91,;17.35,-29.14,;18.68,-29.91,;18.68,-31.45,;22.68,-32.22,;24.02,-31.45,)| Show InChI InChI=1S/C23H27NO5/c25-22(26)17-28-15-18-11-13-19(14-12-18)16-29-23(27)24(20-7-3-1-4-8-20)21-9-5-2-6-10-21/h1-10,18-19H,11-17H2,(H,25,26)/t18-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Prostaglandin I2 receptor binding by displacement of [3H]-iloprost from human platelets |
Bioorg Med Chem Lett 5: 1065-1070 (1995)
Article DOI: 10.1016/0960-894X(95)00167-R
BindingDB Entry DOI: 10.7270/Q2FF3S9T |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from Prostaglandin I2 receptor of human platelets |
Bioorg Med Chem Lett 5: 1071-1076 (1995)
Article DOI: 10.1016/0960-894X(95)00168-S
BindingDB Entry DOI: 10.7270/Q29P31MX |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235389

(CHEMBL3983767)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccc(F)cc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(30.69,-33.67,;29.35,-34.44,;29.35,-35.98,;28.02,-33.67,;28.02,-32.13,;26.69,-31.36,;25.35,-32.13,;25.35,-33.67,;24.02,-34.44,;22.69,-33.67,;21.35,-34.44,;20.02,-33.67,;18.68,-34.44,;18.68,-35.98,;17.35,-33.67,;17.35,-32.13,;16.02,-31.36,;16.02,-29.82,;17.35,-29.05,;17.35,-27.51,;18.68,-29.82,;18.68,-31.36,;16.02,-34.44,;14.68,-33.67,;13.35,-34.44,;13.35,-35.98,;14.68,-36.75,;14.68,-38.29,;16.02,-35.98,;22.69,-32.13,;24.02,-31.36,)| Show InChI InChI=1S/C23H25F2NO5/c24-18-8-10-20(11-9-18)26(21-3-1-2-19(25)12-21)23(29)31-14-17-6-4-16(5-7-17)13-30-15-22(27)28/h1-3,8-12,16-17H,4-7,13-15H2,(H,27,28)/t16-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM85602
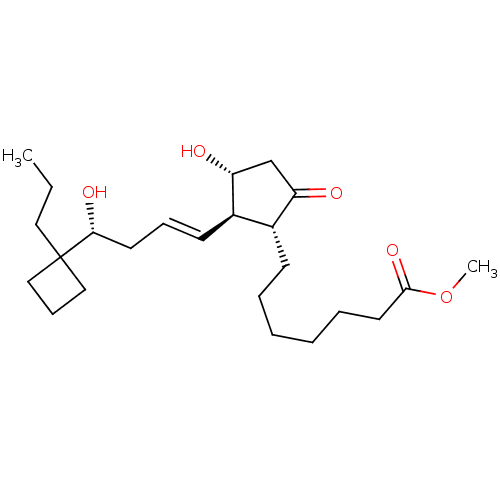
((R)-BUTAPROST | Butaprost (Free Acid) | CAS_69648-...)Show SMILES CCCC1(CCC1)[C@H](O)C\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(=O)OC |r| Show InChI InChI=1S/C24H40O5/c1-3-14-24(15-9-16-24)22(27)12-8-11-19-18(20(25)17-21(19)26)10-6-4-5-7-13-23(28)29-2/h8,11,18-19,21-22,26-27H,3-7,9-10,12-17H2,1-2H3/b11-8+/t18-,19-,21-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Agonist activity at prostanoid IP receptor (unknown origin) by functional assay |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50191135

(CHEMBL215442 | sodium 2-(3-(((1S,2R)-2-(4,5-diphen...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCC[C@H]2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C29H27NO4/c31-26(32)19-33-24-15-7-9-20(18-24)17-23-14-8-16-25(23)29-30-27(21-10-3-1-4-11-21)28(34-29)22-12-5-2-6-13-22/h1-7,9-13,15,18,23,25H,8,14,16-17,19H2,(H,31,32)/p-1/t23-,25+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PGI2 receptor assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235374
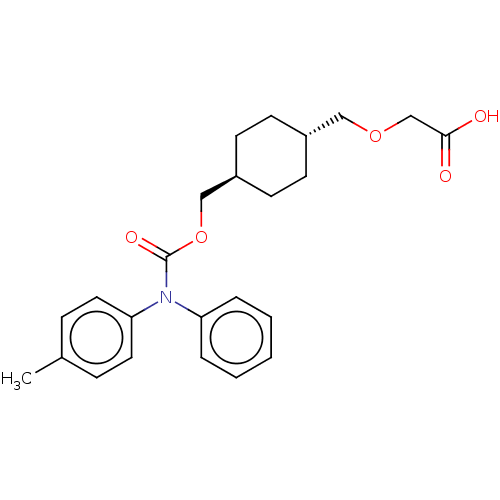
(CHEMBL3935924)Show SMILES Cc1ccc(cc1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1ccccc1 |r,wU:15.16,wD:12.12,(13.2,-36.56,;14.53,-35.79,;15.86,-36.56,;17.2,-35.79,;17.2,-34.25,;15.86,-33.48,;14.53,-34.25,;18.53,-33.48,;19.87,-34.25,;19.87,-35.79,;21.2,-33.48,;22.53,-34.25,;23.87,-33.48,;25.2,-34.25,;26.53,-33.48,;26.53,-31.94,;27.87,-31.17,;29.2,-31.94,;29.2,-33.48,;30.53,-34.25,;31.87,-33.48,;30.53,-35.79,;25.2,-31.17,;23.87,-31.94,;18.53,-31.94,;17.2,-31.17,;17.2,-29.63,;18.53,-28.86,;19.87,-29.63,;19.87,-31.17,)| Show InChI InChI=1S/C24H29NO5/c1-18-7-13-22(14-8-18)25(21-5-3-2-4-6-21)24(28)30-16-20-11-9-19(10-12-20)15-29-17-23(26)27/h2-8,13-14,19-20H,9-12,15-17H2,1H3,(H,26,27)/t19-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50284378

(CHEMBL11765 | [6-(4-Benzhydryl-pyrazol-1-ylmethyl)...)Show SMILES OC(=O)COc1cccc2CC(Cn3cc(cn3)C(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C29H28N2O3/c32-28(33)20-34-27-13-7-12-24-16-21(14-15-26(24)27)18-31-19-25(17-30-31)29(22-8-3-1-4-9-22)23-10-5-2-6-11-23/h1-13,17,19,21,29H,14-16,18,20H2,(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against Prostaglandin I2 receptor using conventional ligand assay by the displacement of [3H]-iloprost f... |
Bioorg Med Chem Lett 5: 1083-1086 (1995)
Article DOI: 10.1016/0960-894X(95)00170-X
BindingDB Entry DOI: 10.7270/Q22807KH |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235379
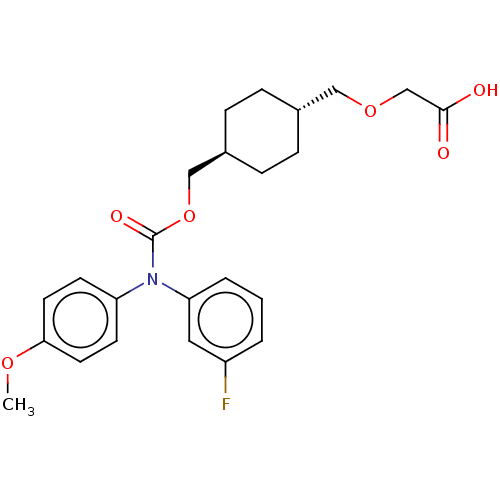
(CHEMBL3932106 | US10668033, Compound 55)Show SMILES COc1ccc(cc1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1cccc(F)c1 |r,wU:16.17,wD:13.13,(31.51,-37.36,;31.51,-35.82,;30.18,-35.05,;30.18,-33.51,;28.85,-32.74,;27.51,-33.51,;27.51,-35.05,;28.85,-35.82,;26.18,-32.74,;24.85,-33.51,;23.51,-32.74,;24.85,-35.05,;23.51,-35.82,;22.18,-35.05,;22.18,-33.51,;20.84,-32.74,;19.51,-33.51,;18.18,-32.74,;16.84,-33.51,;15.51,-32.74,;14.18,-33.51,;12.84,-32.74,;14.18,-35.05,;19.51,-35.05,;20.84,-35.82,;26.18,-31.2,;27.51,-30.43,;27.51,-28.89,;26.18,-28.12,;24.85,-28.89,;23.51,-28.12,;24.85,-30.43,)| Show InChI InChI=1S/C24H28FNO6/c1-30-22-11-9-20(10-12-22)26(21-4-2-3-19(25)13-21)24(29)32-15-18-7-5-17(6-8-18)14-31-16-23(27)28/h2-4,9-13,17-18H,5-8,14-16H2,1H3,(H,27,28)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50191133

(CHEMBL439357 | sodium (R)-2-(3-((2-(4,5-diphenylox...)Show SMILES [O-]C(=O)COc1cccc(CN2CCC[C@@H]2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C28H26N2O4/c31-25(32)19-33-23-14-7-9-20(17-23)18-30-16-8-15-24(30)28-29-26(21-10-3-1-4-11-21)27(34-28)22-12-5-2-6-13-22/h1-7,9-14,17,24H,8,15-16,18-19H2,(H,31,32)/p-1/t24-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PGI2 receptor assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50191134

(CHEMBL379203 | sodium 2-(3-(((1S,2S)-2-(4,5-diphen...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCC[C@@H]2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C29H27NO4/c31-26(32)19-33-24-15-7-9-20(18-24)17-23-14-8-16-25(23)29-30-27(21-10-3-1-4-11-21)28(34-29)22-12-5-2-6-13-22/h1-7,9-13,15,18,23,25H,8,14,16-17,19H2,(H,31,32)/p-1/t23-,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PGI2 receptor assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23960

(5-(4-phenylbenzyl)oxazole-4-carboxamide, 6g | 5-{[...)Show SMILES C[C@@H](CNC(=O)c1ncoc1Cc1ccc(cc1)-c1cccc(NC(C)=O)c1)c1ccccc1 |r| Show InChI InChI=1S/C28H27N3O3/c1-19(22-7-4-3-5-8-22)17-29-28(33)27-26(34-18-30-27)15-21-11-13-23(14-12-21)24-9-6-10-25(16-24)31-20(2)32/h3-14,16,18-19H,15,17H2,1-2H3,(H,29,33)(H,31,32)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | 51 | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc.
| Assay Description
IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... |
Bioorg Med Chem Lett 17: 1211-5 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.025
BindingDB Entry DOI: 10.7270/Q2RV0M0H |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235372
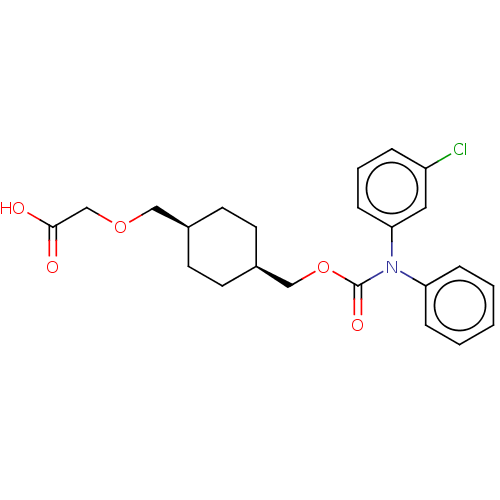
(CHEMBL3966307)Show SMILES OC(=O)COC[C@H]1CC[C@@H](COC(=O)N(c2ccccc2)c2cccc(Cl)c2)CC1 |r,wU:9.9,6.5,(14.02,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.02,-35.33,;19.36,-34.56,;19.36,-33.02,;20.69,-32.25,;22.03,-33.02,;23.36,-32.25,;24.69,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.69,-35.33,;28.69,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.69,-32.25,;30.03,-33.02,;31.36,-32.25,;31.36,-30.71,;30.03,-29.94,;30.03,-28.4,;28.69,-30.71,;22.03,-34.56,;20.69,-35.33,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235384
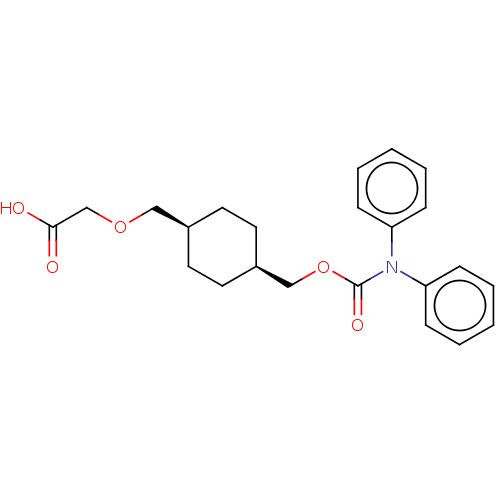
(CHEMBL3900038)Show SMILES OC(=O)COC[C@H]1CC[C@@H](COC(=O)N(c2ccccc2)c2ccccc2)CC1 |r,wU:9.9,6.5,(30.68,-33.76,;29.35,-34.53,;29.35,-36.07,;28.02,-33.76,;28.02,-32.22,;26.68,-31.45,;25.35,-32.22,;25.35,-33.76,;24.02,-34.53,;22.68,-33.76,;21.35,-34.53,;20.01,-33.76,;18.68,-34.53,;18.68,-36.07,;17.35,-33.76,;16.01,-34.53,;16.01,-36.07,;14.68,-36.84,;13.35,-36.07,;13.35,-34.53,;14.68,-33.76,;17.35,-32.22,;16.01,-31.45,;16.01,-29.91,;17.35,-29.14,;18.68,-29.91,;18.68,-31.45,;22.68,-32.22,;24.02,-31.45,)| Show InChI InChI=1S/C23H27NO5/c25-22(26)17-28-15-18-11-13-19(14-12-18)16-29-23(27)24(20-7-3-1-4-8-20)21-9-5-2-6-10-21/h1-10,18-19H,11-17H2,(H,25,26)/t18-,19+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50191132
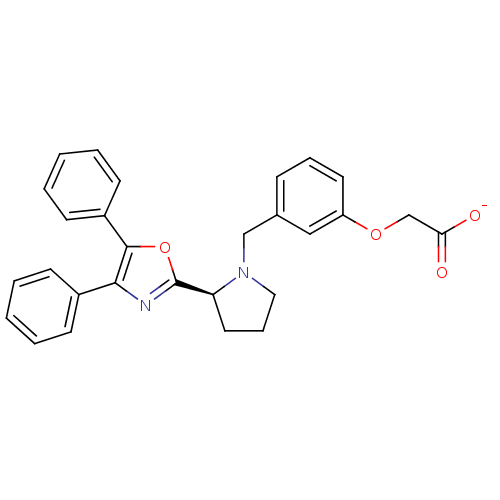
(CHEMBL438092 | sodium (S)-2-(3-((2-(4,5-diphenylox...)Show SMILES [O-]C(=O)COc1cccc(CN2CCC[C@H]2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C28H26N2O4/c31-25(32)19-33-23-14-7-9-20(17-23)18-30-16-8-15-24(30)28-29-26(21-10-3-1-4-11-21)27(34-28)22-12-5-2-6-13-22/h1-7,9-14,17,24H,8,15-16,18-19H2,(H,31,32)/p-1/t24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PGI2 receptor assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50136234

(CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...)Show SMILES [O-]C(=O)COc1cccc(CC2CCCC=C2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 |c:15| Show InChI InChI=1S/C30H27NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16-17,19,24H,7-8,15,18,20H2,(H,32,33)/p-1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PGI2 receptor assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235383
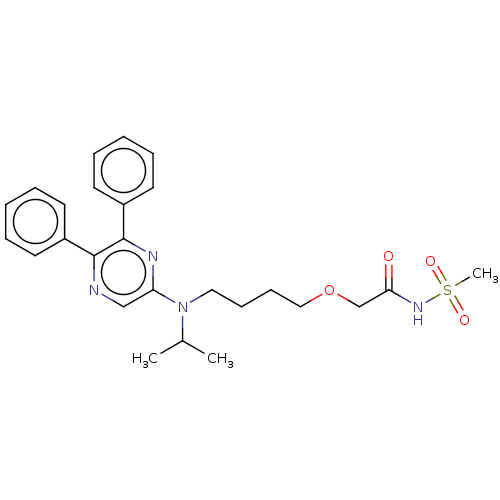
(ACT-293987 | NS-304 | Selexipag | Uptravi)Show SMILES CC(C)N(CCCCOCC(=O)NS(C)(=O)=O)c1cnc(-c2ccccc2)c(n1)-c1ccccc1 Show InChI InChI=1S/C26H32N4O4S/c1-20(2)30(16-10-11-17-34-19-24(31)29-35(3,32)33)23-18-27-25(21-12-6-4-7-13-21)26(28-23)22-14-8-5-9-15-22/h4-9,12-15,18,20H,10-11,16-17,19H2,1-3H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103396
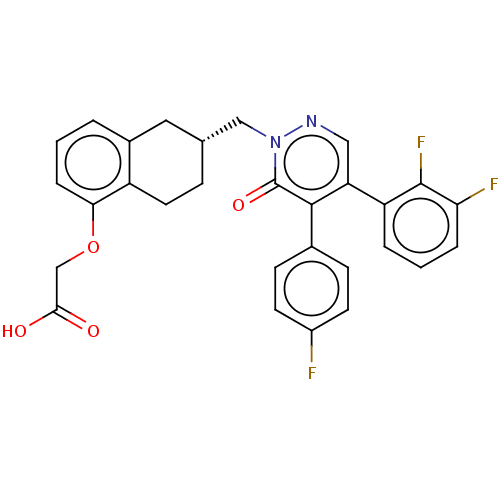
(CHEMBL3398236)Show SMILES OC(=O)COc1cccc2C[C@H](Cn3ncc(-c4cccc(F)c4F)c(-c4ccc(F)cc4)c3=O)CCc12 |r| Show InChI InChI=1S/C29H23F3N2O4/c30-20-10-8-18(9-11-20)27-23(22-4-2-5-24(31)28(22)32)14-33-34(29(27)37)15-17-7-12-21-19(13-17)3-1-6-25(21)38-16-26(35)36/h1-6,8-11,14,17H,7,12-13,15-16H2,(H,35,36)/t17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103395
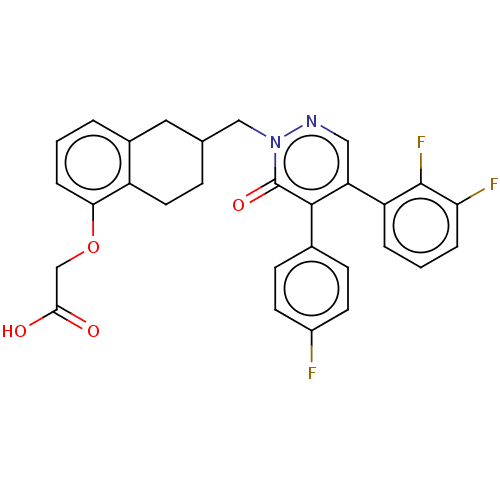
(CHEMBL3398235)Show SMILES OC(=O)COc1cccc2CC(Cn3ncc(-c4cccc(F)c4F)c(-c4ccc(F)cc4)c3=O)CCc12 Show InChI InChI=1S/C29H23F3N2O4/c30-20-10-8-18(9-11-20)27-23(22-4-2-5-24(31)28(22)32)14-33-34(29(27)37)15-17-7-12-21-19(13-17)3-1-6-25(21)38-16-26(35)36/h1-6,8-11,14,17H,7,12-13,15-16H2,(H,35,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103394
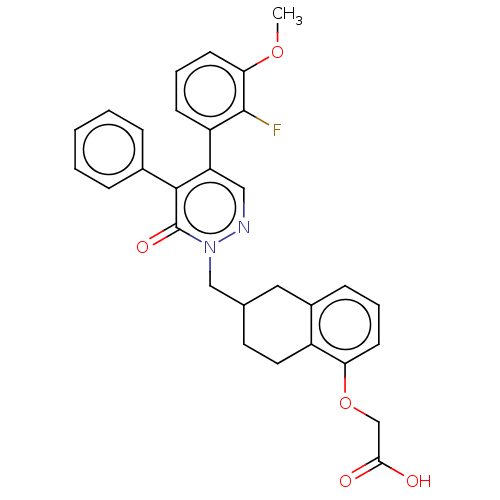
(CHEMBL3398229)Show SMILES COc1cccc(c1F)-c1cnn(CC2CCc3c(C2)cccc3OCC(O)=O)c(=O)c1-c1ccccc1 Show InChI InChI=1S/C30H27FN2O5/c1-37-26-12-6-10-23(29(26)31)24-16-32-33(30(36)28(24)20-7-3-2-4-8-20)17-19-13-14-22-21(15-19)9-5-11-25(22)38-18-27(34)35/h2-12,16,19H,13-15,17-18H2,1H3,(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23953

(5-(4-phenylbenzyl)oxazole-4-carboxamide, 6a | 5-{[...)Show SMILES CC(CNC(=O)c1ncoc1Cc1ccc(cc1)-c1cccc(NC(C)=O)c1)c1ccccc1 Show InChI InChI=1S/C28H27N3O3/c1-19(22-7-4-3-5-8-22)17-29-28(33)27-26(34-18-30-27)15-21-11-13-23(14-12-21)24-9-6-10-25(16-24)31-20(2)32/h3-14,16,18-19H,15,17H2,1-2H3,(H,29,33)(H,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pharmacopeia Drug Discovery Inc.
| Assay Description
IP receptor binding activity was quantified via a filter binding assay measuring displacement of [3H]-Iloprost binding to human platelet membranes. R... |
Bioorg Med Chem Lett 17: 1211-5 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.025
BindingDB Entry DOI: 10.7270/Q2RV0M0H |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50095203

(CHEMBL94751 | [(S)-6-(3-Benzhydryl-6-oxo-6H-pyrida...)Show SMILES OC(=O)COc1cccc2C[C@@H](Cn3nc(ccc3=O)C(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C30H28N2O4/c33-28-17-16-26(30(22-8-3-1-4-9-22)23-10-5-2-6-11-23)31-32(28)19-21-14-15-25-24(18-21)12-7-13-27(25)36-20-29(34)35/h1-13,16-17,21,30H,14-15,18-20H2,(H,34,35)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at PGI2 receptor in Homo sapiens (human) platelets assessed as inhibition of ADP-induced aggregation |
Citation and Details
Article DOI: 10.1007/s00044-012-0261-1
BindingDB Entry DOI: 10.7270/Q2VX0KD3 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50095203

(CHEMBL94751 | [(S)-6-(3-Benzhydryl-6-oxo-6H-pyrida...)Show SMILES OC(=O)COc1cccc2C[C@@H](Cn3nc(ccc3=O)C(c3ccccc3)c3ccccc3)CCc12 Show InChI InChI=1S/C30H28N2O4/c33-28-17-16-26(30(22-8-3-1-4-9-22)23-10-5-2-6-11-23)31-32(28)19-21-14-15-25-24(18-21)12-7-13-27(25)36-20-29(34)35/h1-13,16-17,21,30H,14-15,18-20H2,(H,34,35)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Agonist activity at PGI2 in human platelet-rich plasma assessed as inhibition of ADP-induced platelet aggregation |
Eur J Med Chem 123: 256-281 (2016)
Article DOI: 10.1016/j.ejmech.2016.07.061
BindingDB Entry DOI: 10.7270/Q2Q81G2Z |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103404
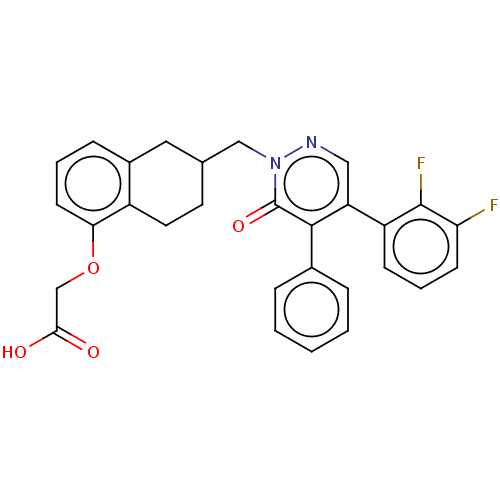
(CHEMBL3398228)Show SMILES OC(=O)COc1cccc2CC(Cn3ncc(-c4cccc(F)c4F)c(-c4ccccc4)c3=O)CCc12 Show InChI InChI=1S/C29H24F2N2O4/c30-24-10-5-9-22(28(24)31)23-15-32-33(29(36)27(23)19-6-2-1-3-7-19)16-18-12-13-21-20(14-18)8-4-11-25(21)37-17-26(34)35/h1-11,15,18H,12-14,16-17H2,(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235371
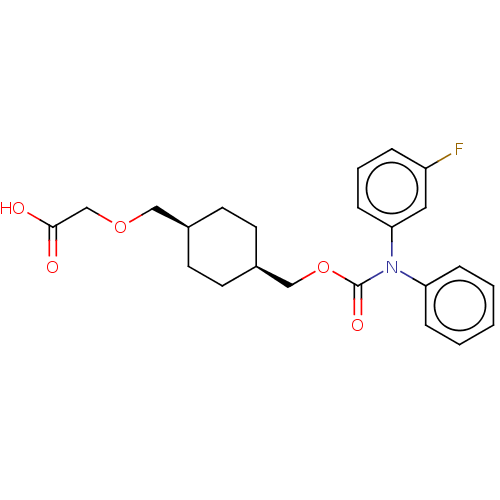
(CHEMBL3922000)Show SMILES OC(=O)COC[C@H]1CC[C@@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:9.9,6.5,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50191137
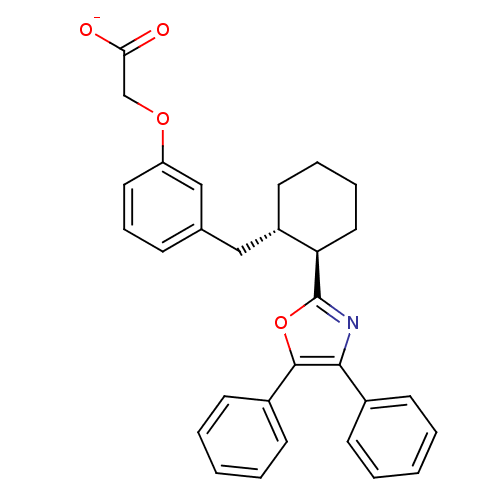
(CHEMBL212943 | sodium 2-(3-(((1S,2R)-2-(4,5-diphen...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC[C@H]2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C30H29NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16,19,24,26H,7-8,15,17-18,20H2,(H,32,33)/p-1/t24-,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PGI2 receptor assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50191138

(CHEMBL405770 | sodium 2-(3-(((1S,2S)-2-(4,5-diphen...)Show SMILES [O-]C(=O)COc1cccc(C[C@@H]2CCCC[C@@H]2c2nc(c(o2)-c2ccccc2)-c2ccccc2)c1 Show InChI InChI=1S/C30H29NO4/c32-27(33)20-34-25-16-9-10-21(19-25)18-24-15-7-8-17-26(24)30-31-28(22-11-3-1-4-12-22)29(35-30)23-13-5-2-6-14-23/h1-6,9-14,16,19,24,26H,7-8,15,17-18,20H2,(H,32,33)/p-1/t24-,26-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Agonist activity at human PGI2 receptor assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 16: 4861-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.076
BindingDB Entry DOI: 10.7270/Q2R49QD9 |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50163296
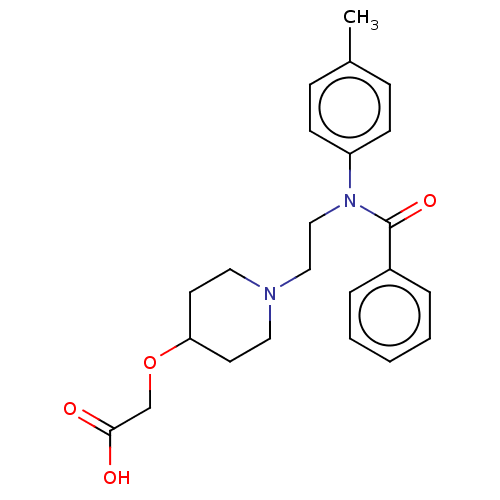
(CHEMBL3793911)Show SMILES Cc1ccc(cc1)N(CCN1CCC(CC1)OCC(O)=O)C(=O)c1ccccc1 Show InChI InChI=1S/C23H28N2O4/c1-18-7-9-20(10-8-18)25(23(28)19-5-3-2-4-6-19)16-15-24-13-11-21(12-14-24)29-17-22(26)27/h2-10,21H,11-17H2,1H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in platelet rich plasma assessed as inhibition of ADP-induced platelet aggregation preincubated for 1 min follo... |
Bioorg Med Chem Lett 26: 2360-4 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.009
BindingDB Entry DOI: 10.7270/Q2Q52RJW |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235377
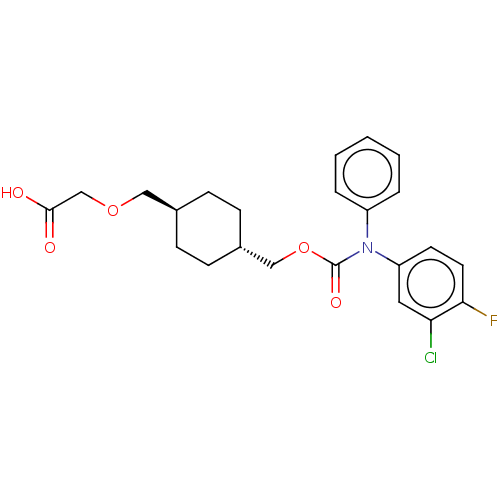
(CHEMBL3890685)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(F)c(Cl)c2)CC1 |r,wU:6.5,wD:9.9,(31.35,-32.86,;30.02,-33.63,;30.02,-35.17,;28.69,-32.86,;28.69,-31.32,;27.35,-30.55,;26.02,-31.32,;26.02,-32.86,;24.69,-33.63,;23.35,-32.86,;22.02,-33.63,;20.68,-32.86,;19.35,-33.63,;19.35,-35.17,;18.02,-32.86,;18.02,-31.32,;16.68,-30.55,;16.68,-29.01,;18.02,-28.24,;19.35,-29.01,;19.35,-30.55,;16.68,-33.63,;15.35,-32.86,;14.02,-33.63,;14.02,-35.17,;12.68,-35.94,;15.35,-35.94,;15.35,-37.48,;16.68,-35.17,;23.35,-31.32,;24.69,-30.55,)| Show InChI InChI=1S/C23H25ClFNO5/c24-20-12-19(10-11-21(20)25)26(18-4-2-1-3-5-18)23(29)31-14-17-8-6-16(7-9-17)13-30-15-22(27)28/h1-5,10-12,16-17H,6-9,13-15H2,(H,27,28)/t16-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50284366
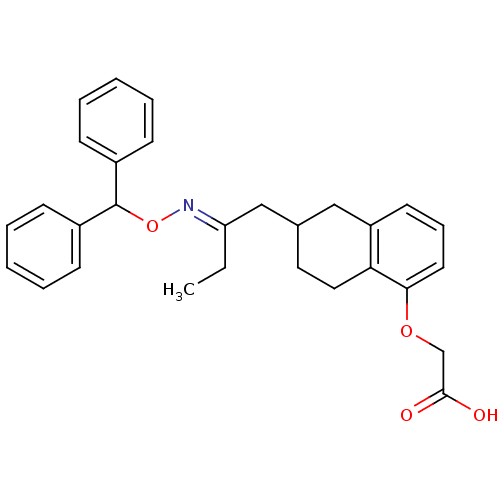
((6-{2-[(E)-Benzhydryloxyimino]-butyl}-5,6,7,8-tetr...)Show SMILES CC\C(CC1CCc2c(C1)cccc2OCC(O)=O)=N/OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H31NO4/c1-2-25(30-34-29(22-10-5-3-6-11-22)23-12-7-4-8-13-23)19-21-16-17-26-24(18-21)14-9-15-27(26)33-20-28(31)32/h3-15,21,29H,2,16-20H2,1H3,(H,31,32)/b30-25+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from Prostaglandin I2 receptor of human platelets |
Bioorg Med Chem Lett 5: 1071-1076 (1995)
Article DOI: 10.1016/0960-894X(95)00168-S
BindingDB Entry DOI: 10.7270/Q29P31MX |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50095205
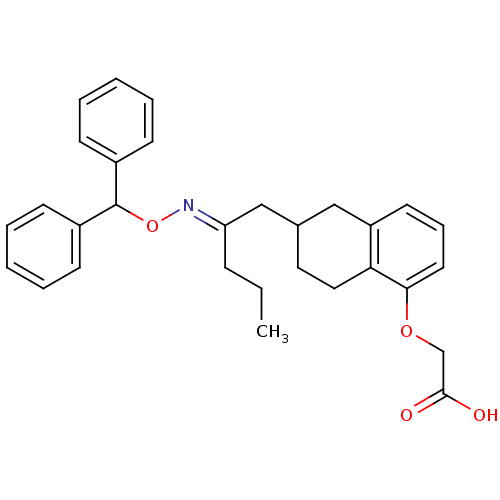
((6-{2-[(E)-Benzhydryloxyimino]-pentyl}-5,6,7,8-tet...)Show SMILES CCC\C(CC1CCc2c(C1)cccc2OCC(O)=O)=N/OC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C30H33NO4/c1-2-10-26(31-35-30(23-11-5-3-6-12-23)24-13-7-4-8-14-24)20-22-17-18-27-25(19-22)15-9-16-28(27)34-21-29(32)33/h3-9,11-16,22,30H,2,10,17-21H2,1H3,(H,32,33)/b31-26+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from Prostaglandin I2 receptor of human platelets |
Bioorg Med Chem Lett 5: 1071-1076 (1995)
Article DOI: 10.1016/0960-894X(95)00168-S
BindingDB Entry DOI: 10.7270/Q29P31MX |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50002401

(8-(4,5-Diphenyl-pyrazol-1-yl)-octanoic acid | CHEM...)Show InChI InChI=1S/C23H26N2O2/c26-22(27)16-10-2-1-3-11-17-25-23(20-14-8-5-9-15-20)21(18-24-25)19-12-6-4-7-13-19/h4-9,12-15,18H,1-3,10-11,16-17H2,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
IC50 value of the compound was evaluated by measuring the displacement of [3H]-iloprost from Prostaglandin I2 receptor |
J Med Chem 35: 389-97 (1992)
BindingDB Entry DOI: 10.7270/Q29K4966 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data