 Found 637 hits of ic50 for UniProtKB: Q969S8
Found 637 hits of ic50 for UniProtKB: Q969S8 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50101331
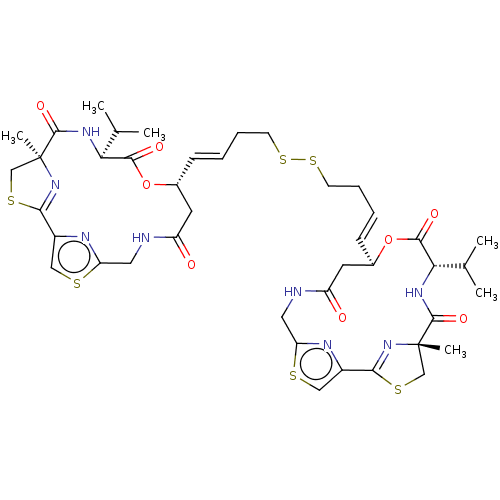
(CHEMBL3329621)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSCC\C=C\[C@@H]1CC(=O)NCc3nc(cs3)C3=N[C@@](C)(CS3)C(=O)N[C@@H](C(C)C)C(=O)O1)n2 |r,c:11,t:50| Show InChI InChI=1S/C42H54N8O8S6/c1-23(2)33-37(53)57-25(15-29(51)43-17-31-45-27(19-59-31)35-49-41(5,21-61-35)39(55)47-33)11-7-9-13-63-64-14-10-8-12-26-16-30(52)44-18-32-46-28(20-60-32)36-50-42(6,22-62-36)40(56)48-34(24(3)4)38(54)58-26/h7-8,11-12,19-20,23-26,33-34H,9-10,13-18,21-22H2,1-6H3,(H,43,51)(H,44,52)(H,47,55)(H,48,56)/b11-7+,12-8+/t25-,26-,33+,34+,41+,42+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50020912

(Largazole Thiol)Show SMILES [H][C@]1(CC(=O)NCc2nc(cs2)C2=N[C@@](C)(CS2)C(=O)N[C@H](C(C)C)C(=O)O1)\C=C\CCS |r,t:13| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17-,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and in absence of DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC10 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50101330
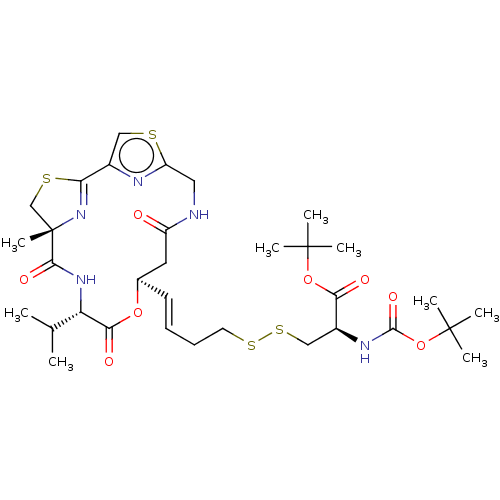
(CHEMBL3329622)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSSC[C@H](NC(=O)OC(C)(C)C)C(=O)OC(C)(C)C)n2 |r,c:11| Show InChI InChI=1S/C33H49N5O8S4/c1-19(2)25-28(41)44-20(14-23(39)34-15-24-35-21(16-47-24)26-38-33(9,18-48-26)29(42)37-25)12-10-11-13-49-50-17-22(27(40)45-31(3,4)5)36-30(43)46-32(6,7)8/h10,12,16,19-20,22,25H,11,13-15,17-18H2,1-9H3,(H,34,39)(H,36,43)(H,37,42)/b12-10+/t20-,22+,25+,33+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50098414

(CHEMBL3593247)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCS)n2 |r,c:11| Show InChI InChI=1S/C21H28N4O4S3/c1-12(2)17-19(27)29-13(6-4-5-7-30)8-15(26)22-9-16-23-14(10-31-16)18-25-21(3,11-32-18)20(28)24-17/h4,6,10,12-13,17,30H,5,7-9,11H2,1-3H3,(H,22,26)(H,24,28)/b6-4+/t13-,17+,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged/C-terminal His-tagged human HDAC10 (1 to 481 residues) expressed in baculovirus infected Sf9 insect cells using B... |
Bioorg Med Chem 25: 3077-3086 (2017)
Article DOI: 10.1016/j.bmc.2017.03.071
BindingDB Entry DOI: 10.7270/Q2M047MP |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50530002
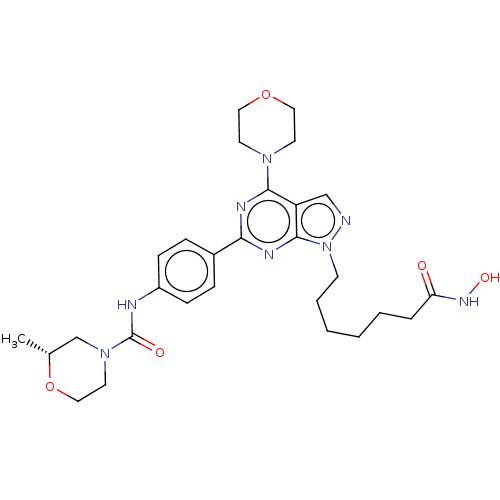
(CHEMBL4532398)Show SMILES C[C@@H]1CN(CCO1)C(=O)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cnn(CCCCCCC(=O)NO)c2n1 |r| Show InChI InChI=1S/C28H38N8O5/c1-20-19-35(14-17-41-20)28(38)30-22-9-7-21(8-10-22)25-31-26(34-12-15-40-16-13-34)23-18-29-36(27(23)32-25)11-5-3-2-4-6-24(37)33-39/h7-10,18,20,39H,2-6,11-17,19H2,1H3,(H,30,38)(H,33,37)/t20-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length N-terminal FLAG-tagged human HDAC10 expressed in baculovirus infected sf9 cells using Ac-peptide-AMC as substra... |
J Med Chem 62: 1577-1592 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01825
BindingDB Entry DOI: 10.7270/Q20868S8 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50530002

(CHEMBL4532398)Show SMILES C[C@@H]1CN(CCO1)C(=O)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cnn(CCCCCCC(=O)NO)c2n1 |r| Show InChI InChI=1S/C28H38N8O5/c1-20-19-35(14-17-41-20)28(38)30-22-9-7-21(8-10-22)25-31-26(34-12-15-40-16-13-34)23-18-29-36(27(23)32-25)11-5-3-2-4-6-24(37)33-39/h7-10,18,20,39H,2-6,11-17,19H2,1H3,(H,30,38)(H,33,37)/t20-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length N-terminal FLAG-tagged human HDAC10 expressed in baculovirus infected sf9 cells using Ac-peptide-AMC as substra... |
J Med Chem 62: 1577-1592 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01825
BindingDB Entry DOI: 10.7270/Q20868S8 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50188899
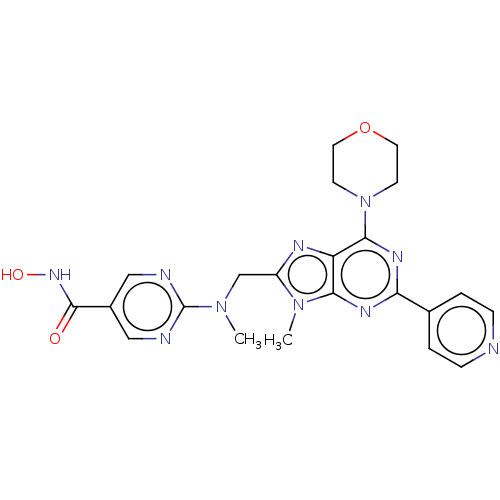
(CHEMBL3827517)Show SMILES CN(Cc1nc2c(nc(nc2n1C)-c1ccncc1)N1CCOCC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C22H24N10O3/c1-30(22-24-11-15(12-25-22)21(33)29-34)13-16-26-17-19(31(16)2)27-18(14-3-5-23-6-4-14)28-20(17)32-7-9-35-10-8-32/h3-6,11-12,34H,7-10,13H2,1-2H3,(H,29,33) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
West China Hospital of Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 expressed in baculovirus infected insect Sf9 cells using Ac-peptide-AMC as substrate assessed as release of AM... |
J Med Chem 59: 5488-504 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00579
BindingDB Entry DOI: 10.7270/Q2T155K0 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50354086

(FK-228 | Istodax | ROMIDEPSIN)Show SMILES C\C=C1/NC(=O)[C@@H](CS)NC(=O)[C@H](CC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C25H39N3O6S2/c1-6-19-23(31)28-21(15(4)5)25(33)34-17(9-7-8-10-35)11-16(29)12-18(14(2)3)22(30)27-20(13-36)24(32)26-19/h6-7,9,14-15,17-18,20-21,35-36H,8,10-13H2,1-5H3,(H,26,32)(H,27,30)(H,28,31)/b9-7+,19-6-/t17-,18-,20-,21+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) incubated for 30 mins in presence of BSA and DTT by fluorescence assay |
ACS Med Chem Lett 5: 905-10 (2014)
Article DOI: 10.1021/ml500170r
BindingDB Entry DOI: 10.7270/Q2RV0QFK |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50027494

(CHEMBL3356916)Show SMILES Cc1cccc(NC(=O)[C@H](CCCCCS)NC(=O)[C@@H]2CCCC(=O)N2)c1 |r| Show InChI InChI=1S/C20H29N3O3S/c1-14-7-5-8-15(13-14)21-19(25)17(9-3-2-4-12-27)23-20(26)16-10-6-11-18(24)22-16/h5,7-8,13,16-17,27H,2-4,6,9-12H2,1H3,(H,21,25)(H,22,24)(H,23,26)/t16-,17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50048864

(CHEMBL3310505)Show SMILES [H][C@@]12CC(=O)N[C@H](C(C)C)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O2 |r,t:20| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17+,19+,20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC10 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50530003
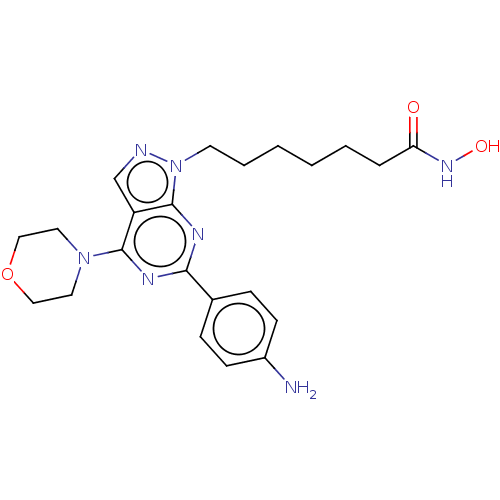
(CHEMBL4552057)Show SMILES Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cnn(CCCCCCC(=O)NO)c2n1 Show InChI InChI=1S/C22H29N7O3/c23-17-8-6-16(7-9-17)20-25-21(28-11-13-32-14-12-28)18-15-24-29(22(18)26-20)10-4-2-1-3-5-19(30)27-31/h6-9,15,31H,1-5,10-14,23H2,(H,27,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length N-terminal FLAG-tagged human HDAC10 expressed in baculovirus infected sf9 cells using Ac-peptide-AMC as substra... |
J Med Chem 62: 1577-1592 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01825
BindingDB Entry DOI: 10.7270/Q20868S8 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50530003

(CHEMBL4552057)Show SMILES Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cnn(CCCCCCC(=O)NO)c2n1 Show InChI InChI=1S/C22H29N7O3/c23-17-8-6-16(7-9-17)20-25-21(28-11-13-32-14-12-28)18-15-24-29(22(18)26-20)10-4-2-1-3-5-19(30)27-31/h6-9,15,31H,1-5,10-14,23H2,(H,27,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length N-terminal FLAG-tagged human HDAC10 expressed in baculovirus infected sf9 cells using Ac-peptide-AMC as substra... |
J Med Chem 62: 1577-1592 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01825
BindingDB Entry DOI: 10.7270/Q20868S8 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human HDAC10 expressed in baculovirus infected Sf9 cells using FAM-RHKK-Ac as substrate incubated for 17 hrs by... |
ACS Med Chem Lett 11: 56-64 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00471
BindingDB Entry DOI: 10.7270/Q26976Z8 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50555460
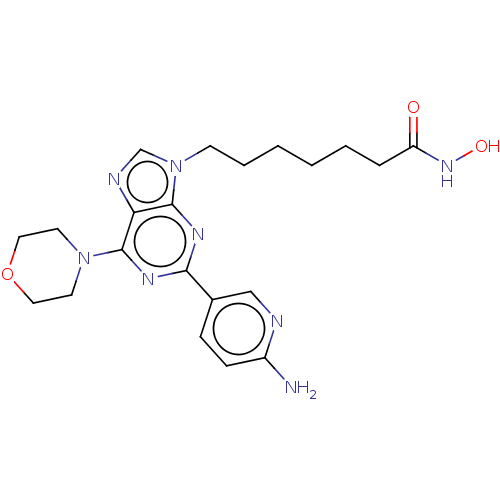
(CHEMBL4785064)Show SMILES Nc1ccc(cn1)-c1nc(N2CCOCC2)c2ncn(CCCCCCC(=O)NO)c2n1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal FLAG-tagged human HDAC10 (2 to 631 residues) expressed in baculovirus infected Sf9 cells using fluorogenic HDAC substrate 3 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.7b01465
BindingDB Entry DOI: 10.7270/Q2765K0S |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM323709
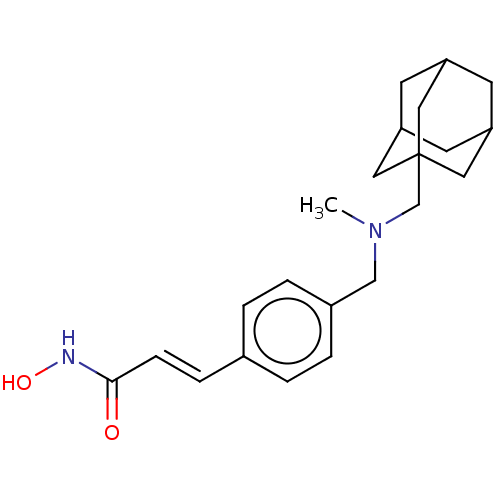
(US10188756, Compound CN133 | US11207431, Martinost...)Show SMILES CN(Cc1ccc(\C=C\C(=O)NO)cc1)CC12CC3CC(CC(C3)C1)C2 |TLB:19:20:24:18.23.17,23:18:25:24.22.21,23:22:25:18.19.17,THB:19:18:24:25.20.21| Show InChI InChI=1S/C22H30N2O2/c1-24(14-17-4-2-16(3-5-17)6-7-21(25)23-26)15-22-11-18-8-19(12-22)10-20(9-18)13-22/h2-7,18-20,26H,8-15H2,1H3,(H,23,25)/b7-6+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
IC50 measurements were conducted by BPS Biosciences (Table 1) or by Nanosyn (Table 1A) with an established fluorescence assay. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2639SXJ |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human HDAC10 expressed in SF9 baculovirus using FAM- labelled acetylated peptide as substrate measured by Elect... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112411
BindingDB Entry DOI: 10.7270/Q2JH3QVS |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro antagonistic activity against kinin-induced rabbit jugular vein contraction. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50027519
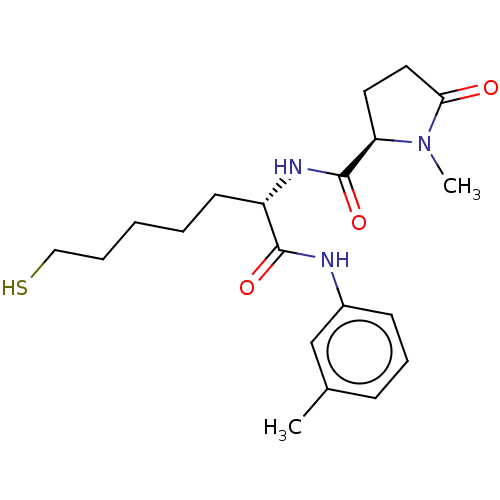
(CHEMBL3356924)Show SMILES CN1[C@H](CCC1=O)C(=O)N[C@@H](CCCCCS)C(=O)Nc1cccc(C)c1 |r| Show InChI InChI=1S/C20H29N3O3S/c1-14-7-6-8-15(13-14)21-19(25)16(9-4-3-5-12-27)22-20(26)17-10-11-18(24)23(17)2/h6-8,13,16-17,27H,3-5,9-12H2,1-2H3,(H,21,25)(H,22,26)/t16-,17+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50514463

(CHEMBL4460552)Show SMILES C[C@H](NC(=O)c1nc(sc1C1CC1)-c1ncc(s1)-c1ccccc1)c1ccc(cc1)C(=O)NO |r| Show InChI InChI=1S/C25H22N4O3S2/c1-14(15-7-11-18(12-8-15)22(30)29-32)27-23(31)20-21(17-9-10-17)34-25(28-20)24-26-13-19(33-24)16-5-3-2-4-6-16/h2-8,11-14,17,32H,9-10H2,1H3,(H,27,31)(H,29,30)/t14-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His6/GST-tagged HDAC10 expressed in baculovirus infected High5 insect cells using Ac-Lys-Tyr-Lys(epsilon-acetyl)-AMC ... |
J Med Chem 63: 804-815 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01792
BindingDB Entry DOI: 10.7270/Q21839VR |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human HDAC10 expressed in baculovirus infected Sf9 cells using FAM-RHKK-Ac as substrate incubated for 17 hrs by... |
ACS Med Chem Lett 11: 56-64 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00471
BindingDB Entry DOI: 10.7270/Q26976Z8 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50526161

(CHEMBL4464421)Show SMILES CCOC(=O)Nc1cccc(c1)-c1csc(NC(=O)CCCCCC(=O)NO)n1 Show InChI InChI=1S/C19H24N4O5S/c1-2-28-19(26)20-14-8-6-7-13(11-14)15-12-29-18(21-15)22-16(24)9-4-3-5-10-17(25)23-27/h6-8,11-12,27H,2-5,9-10H2,1H3,(H,20,26)(H,23,25)(H,21,22,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay |
Eur J Med Chem 166: 369-380 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.077
BindingDB Entry DOI: 10.7270/Q2K93BZT |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Qingdao University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) |
J Med Chem 62: 3171-3183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00189
BindingDB Entry DOI: 10.7270/Q27H1NWV |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50555464
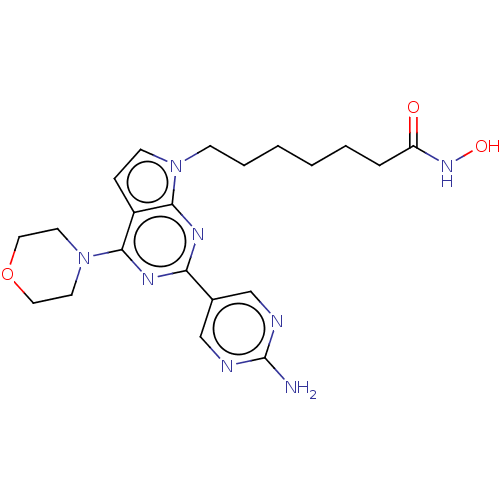
(CHEMBL4744689)Show SMILES Nc1ncc(cn1)-c1nc(N2CCOCC2)c2ccn(CCCCCCC(=O)NO)c2n1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal FLAG-tagged human HDAC10 (2 to 631 residues) expressed in baculovirus infected Sf9 cells using fluorogenic HDAC substrate 3 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.7b01465
BindingDB Entry DOI: 10.7270/Q2765K0S |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC10 using Boc-Lys(triflouroacetyI)-AMC substrate incubated for 2 hrs by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01472
BindingDB Entry DOI: 10.7270/Q28W3J6H |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50555461
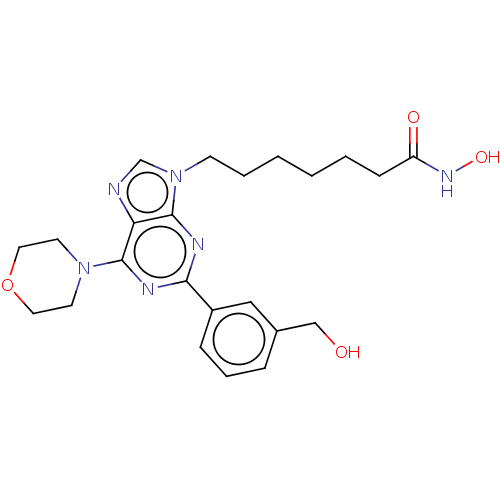
(CHEMBL4749655)Show SMILES OCc1cccc(c1)-c1nc(N2CCOCC2)c2ncn(CCCCCCC(=O)NO)c2n1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal FLAG-tagged human HDAC10 (2 to 631 residues) expressed in baculovirus infected Sf9 cells using fluorogenic HDAC substrate 3 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.7b01465
BindingDB Entry DOI: 10.7270/Q2765K0S |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hunan University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using Color de lys as substrate by HTS assay |
Eur J Med Chem 136: 195-211 (2017)
Article DOI: 10.1016/j.ejmech.2017.05.016
BindingDB Entry DOI: 10.7270/Q2D79DX7 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
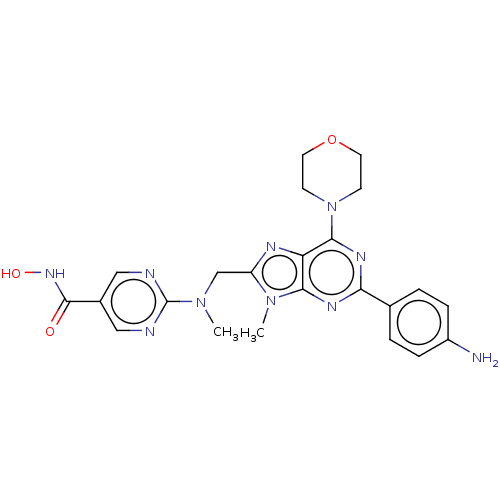
(Homo sapiens (Human)) | BDBM50188637
(CHEMBL3827814)Show SMILES CN(Cc1nc2c(nc(nc2n1C)-c1ccc(N)cc1)N1CCOCC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C23H26N10O3/c1-31(23-25-11-15(12-26-23)22(34)30-35)13-17-27-18-20(32(17)2)28-19(14-3-5-16(24)6-4-14)29-21(18)33-7-9-36-10-8-33/h3-6,11-12,35H,7-10,13,24H2,1-2H3,(H,30,34) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
West China Hospital of Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 expressed in baculovirus infected insect Sf9 cells using Ac-peptide-AMC as substrate assessed as release of AM... |
J Med Chem 59: 5488-504 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00579
BindingDB Entry DOI: 10.7270/Q2T155K0 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
West China Hospital of Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 expressed in baculovirus infected insect Sf9 cells using Ac-peptide-AMC as substrate assessed as release of AM... |
J Med Chem 59: 5488-504 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00579
BindingDB Entry DOI: 10.7270/Q2T155K0 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
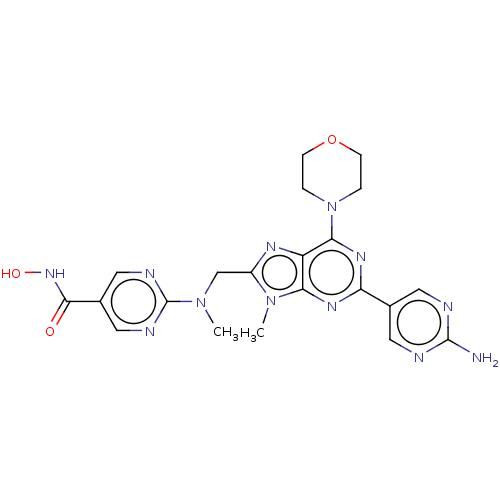
(Homo sapiens (Human)) | BDBM50188959
(CHEMBL3827894)Show SMILES CN(Cc1nc2c(nc(nc2n1C)-c1cnc(N)nc1)N1CCOCC1)c1ncc(cn1)C(=O)NO Show InChI InChI=1S/C21H24N12O3/c1-31(21-25-9-13(10-26-21)19(34)30-35)11-14-27-15-17(32(14)2)28-16(12-7-23-20(22)24-8-12)29-18(15)33-3-5-36-6-4-33/h7-10,35H,3-6,11H2,1-2H3,(H,30,34)(H2,22,23,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
West China Hospital of Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 expressed in baculovirus infected insect Sf9 cells using Ac-peptide-AMC as substrate assessed as release of AM... |
J Med Chem 59: 5488-504 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00579
BindingDB Entry DOI: 10.7270/Q2T155K0 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Qingdao University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) |
J Med Chem 62: 3171-3183 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00189
BindingDB Entry DOI: 10.7270/Q27H1NWV |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | CHEMBL5278968
Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-15(18)11-19-16-8-7-13-5-3-4-6-14(13)9-16/h3-9,12,15,17-18H,10-11H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114039
BindingDB Entry DOI: 10.7270/Q2TT4VZR |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC10 (unknown origin) by color de Lys colorimetric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50242139

(CHEMBL4078721)Show SMILES CC(C)[C@@H]1NC(=O)[C@]2(C)CSC(=N2)c2csc(CNC(=O)C[C@H](OC1=O)\C=C\CCSCCS)n2 |r,c:11| Show InChI InChI=1S/C23H32N4O4S4/c1-14(2)19-21(29)31-15(6-4-5-8-33-9-7-32)10-17(28)24-11-18-25-16(12-34-18)20-27-23(3,13-35-20)22(30)26-19/h4,6,12,14-15,19,32H,5,7-11,13H2,1-3H3,(H,24,28)(H,26,30)/b6-4+/t15-,19+,23+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged/C-terminal His-tagged human HDAC10 (1 to 481 residues) expressed in baculovirus infected Sf9 insect cells using B... |
Bioorg Med Chem 25: 3077-3086 (2017)
Article DOI: 10.1016/j.bmc.2017.03.071
BindingDB Entry DOI: 10.7270/Q2M047MP |
More data for this
Ligand-Target Pair | |
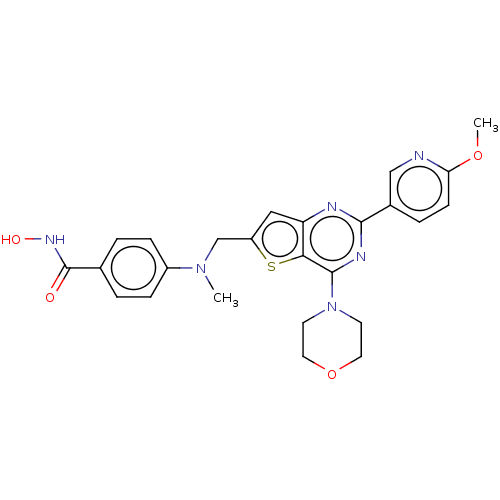
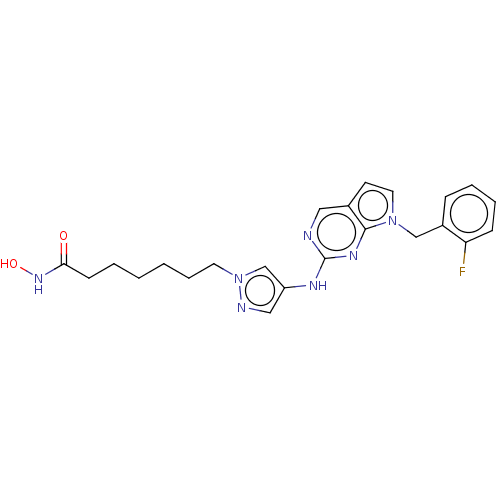
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50582354
(CHEMBL5076728)Show SMILES ONC(=O)CCCCCCn1cc(Nc2ncc3ccn(Cc4ccccc4F)c3n2)cn1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC10 (unknown origin) using Ac-peptide-AMC as substrate incubated for 240 mins by microplate reader analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02111
BindingDB Entry DOI: 10.7270/Q26M3BQC |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50526156

(CHEMBL1630208 | WR301861)Show InChI InChI=1S/C16H20N4O3S/c17-12-6-4-5-11(9-12)13-10-24-16(18-13)19-14(21)7-2-1-3-8-15(22)20-23/h4-6,9-10,23H,1-3,7-8,17H2,(H,20,22)(H,18,19,21) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay |
Eur J Med Chem 166: 369-380 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.077
BindingDB Entry DOI: 10.7270/Q2K93BZT |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50123617
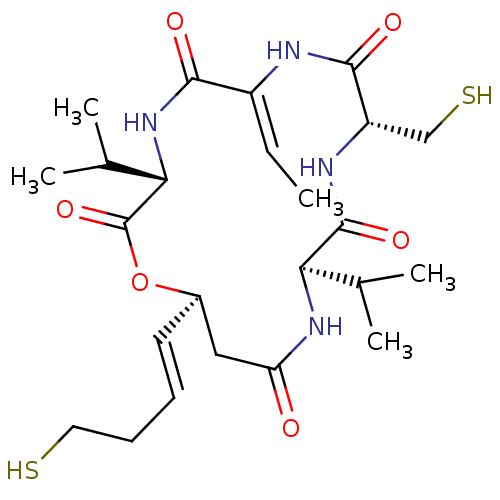
(CHEMBL3622726)Show SMILES C\C=C1/NC(=O)[C@H](CS)NC(=O)[C@H](NC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C24H38N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15(9-7-8-10-35)11-18(29)27-19(13(2)3)23(32)26-17(12-36)22(31)25-16/h6-7,9,13-15,17,19-20,35-36H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17+,19-,20+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC10 after 60 mins by fluorescence assay |
J Med Chem 58: 7672-80 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01044
BindingDB Entry DOI: 10.7270/Q2DF6T01 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50232354
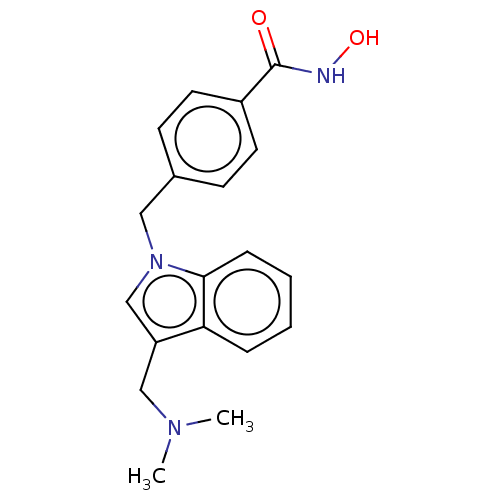
(CHEMBL4066920)Show InChI InChI=1S/C19H21N3O2/c1-21(2)12-16-13-22(18-6-4-3-5-17(16)18)11-14-7-9-15(10-8-14)19(23)20-24/h3-10,13,24H,11-12H2,1-2H3,(H,20,23) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
European Molecular Biology Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of dye-labeled tracer binding to HDAC10 (unknown origin) transfected in human HeLa cells measured after 2 hrs by nano-luciferase reporter ... |
J Med Chem 62: 4426-4443 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01936
BindingDB Entry DOI: 10.7270/Q26H4MTC |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
European Molecular Biology Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of tubastatin-Alexa647-tracer binding to recombinant GST-tagged HDAC10 (unknown origin) measured after 1 hr by TR-FRET assay |
J Med Chem 62: 4426-4443 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01936
BindingDB Entry DOI: 10.7270/Q26H4MTC |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
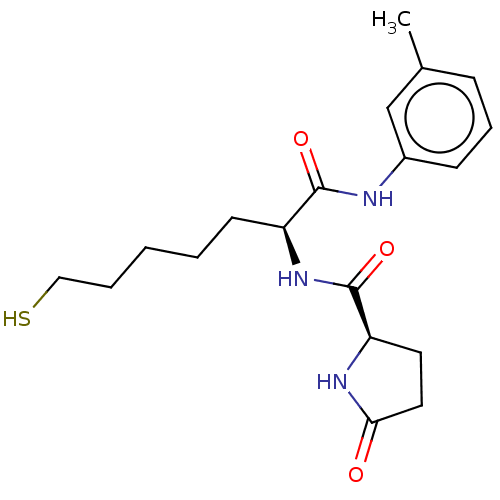
(Homo sapiens (Human)) | BDBM50027501
(CHEMBL3356923)Show SMILES Cc1cccc(NC(=O)[C@H](CCCCCS)NC(=O)[C@H]2CCC(=O)N2)c1 |r| Show InChI InChI=1S/C19H27N3O3S/c1-13-6-5-7-14(12-13)20-18(24)15(8-3-2-4-11-26)22-19(25)16-9-10-17(23)21-16/h5-7,12,15-16,26H,2-4,8-11H2,1H3,(H,20,24)(H,21,23)(H,22,25)/t15-,16+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
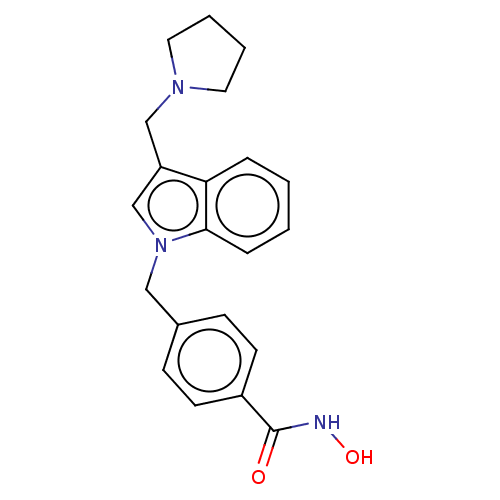
(Homo sapiens (Human)) | BDBM50521588
(CHEMBL4578278)Show InChI InChI=1S/C21H23N3O2/c25-21(22-26)17-9-7-16(8-10-17)13-24-15-18(14-23-11-3-4-12-23)19-5-1-2-6-20(19)24/h1-2,5-10,15,26H,3-4,11-14H2,(H,22,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
European Molecular Biology Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of tubastatin-Alexa647-tracer binding to recombinant GST-tagged HDAC10 (unknown origin) measured after 1 hr by TR-FRET assay |
J Med Chem 62: 4426-4443 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01936
BindingDB Entry DOI: 10.7270/Q26H4MTC |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HDAC10 (unknown origin) using AMC labeled AC-peptide as substrate incubated for 1 hr by fluorescence analysis |
J Med Chem 59: 1455-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01342
BindingDB Entry DOI: 10.7270/Q2T155H3 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
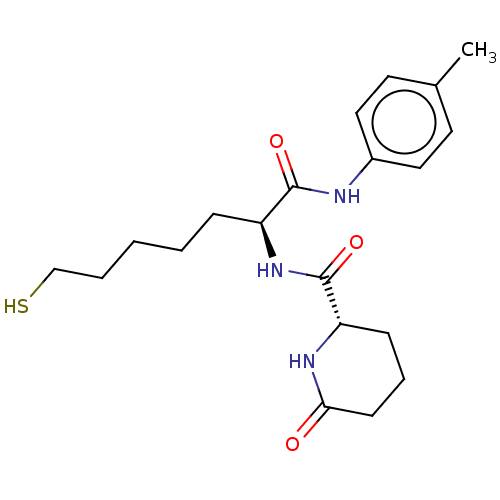
(Homo sapiens (Human)) | BDBM50027495
(CHEMBL3356917)Show SMILES Cc1ccc(NC(=O)[C@H](CCCCCS)NC(=O)[C@@H]2CCCC(=O)N2)cc1 |r| Show InChI InChI=1S/C20H29N3O3S/c1-14-9-11-15(12-10-14)21-19(25)17(6-3-2-4-13-27)23-20(26)16-7-5-8-18(24)22-16/h9-12,16-17,27H,2-8,13H2,1H3,(H,21,25)(H,22,24)(H,23,26)/t16-,17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau Industrie Farmaceutiche Riunite SpA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) using fluorogenic tetrapeptide RHKK(Ac) substrate by fluorescence assay |
J Med Chem 57: 8358-77 (2014)
Article DOI: 10.1021/jm5008209
BindingDB Entry DOI: 10.7270/Q2JW8GGN |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50521588

(CHEMBL4578278)Show InChI InChI=1S/C21H23N3O2/c25-21(22-26)17-9-7-16(8-10-17)13-24-15-18(14-23-11-3-4-12-23)19-5-1-2-6-20(19)24/h1-2,5-10,15,26H,3-4,11-14H2,(H,22,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
European Molecular Biology Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of dye-labeled tracer binding to HDAC10 (unknown origin) transfected in human HeLa cells measured after 2 hrs by nano-luciferase reporter ... |
J Med Chem 62: 4426-4443 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01936
BindingDB Entry DOI: 10.7270/Q26H4MTC |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50188961

(CHEMBL3622533)Show SMILES COc1ccc(cn1)-c1nc(N2CCOCC2)c2sc(CN(C)c3ncc(cn3)C(=O)NO)cc2n1 Show InChI InChI=1S/C23H24N8O4S/c1-30(23-25-11-15(12-26-23)22(32)29-33)13-16-9-17-19(36-16)21(31-5-7-35-8-6-31)28-20(27-17)14-3-4-18(34-2)24-10-14/h3-4,9-12,33H,5-8,13H2,1-2H3,(H,29,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal FLAG-tagged human HDAC10 (2 to 631 residues) expressed in baculovirus infected Sf9 cells using fluorogenic HDAC substrate 3 ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.7b01465
BindingDB Entry DOI: 10.7270/Q2765K0S |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50304782

(CHEMBL609583 | N-hydroxy-2-(4-(naphthalen-2-ylsulf...)Show SMILES ONC(=O)c1cnc(nc1)N1CCN(CC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C19H19N5O4S/c25-18(22-26)16-12-20-19(21-13-16)23-7-9-24(10-8-23)29(27,28)17-6-5-14-3-1-2-4-15(14)11-17/h1-6,11-13,26H,7-10H2,(H,22,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ortho-Biotech Oncology Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC10 |
Bioorg Med Chem Lett 20: 294-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.118
BindingDB Entry DOI: 10.7270/Q2DB81ZS |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
West China Hospital of Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 expressed in baculovirus infected insect Sf9 cells using Ac-peptide-AMC as substrate assessed as release of AM... |
J Med Chem 59: 5488-504 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00579
BindingDB Entry DOI: 10.7270/Q2T155K0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data