 Found 45 hits of ki data for polymerid = 2538
Found 45 hits of ki data for polymerid = 2538 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50105327
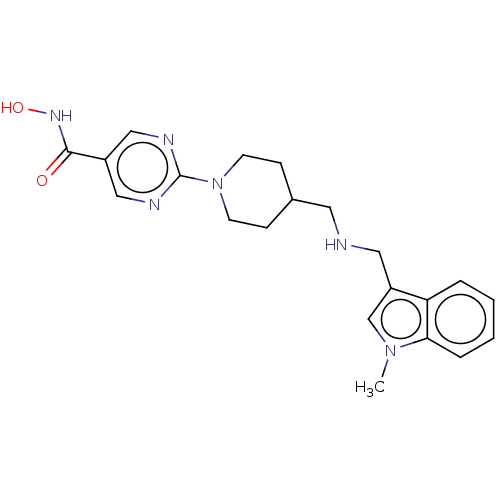
(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC10 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50463739
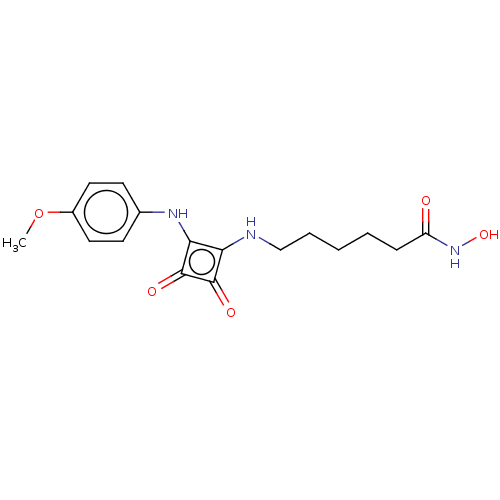
(CHEMBL4237803)Show InChI InChI=1S/C17H21N3O5/c1-25-12-8-6-11(7-9-12)19-15-14(16(22)17(15)23)18-10-4-2-3-5-13(21)20-24/h6-9,18-19,24H,2-5,10H2,1H3,(H,20,21) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using fluorogenic HDAC substrate after 45 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50463743
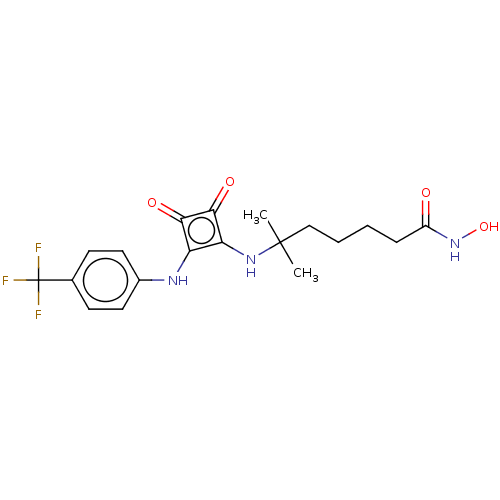
(CHEMBL4241370)Show SMILES CC(C)(CCCCC(=O)NO)Nc1c(Nc2ccc(cc2)C(F)(F)F)c(=O)c1=O Show InChI InChI=1S/C19H22F3N3O4/c1-18(2,10-4-3-5-13(26)25-29)24-15-14(16(27)17(15)28)23-12-8-6-11(7-9-12)19(20,21)22/h6-9,23-24,29H,3-5,10H2,1-2H3,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using fluorogenic HDAC substrate after 45 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using fluorogenic HDAC substrate after 45 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC10 expressed in baculovirus-infected insect cells using fluorogenic peptide RHKKAc as substrate by fluorimeter |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using fluorogenic HDAC substrate after 45 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM19130
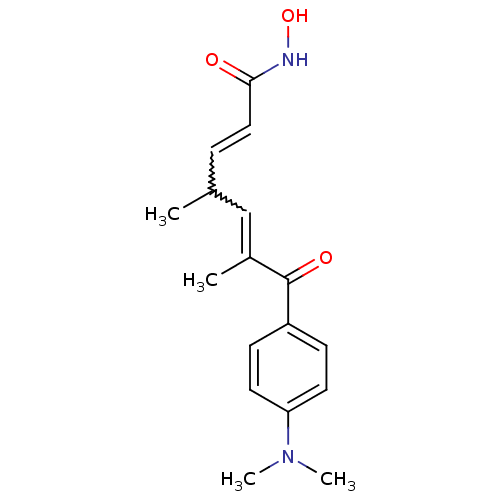
((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Poitiers
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 |
Eur J Med Chem 45: 2095-116 (2010)
Article DOI: 10.1016/j.ejmech.2010.02.030
BindingDB Entry DOI: 10.7270/Q2BR8SCS |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50319209
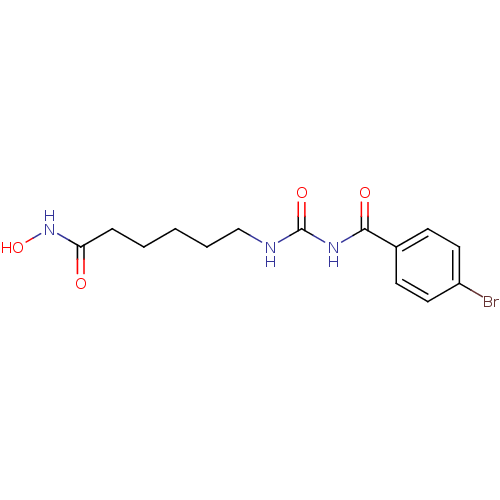
(6-[3-(4-Bromo-benzoyl)-ureido]-hexanoic acid hydro...)Show InChI InChI=1S/C14H18BrN3O4/c15-11-7-5-10(6-8-11)13(20)17-14(21)16-9-3-1-2-4-12(19)18-22/h5-8,22H,1-4,9H2,(H,18,19)(H2,16,17,20,21) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 |
Bioorg Med Chem Lett 20: 3314-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.041
BindingDB Entry DOI: 10.7270/Q2GT5NCX |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
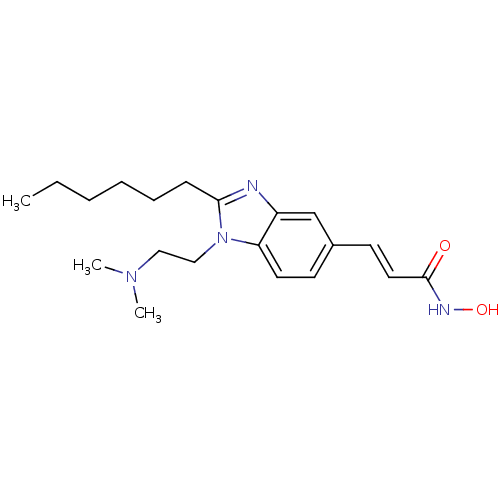
(Homo sapiens (Human)) | BDBM50353232
(CHEMBL1830424)Show InChI InChI=1S/C20H30N4O2/c1-4-5-6-7-8-19-21-17-15-16(10-12-20(25)22-26)9-11-18(17)24(19)14-13-23(2)3/h9-12,15,26H,4-8,13-14H2,1-3H3,(H,22,25)/b12-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
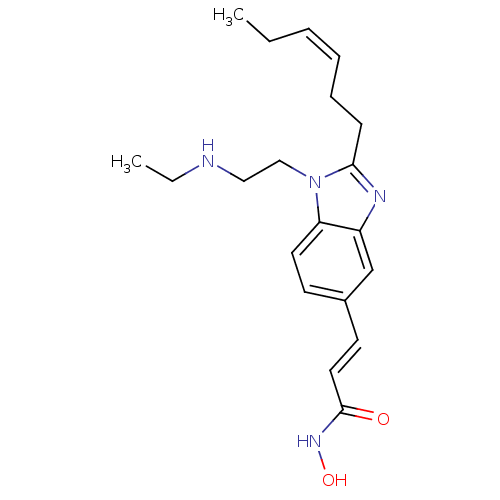
(Homo sapiens (Human)) | BDBM50353233
(CHEMBL1830536)Show SMILES CCNCCn1c(CC\C=C/CC)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C20H28N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h5-6,9-12,15,21,26H,3-4,7-8,13-14H2,1-2H3,(H,23,25)/b6-5-,12-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
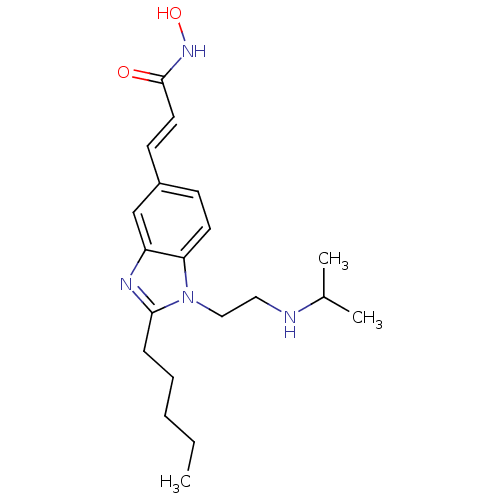
(Homo sapiens (Human)) | BDBM50353234
(CHEMBL1830537)Show InChI InChI=1S/C20H30N4O2/c1-4-5-6-7-19-22-17-14-16(9-11-20(25)23-26)8-10-18(17)24(19)13-12-21-15(2)3/h8-11,14-15,21,26H,4-7,12-13H2,1-3H3,(H,23,25)/b11-9+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50353227
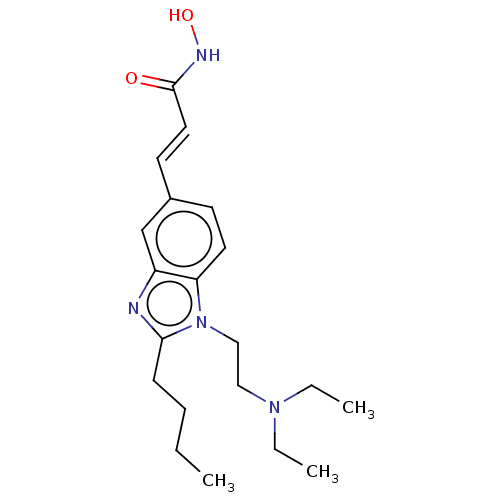
(CHEMBL3215861)Show SMILES Cl.Cl.CCCCc1nc2cc(\C=C\C(=O)NO)ccc2n1CCN(CC)CC Show InChI InChI=1S/C20H30N4O2/c1-4-7-8-19-21-17-15-16(10-12-20(25)22-26)9-11-18(17)24(19)14-13-23(5-2)6-3/h9-12,15,26H,4-8,13-14H2,1-3H3,(H,22,25)/b12-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50248476
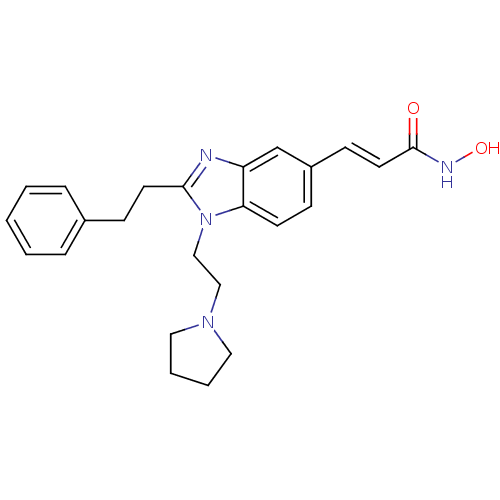
(CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...)Show SMILES ONC(=O)\C=C\c1ccc2n(CCN3CCCC3)c(CCc3ccccc3)nc2c1 Show InChI InChI=1S/C24H28N4O2/c29-24(26-30)13-10-20-8-11-22-21(18-20)25-23(12-9-19-6-2-1-3-7-19)28(22)17-16-27-14-4-5-15-27/h1-3,6-8,10-11,13,18,30H,4-5,9,12,14-17H2,(H,26,29)/b13-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50248476

(CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...)Show SMILES ONC(=O)\C=C\c1ccc2n(CCN3CCCC3)c(CCc3ccccc3)nc2c1 Show InChI InChI=1S/C24H28N4O2/c29-24(26-30)13-10-20-8-11-22-21(18-20)25-23(12-9-19-6-2-1-3-7-19)28(22)17-16-27-14-4-5-15-27/h1-3,6-8,10-11,13,18,30H,4-5,9,12,14-17H2,(H,26,29)/b13-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HDAC10 by fluorimetric assay |
Bioorg Med Chem Lett 19: 1403-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.041
BindingDB Entry DOI: 10.7270/Q2FT8KX3 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human HDAC10 (1 to 631 residues) expressed in baculovirus expression system in Sf9 cells RHKAcKAc after 2 hrs by ... |
Eur J Med Chem 135: 174-195 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.013
BindingDB Entry DOI: 10.7270/Q2G44SQS |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50248522
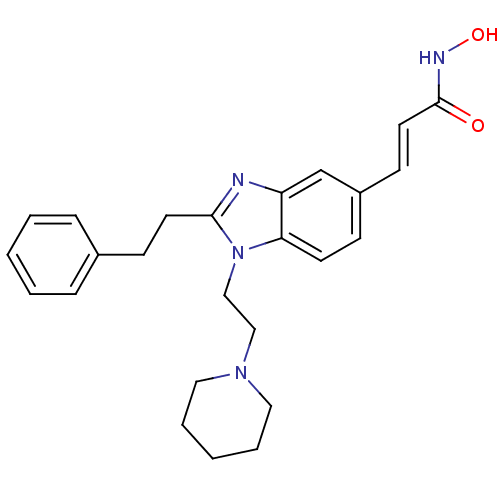
(CHEMBL489332 | N-hydroxy-3-(2-phenethyl-1-(2-(pipe...)Show SMILES ONC(=O)\C=C\c1ccc2n(CCN3CCCCC3)c(CCc3ccccc3)nc2c1 Show InChI InChI=1S/C25H30N4O2/c30-25(27-31)14-11-21-9-12-23-22(19-21)26-24(13-10-20-7-3-1-4-8-20)29(23)18-17-28-15-5-2-6-16-28/h1,3-4,7-9,11-12,14,19,31H,2,5-6,10,13,15-18H2,(H,27,30)/b14-11+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50105330
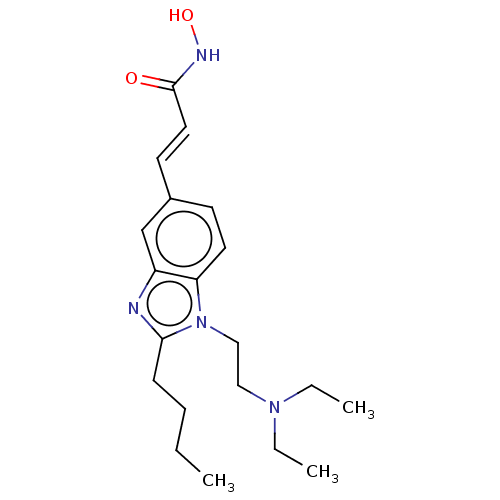
(CHEMBL1851943)Show InChI InChI=1S/C20H30N4O2/c1-4-7-8-19-21-17-15-16(10-12-20(25)22-26)9-11-18(17)24(19)14-13-23(5-2)6-3/h9-12,15,26H,4-8,13-14H2,1-3H3,(H,22,25)/b12-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC10 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50248570
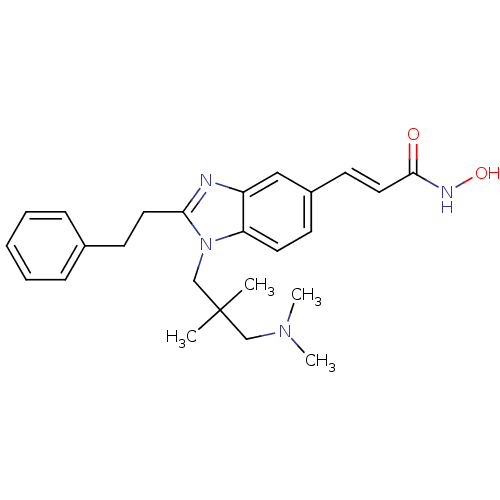
(3-(1-(3-(dimethylamino)-2,2-dimethylpropyl)-2-phen...)Show SMILES CN(C)CC(C)(C)Cn1c(CCc2ccccc2)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C25H32N4O2/c1-25(2,17-28(3)4)18-29-22-13-10-20(12-15-24(30)27-31)16-21(22)26-23(29)14-11-19-8-6-5-7-9-19/h5-10,12-13,15-16,31H,11,14,17-18H2,1-4H3,(H,27,30)/b15-12+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC10 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | -10.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50353230
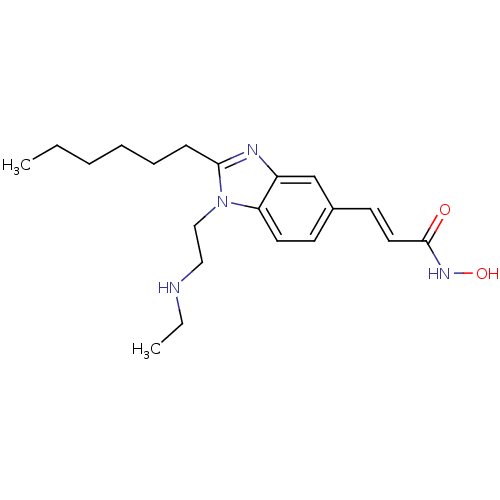
(CHEMBL1830420)Show InChI InChI=1S/C20H30N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h9-12,15,21,26H,3-8,13-14H2,1-2H3,(H,23,25)/b12-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50353231
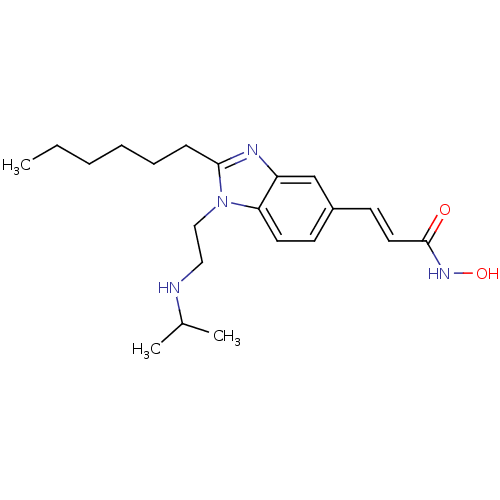
(CHEMBL1830422)Show InChI InChI=1S/C21H32N4O2/c1-4-5-6-7-8-20-23-18-15-17(10-12-21(26)24-27)9-11-19(18)25(20)14-13-22-16(2)3/h9-12,15-16,22,27H,4-8,13-14H2,1-3H3,(H,24,26)/b12-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50353228
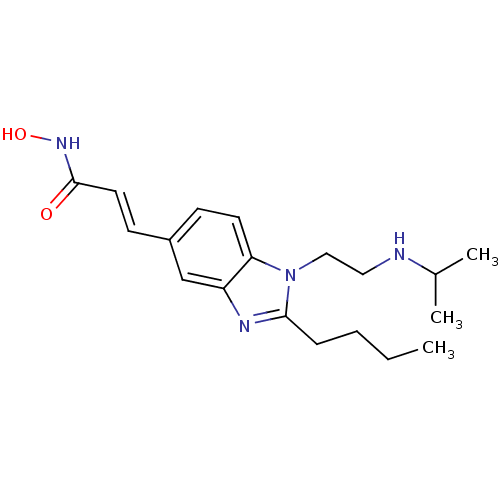
(CHEMBL1830396)Show InChI InChI=1S/C19H28N4O2/c1-4-5-6-18-21-16-13-15(8-10-19(24)22-25)7-9-17(16)23(18)12-11-20-14(2)3/h7-10,13-14,20,25H,4-6,11-12H2,1-3H3,(H,22,24)/b10-8+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50353229
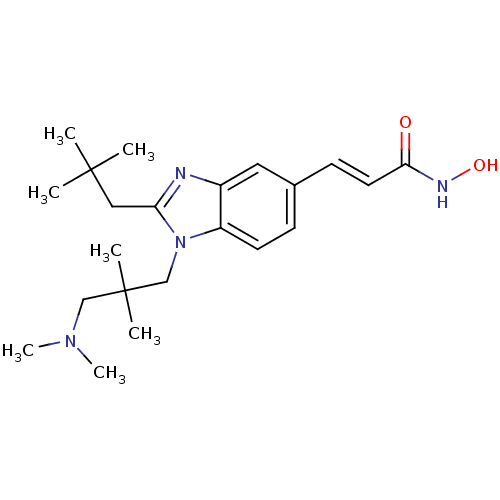
(CHEMBL1830397)Show SMILES CN(C)CC(C)(C)Cn1c(CC(C)(C)C)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C22H34N4O2/c1-21(2,3)13-19-23-17-12-16(9-11-20(27)24-28)8-10-18(17)26(19)15-22(4,5)14-25(6)7/h8-12,28H,13-15H2,1-7H3,(H,24,27)/b11-9+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50319235
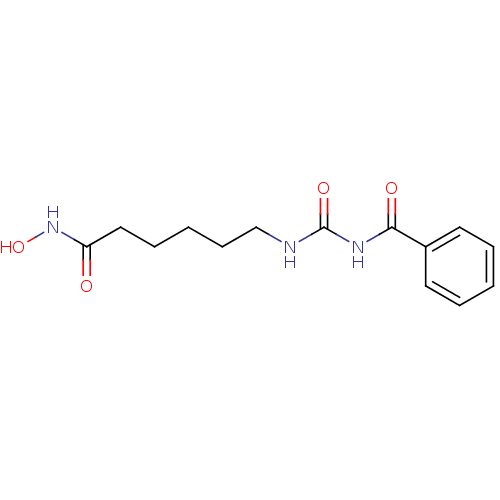
(6-(3-Benzoyl-ureido)-hexanoic acid hydroxyamide | ...)Show InChI InChI=1S/C14H19N3O4/c18-12(17-21)9-5-2-6-10-15-14(20)16-13(19)11-7-3-1-4-8-11/h1,3-4,7-8,21H,2,5-6,9-10H2,(H,17,18)(H2,15,16,19,20) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 |
Bioorg Med Chem Lett 20: 3314-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.041
BindingDB Entry DOI: 10.7270/Q2GT5NCX |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 |
Bioorg Med Chem Lett 20: 3314-21 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.041
BindingDB Entry DOI: 10.7270/Q2GT5NCX |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50463738
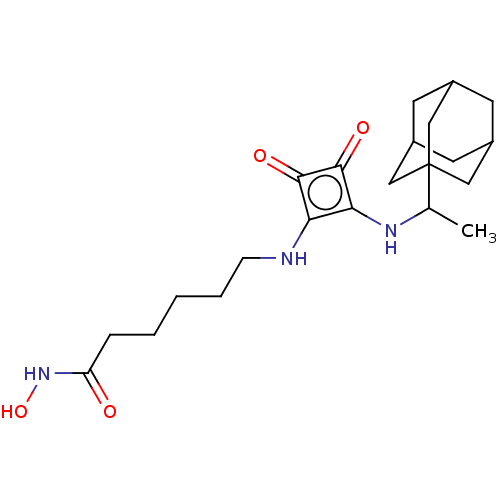
(CHEMBL4244764)Show SMILES CC(Nc1c(NCCCCCC(=O)NO)c(=O)c1=O)C12CC3CC(CC(C3)C1)C2 |THB:24:23:20:26.25.27,24:25:22.23.28:20,27:25:22:28.19.20,27:19:22:26.24.25| Show InChI InChI=1S/C22H33N3O4/c1-13(22-10-14-7-15(11-22)9-16(8-14)12-22)24-19-18(20(27)21(19)28)23-6-4-2-3-5-17(26)25-29/h13-16,23-24,29H,2-12H2,1H3,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health R&D
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using fluorogenic HDAC substrate after 45 mins by fluorimetrc method |
Bioorg Med Chem Lett 28: 2985-2992 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.029
BindingDB Entry DOI: 10.7270/Q2M0484T |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM25150

((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032270
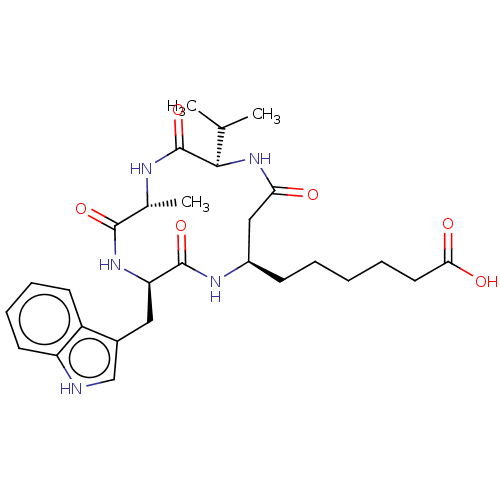
(CHEMBL3352995)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H39N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h7-8,10-11,15-17,19,22,25,29H,4-6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/t17-,19-,22-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032267

(CHEMBL3352997)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h4-5,7-8,10-11,15-17,19,22,25,29H,6,9,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b5-4-/t17-,19-,22-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032266
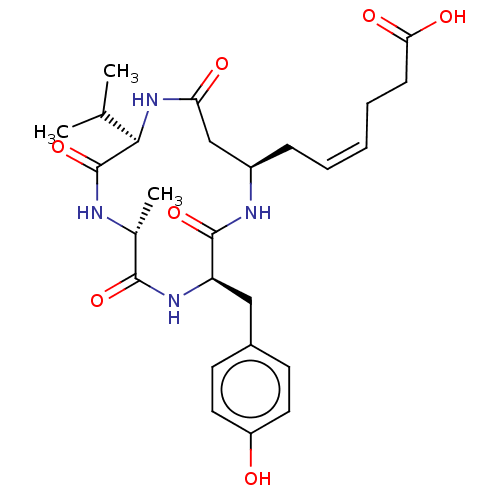
(CHEMBL3352823)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h4-5,9-12,15-16,18,20,23,31H,6-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b5-4-/t16-,18-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032272
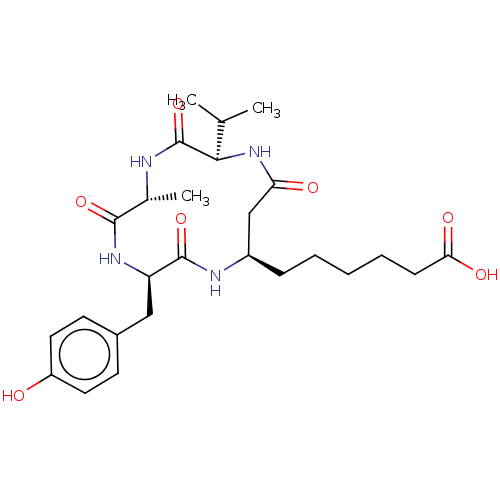
(CHEMBL3352994)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H38N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h9-12,15-16,18,20,23,31H,4-8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/t16-,18-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM22449
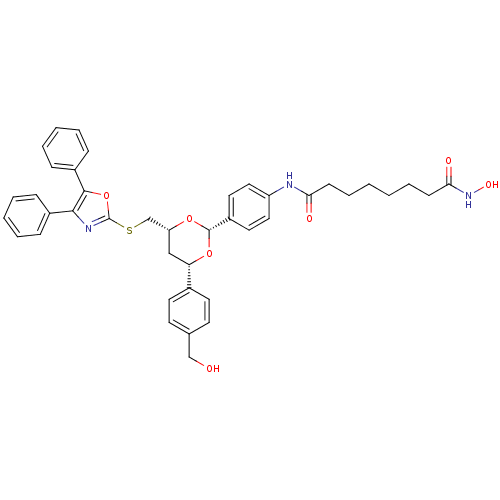
(CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...)Show SMILES OCc1ccc(cc1)[C@@H]1C[C@H](CSc2nc(c(o2)-c2ccccc2)-c2ccccc2)O[C@@H](O1)c1ccc(NC(=O)CCCCCCC(=O)NO)cc1 |r| Show InChI InChI=1S/C41H43N3O7S/c45-26-28-17-19-29(20-18-28)35-25-34(27-52-41-43-38(30-11-5-3-6-12-30)39(51-41)31-13-7-4-8-14-31)49-40(50-35)32-21-23-33(24-22-32)42-36(46)15-9-1-2-10-16-37(47)44-48/h3-8,11-14,17-24,34-35,40,45,48H,1-2,9-10,15-16,25-27H2,(H,42,46)(H,44,47)/t34-,35+,40+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 374 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC10 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50252395
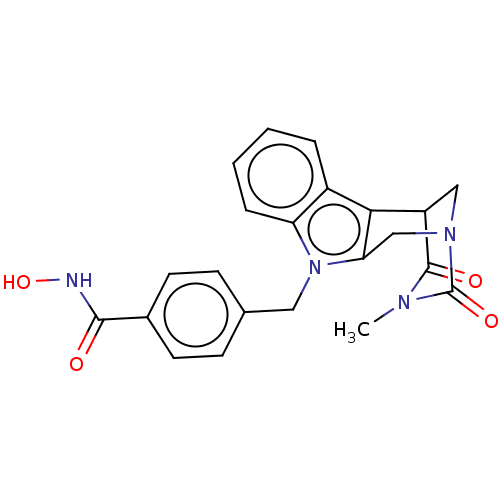
(CHEMBL4087616)Show SMILES CN1C(=O)C2CN(Cc3c2c2ccccc2n3Cc2ccc(cc2)C(=O)NO)C1=O Show InChI InChI=1S/C22H20N4O4/c1-24-21(28)16-11-25(22(24)29)12-18-19(16)15-4-2-3-5-17(15)26(18)10-13-6-8-14(9-7-13)20(27)23-30/h2-9,16,30H,10-12H2,1H3,(H,23,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC10 expressed in baculovirus-infected insect cells using fluorogenic peptide RHKKAc as substrate by fluorimeter |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032269
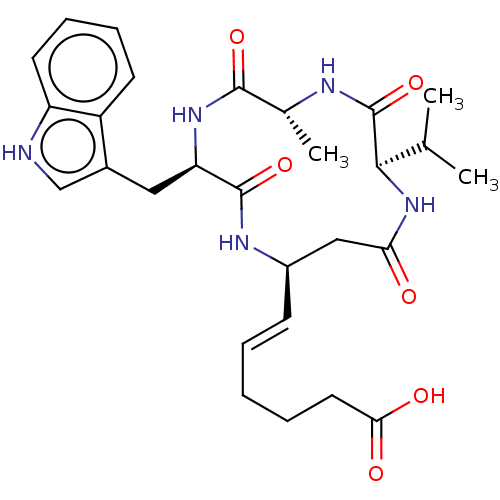
(CHEMBL3352992)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C28H37N5O6/c1-16(2)25-28(39)30-17(3)26(37)32-22(13-18-15-29-21-11-8-7-10-20(18)21)27(38)31-19(14-23(34)33-25)9-5-4-6-12-24(35)36/h5,7-11,15-17,19,22,25,29H,4,6,12-14H2,1-3H3,(H,30,39)(H,31,38)(H,32,37)(H,33,34)(H,35,36)/b9-5+/t17-,19-,22-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032274
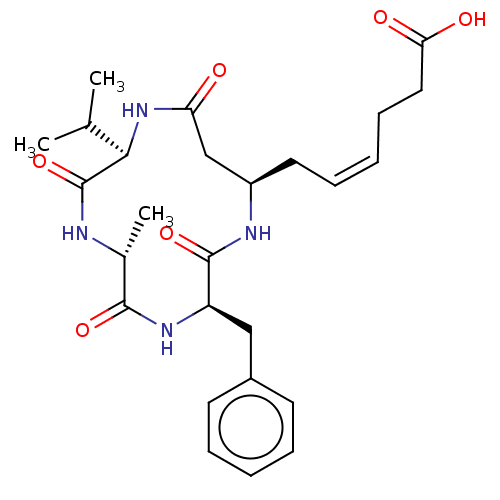
(CHEMBL3352996)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](C\C=C/CCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H36N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4-8,10-11,16-17,19-20,23H,9,12-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/b8-5-/t17-,19-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032271
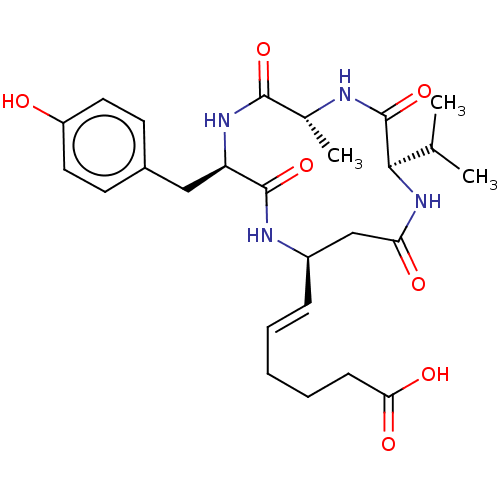
(CHEMBL3352991)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2ccc(O)cc2)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C26H36N4O7/c1-15(2)23-26(37)27-16(3)24(35)29-20(13-17-9-11-19(31)12-10-17)25(36)28-18(14-21(32)30-23)7-5-4-6-8-22(33)34/h5,7,9-12,15-16,18,20,23,31H,4,6,8,13-14H2,1-3H3,(H,27,37)(H,28,36)(H,29,35)(H,30,32)(H,33,34)/b7-5+/t16-,18-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032273
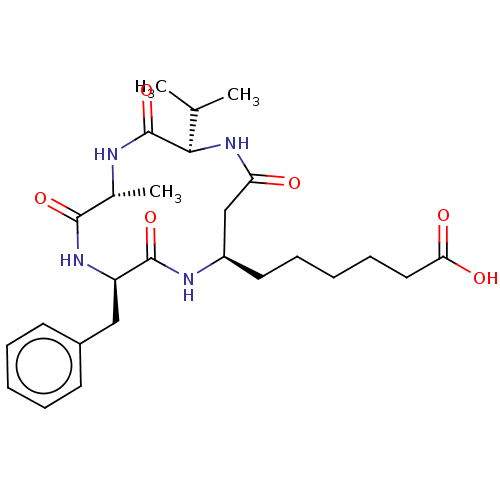
(CHEMBL3352993)Show SMILES CC(C)[C@H]1NC(=O)C[C@@H](CCCCCC(O)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O |r| Show InChI InChI=1S/C26H38N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4,6-7,10-11,16-17,19-20,23H,5,8-9,12-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/t17-,19-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50032268
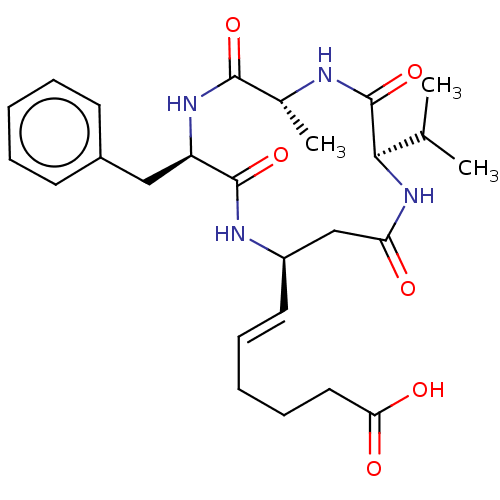
(CHEMBL3352990)Show SMILES CC(C)[C@H]1NC(=O)C[C@H](NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](C)NC1=O)\C=C\CCCC(O)=O |r| Show InChI InChI=1S/C26H36N4O6/c1-16(2)23-26(36)27-17(3)24(34)29-20(14-18-10-6-4-7-11-18)25(35)28-19(15-21(31)30-23)12-8-5-9-13-22(32)33/h4,6-8,10-12,16-17,19-20,23H,5,9,13-15H2,1-3H3,(H,27,36)(H,28,35)(H,29,34)(H,30,31)(H,32,33)/b12-8+/t17-,19-,20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC10 using Ac-Arg-His-Lys(Ac)-Lys(Ac)-AMC substrate incubated for 15 to 30 mins |
J Med Chem 57: 9644-57 (2014)
Article DOI: 10.1021/jm501399d
BindingDB Entry DOI: 10.7270/Q2FJ2JCG |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50446481
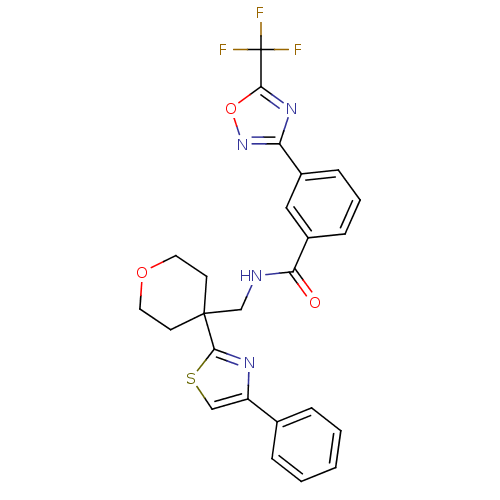
(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC10 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50517219
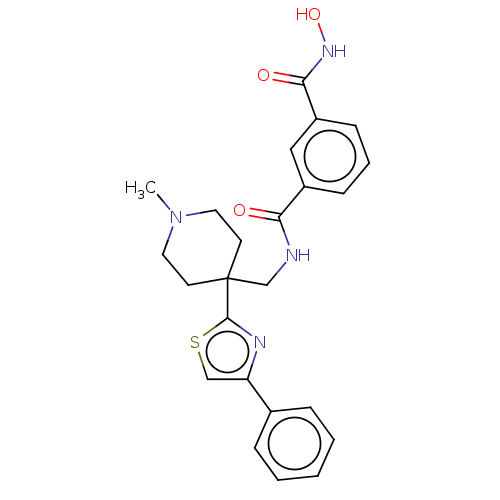
(CHEMBL4456269)Show SMILES CN1CCC(CNC(=O)c2cccc(c2)C(=O)NO)(CC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C24H26N4O3S/c1-28-12-10-24(11-13-28,23-26-20(15-32-23)17-6-3-2-4-7-17)16-25-21(29)18-8-5-9-19(14-18)22(30)27-31/h2-9,14-15,31H,10-13,16H2,1H3,(H,25,29)(H,27,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.115087
BindingDB Entry DOI: 10.7270/Q2VM4GMZ |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50517218
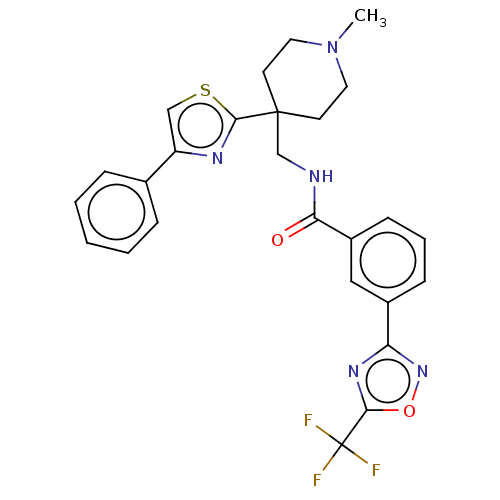
(CHEMBL4525406)Show SMILES CN1CCC(CNC(=O)c2cccc(c2)-c2noc(n2)C(F)(F)F)(CC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C26H24F3N5O2S/c1-34-12-10-25(11-13-34,24-31-20(15-37-24)17-6-3-2-4-7-17)16-30-22(35)19-9-5-8-18(14-19)21-32-23(36-33-21)26(27,28)29/h2-9,14-15H,10-13,16H2,1H3,(H,30,35) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t D£sseldorf
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 (unknown origin) |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.115087
BindingDB Entry DOI: 10.7270/Q2VM4GMZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data