 Found 8534 hits of ic50 data for polymerid = 2556,50005094
Found 8534 hits of ic50 data for polymerid = 2556,50005094 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50261816
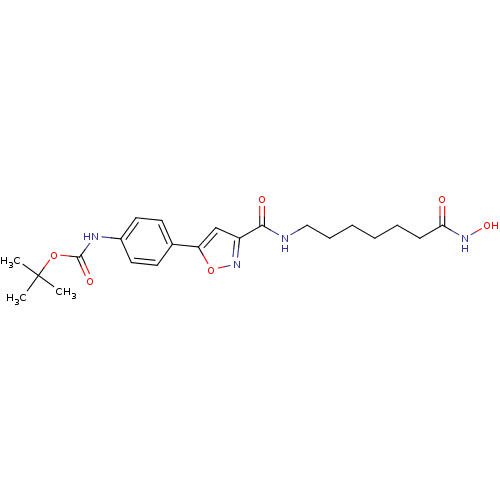
(CHEMBL511749 | tert-butyl 4-(3-((7-(hydroxyamino)-...)Show SMILES CC(C)(C)OC(=O)Nc1ccc(cc1)-c1cc(no1)C(=O)NCCCCCCC(=O)NO Show InChI InChI=1S/C22H30N4O6/c1-22(2,3)31-21(29)24-16-11-9-15(10-12-16)18-14-17(26-32-18)20(28)23-13-7-5-4-6-8-19(27)25-30/h9-12,14,30H,4-8,13H2,1-3H3,(H,23,28)(H,24,29)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 17 hrs |
J Med Chem 51: 4370-3 (2008)
Article DOI: 10.1021/jm8002894
BindingDB Entry DOI: 10.7270/Q27W6C11 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50578549
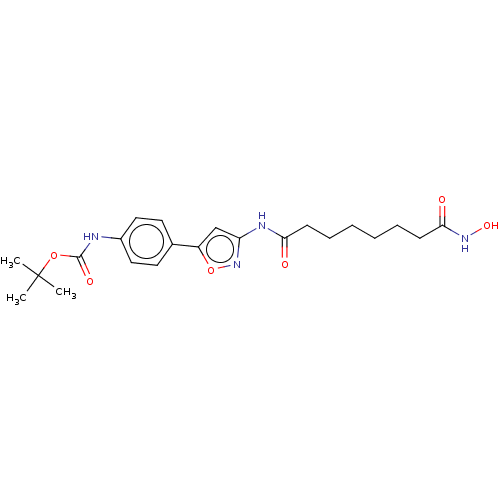
(CHEMBL4876454)Show SMILES CC(C)(C)OC(=O)Nc1ccc(cc1)-c1cc(NC(=O)CCCCCCC(=O)NO)no1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC6 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113526
BindingDB Entry DOI: 10.7270/Q2D79G8J |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50601782
(CHEMBL5198547) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113874
BindingDB Entry DOI: 10.7270/Q2TB1BZK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50587720
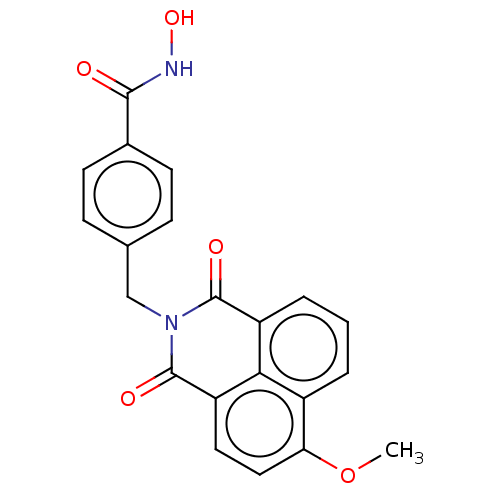
(CHEMBL5195946)Show SMILES COc1ccc2C(=O)N(Cc3ccc(cc3)C(=O)NO)C(=O)c3cccc1c23 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50467405

(CHEMBL4290831)Show SMILES Cl.CC(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(NC(=O)CCCCCCC(=O)NO)cc2)n1 Show InChI InChI=1S/C26H30N6O4/c1-18(33)28-20-10-8-19(9-11-20)23-16-17-27-26(31-23)30-22-14-12-21(13-15-22)29-24(34)6-4-2-3-5-7-25(35)32-36/h8-17,36H,2-7H2,1H3,(H,28,33)(H,29,34)(H,32,35)(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using Arg-His-Lys-Lys(Ac) as substrate preincubated with enzyme followed by substrate addition for 2 hrs and mea... |
Eur J Med Chem 158: 593-619 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.024
BindingDB Entry DOI: 10.7270/Q2Z3229Z |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50250140

(CHEMBL4098975)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(NCCCn2cc(cn2)-c2ncnc3[nH]ccc23)cc1 Show InChI InChI=1S/C26H32N8O3/c35-23(6-3-1-2-4-7-24(36)33-37)32-21-10-8-20(9-11-21)27-13-5-15-34-17-19(16-31-34)25-22-12-14-28-26(22)30-18-29-25/h8-12,14,16-18,27,37H,1-7,13,15H2,(H,32,35)(H,33,36)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST tagged human HDAC6 (1 to 1215 residues) expressed in baculovirus infected insect cells using RHKKAc as substrate in pres... |
J Med Chem 60: 8336-8357 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00678
BindingDB Entry DOI: 10.7270/Q2CN76B6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM218187
(US9249087, 30)Show SMILES CS(=O)(=O)NCCc1cn(Cc2ccc(cc2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C19H21N3O4S/c1-27(25,26)20-11-10-16-13-22(18-5-3-2-4-17(16)18)12-14-6-8-15(9-7-14)19(23)21-24/h2-9,13,20,24H,10-12H2,1H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 8.0 | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS; THE CHILDREN'S HOSPITAL OF PHILADELPHIA
US Patent
| Assay Description
HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC... |
US Patent US9249087 (2016)
BindingDB Entry DOI: 10.7270/Q2Q23Z3W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50526157

(CHEMBL4550526)Show SMILES CC(C)(C)OC(=O)Nc1cccc(c1)-c1csc(NC(=O)CCCCCC(=O)NO)n1 Show InChI InChI=1S/C21H28N4O5S/c1-21(2,3)30-20(28)22-15-9-7-8-14(12-15)16-13-31-19(23-16)24-17(26)10-5-4-6-11-18(27)25-29/h7-9,12-13,29H,4-6,10-11H2,1-3H3,(H,22,28)(H,25,27)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay |
Eur J Med Chem 166: 369-380 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.077
BindingDB Entry DOI: 10.7270/Q2K93BZT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50462724

(CHEMBL4243347)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(Nc2nc(-c3cn[nH]c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C23H26N8O3/c32-19(5-3-1-2-4-6-20(33)31-34)27-16-7-9-17(10-8-16)28-23-29-21(15-13-25-26-14-15)18-11-12-24-22(18)30-23/h7-14,34H,1-6H2,(H,25,26)(H,27,32)(H,31,33)(H2,24,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 using p53 (379 to 382 residues) derived fluorogenic peptide RHKKAc as substrate |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50462726
(CHEMBL4247128)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(Nc2nc(-c3cnn(CCC=C)c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C27H32N8O3/c1-2-3-16-35-18-19(17-29-35)25-22-14-15-28-26(22)33-27(32-25)31-21-12-10-20(11-13-21)30-23(36)8-6-4-5-7-9-24(37)34-38/h2,10-15,17-18,38H,1,3-9,16H2,(H,30,36)(H,34,37)(H2,28,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 using p53 (379 to 382 residues) derived fluorogenic peptide RHKKAc as substrate |
Bioorg Med Chem Lett 28: 2636-2640 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.037
BindingDB Entry DOI: 10.7270/Q2T1568C |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557844
(CHEMBL4748025 | WO2021067859, Compound I-8B)Show SMILES ONC(=O)c1cnc(N[C@@H](C2CC2)c2ccccc2)c(F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557844

(CHEMBL4748025 | WO2021067859, Compound I-8B)Show SMILES ONC(=O)c1cnc(N[C@@H](C2CC2)c2ccccc2)c(F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC6 (unknown origin) using luminescent substrate by HDAC-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00282
BindingDB Entry DOI: 10.7270/Q2D2229N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50247556
(CHEMBL4104117)Show InChI InChI=1S/C17H15N3O2/c21-17(20-22)14-8-6-12(7-9-14)11-19-15-5-1-3-13-4-2-10-18-16(13)15/h1-10,19,22H,11H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50247556

(CHEMBL4104117)Show InChI InChI=1S/C17H15N3O2/c21-17(20-22)14-8-6-12(7-9-14)11-19-15-5-1-3-13-4-2-10-18-16(13)15/h1-10,19,22H,11H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.291 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal GST-tagged HDAC6 expressed in baculovirus infected insect cells using RHK-K(Ac)-AMC as substra... |
J Med Chem 61: 905-917 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01404
BindingDB Entry DOI: 10.7270/Q2Q81GGB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50571526
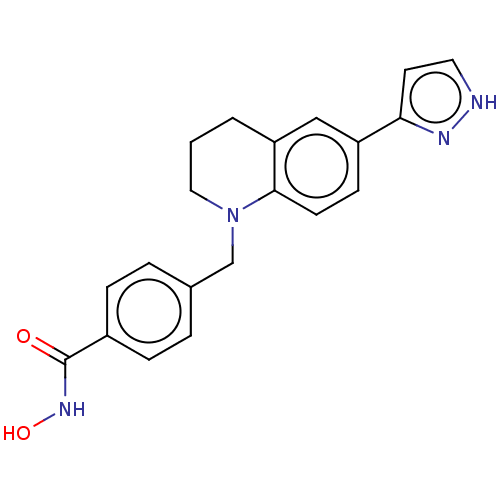
(CHEMBL4863515)Show SMILES ONC(=O)c1ccc(CN2CCCc3cc(ccc23)-c2cc[nH]n2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50274998
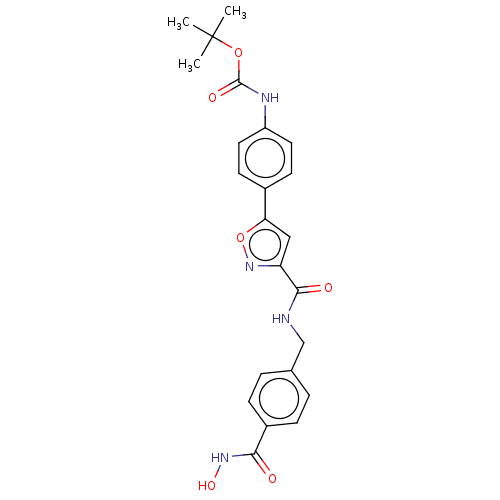
(CHEMBL4126811)Show SMILES CC(C)(C)OC(=O)Nc1ccc(cc1)-c1cc(no1)C(=O)NCc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C23H24N4O6/c1-23(2,3)32-22(30)25-17-10-8-15(9-11-17)19-12-18(27-33-19)21(29)24-13-14-4-6-16(7-5-14)20(28)26-31/h4-12,31H,13H2,1-3H3,(H,24,29)(H,25,30)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human HDAC6 (1 to 1215 residues) expressed in baculovirus infected sf9 insect cells using p53 (379 to... |
J Med Chem 62: 8557-8577 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00946
BindingDB Entry DOI: 10.7270/Q2542RXH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557845
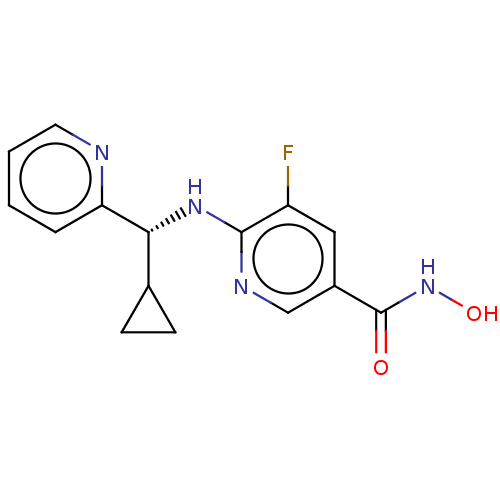
(CHEMBL4756236 | WO2021067859, Compound I-9A)Show SMILES ONC(=O)c1cnc(N[C@H](C2CC2)c2ccccn2)c(F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.351 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC6 (unknown origin) using luminescent substrate by HDAC-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00282
BindingDB Entry DOI: 10.7270/Q2D2229N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557845

(CHEMBL4756236 | WO2021067859, Compound I-9A)Show SMILES ONC(=O)c1cnc(N[C@H](C2CC2)c2ccccn2)c(F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.351 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50560908
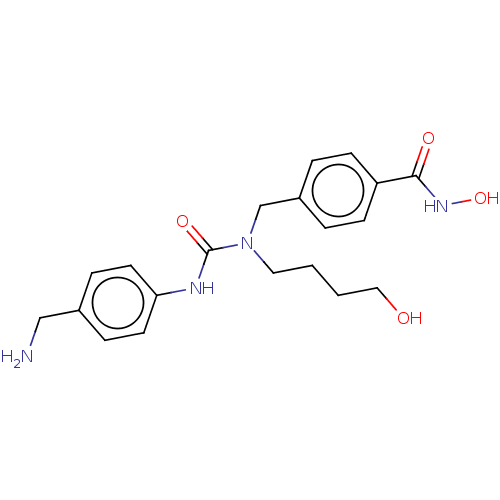
(CHEMBL4777296)Show SMILES NCc1ccc(NC(=O)N(CCCCO)Cc2ccc(cc2)C(=O)NO)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 expressed in HEK293/T17 cells using pre-incubated for 10 mins before Ac-GAK(Ac)-AM substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00567
BindingDB Entry DOI: 10.7270/Q2JH3QW7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
R&D Sigma-Tau SpA
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 |
Bioorg Med Chem Lett 19: 2346-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.029
BindingDB Entry DOI: 10.7270/Q2154GX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50587716
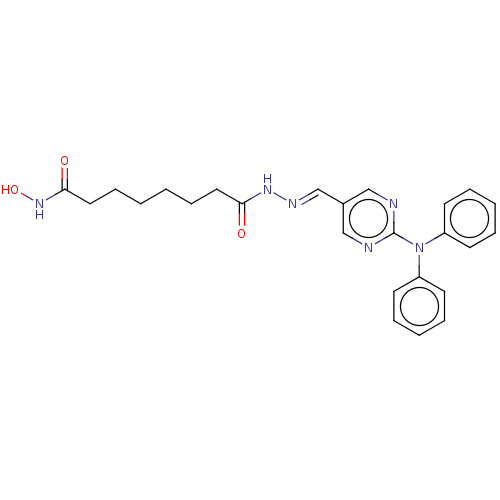
(CHEMBL5188297)Show SMILES ONC(=O)CCCCCCC(=O)N\N=C\c1cnc(nc1)N(c1ccccc1)c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01782
BindingDB Entry DOI: 10.7270/Q2G73JP3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Milano
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 using fluorogenic tetrapeptide RHKKAc as substrate |
Eur J Med Chem 79: 251-9 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.021
BindingDB Entry DOI: 10.7270/Q2V989KV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 by fluorimetric assay |
J Med Chem 53: 8387-8399 (2010)
Article DOI: 10.1021/jm101092u
BindingDB Entry DOI: 10.7270/Q28G8KZJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557847

(CHEMBL4749714 | WO2021067859, Compound I-19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.425 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC6 (unknown origin) using luminescent substrate by HDAC-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00282
BindingDB Entry DOI: 10.7270/Q2D2229N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557847

(CHEMBL4749714 | WO2021067859, Compound I-19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.425 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM119695

(US8685992, 14)Show SMILES ONC(=O)c1ccc(CNC(=O)c2[nH]c(cc2-c2ccsc2)-c2ccc(O)cc2)cc1 Show InChI InChI=1S/C28H27N3O3S/c1-34-23-13-11-20(12-14-23)24-17-25(21-5-3-2-4-6-21)31-26(24)28(33)30-18-19-7-9-22(10-8-19)27(32)29-15-16-35/h2-14,17,31,35H,15-16,18H2,1H3,(H,29,32)(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | 30 |
Universidad del País Vasco; Ikerschem, S.L.
US Patent
| Assay Description
The reactions were carried out in a 96-well microplate for fluorometry in a 50 μl reaction volume. After the deacetylation reaction, Fluor-de-Ly... |
US Patent US8685992 (2014)
BindingDB Entry DOI: 10.7270/Q2CJ8C4X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380385
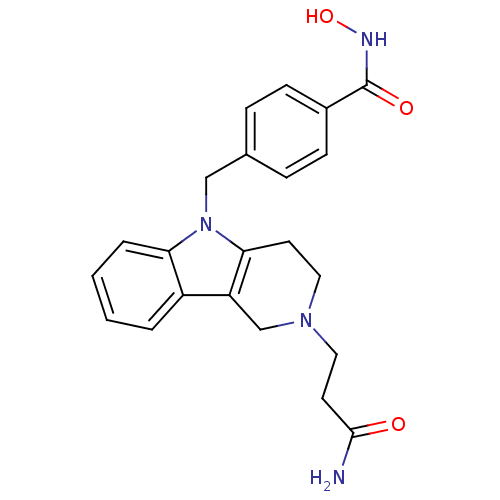
(CHEMBL2018447)Show SMILES NC(=O)CCN1CCc2c(C1)c1ccccc1n2Cc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C22H24N4O3/c23-21(27)10-12-25-11-9-20-18(14-25)17-3-1-2-4-19(17)26(20)13-15-5-7-16(8-6-15)22(28)24-29/h1-8,29H,9-14H2,(H2,23,27)(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.459 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs |
J Med Chem 55: 639-51 (2012)
Article DOI: 10.1021/jm200773h
BindingDB Entry DOI: 10.7270/Q2CJ8FG7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50143887

(CHEMBL3759794)Show InChI InChI=1S/C19H22N4O3/c24-18(6-2-1-3-7-19(25)23-26)22-21-13-15-8-10-16(11-9-15)17-5-4-12-20-14-17/h4-5,8-14,26H,1-3,6-7H2,(H,22,24)(H,23,25)/b21-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 1265-71 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.022
BindingDB Entry DOI: 10.7270/Q2H99714 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50504583
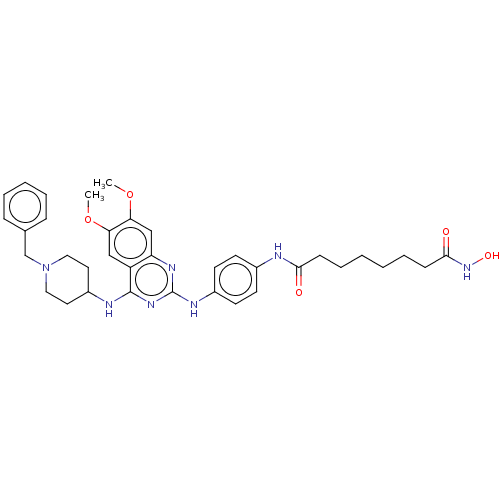
(CHEMBL4444554)Show SMILES COc1cc2nc(Nc3ccc(NC(=O)CCCCCCC(=O)NO)cc3)nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC Show InChI InChI=1S/C36H45N7O5/c1-47-31-22-29-30(23-32(31)48-2)40-36(41-35(29)38-28-18-20-43(21-19-28)24-25-10-6-5-7-11-25)39-27-16-14-26(15-17-27)37-33(44)12-8-3-4-9-13-34(45)42-46/h5-7,10-11,14-17,22-23,28,46H,3-4,8-9,12-13,18-21,24H2,1-2H3,(H,37,44)(H,42,45)(H2,38,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 using Arg-His-Lys-Lys (Ac) as substrate incubated for 2 hrs by fluorescence assay |
Eur J Med Chem 184: (2019)
Article DOI: 10.1016/j.ejmech.2019.111755
BindingDB Entry DOI: 10.7270/Q2ZS30T4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM417049
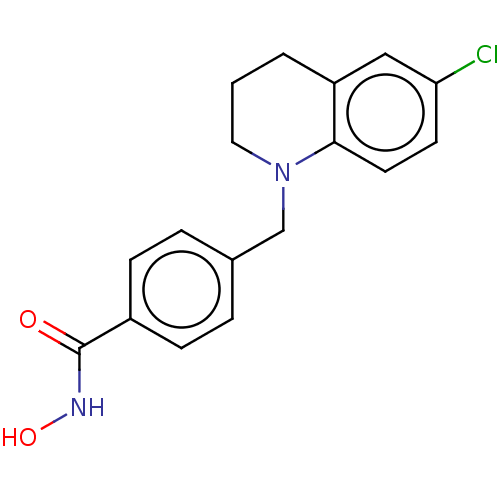
(4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...)Show InChI InChI=1S/C17H17ClN2O2/c18-15-7-8-16-14(10-15)2-1-9-20(16)11-12-3-5-13(6-4-12)17(21)19-22/h3-8,10,22H,1-2,9,11H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50279258
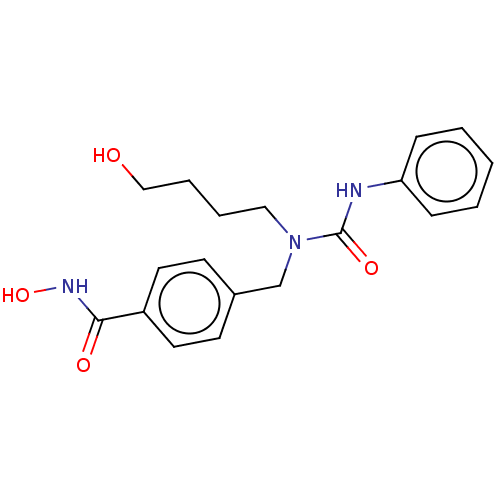
(CHEMBL4177129)Show InChI InChI=1S/C19H23N3O4/c23-13-5-4-12-22(19(25)20-17-6-2-1-3-7-17)14-15-8-10-16(11-9-15)18(24)21-26/h1-3,6-11,23,26H,4-5,12-14H2,(H,20,25)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 expressed in HEK293/T17 cells using pre-incubated for 10 mins before Ac-GAK(Ac)-AM substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00567
BindingDB Entry DOI: 10.7270/Q2JH3QW7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380391
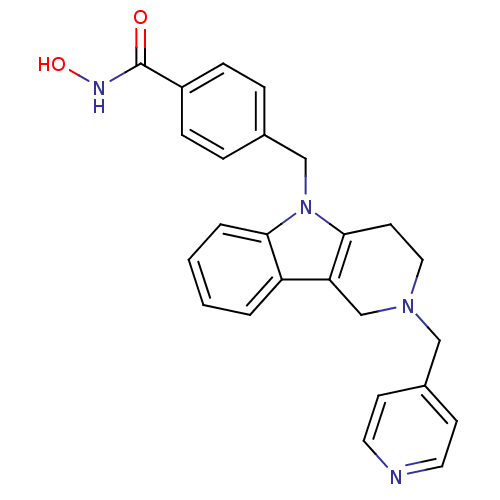
(CHEMBL2018446)Show SMILES ONC(=O)c1ccc(Cn2c3CCN(Cc4ccncc4)Cc3c3ccccc23)cc1 Show InChI InChI=1S/C25H24N4O2/c30-25(27-31)20-7-5-18(6-8-20)16-29-23-4-2-1-3-21(23)22-17-28(14-11-24(22)29)15-19-9-12-26-13-10-19/h1-10,12-13,31H,11,14-17H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.582 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs |
J Med Chem 55: 639-51 (2012)
Article DOI: 10.1021/jm200773h
BindingDB Entry DOI: 10.7270/Q2CJ8FG7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557849
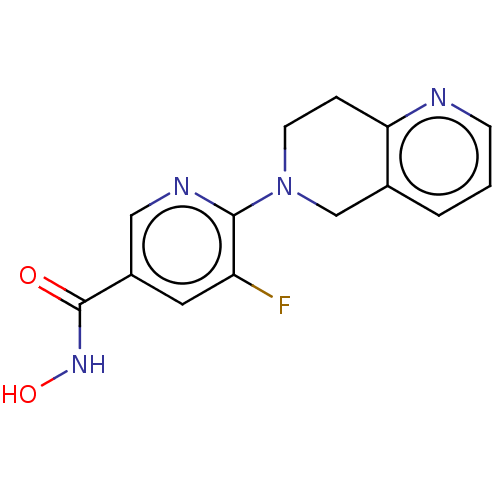
(CHEMBL4751524 | WO2021067859, Compound IV-9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.595 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC6 (unknown origin) using luminescent substrate by HDAC-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00282
BindingDB Entry DOI: 10.7270/Q2D2229N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557849

(CHEMBL4751524 | WO2021067859, Compound IV-9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.595 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50143884
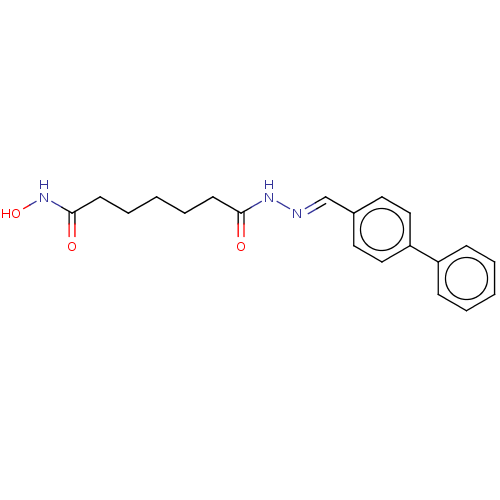
(CHEMBL3759982)Show InChI InChI=1S/C20H23N3O3/c24-19(9-5-2-6-10-20(25)23-26)22-21-15-16-11-13-18(14-12-16)17-7-3-1-4-8-17/h1,3-4,7-8,11-15,26H,2,5-6,9-10H2,(H,22,24)(H,23,25)/b21-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using acetyllysine tripeptide coupled with 7-amino-4-methylcoumarin as substrate by fluorescence assay |
Bioorg Med Chem Lett 26: 1265-71 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.022
BindingDB Entry DOI: 10.7270/Q2H99714 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557848
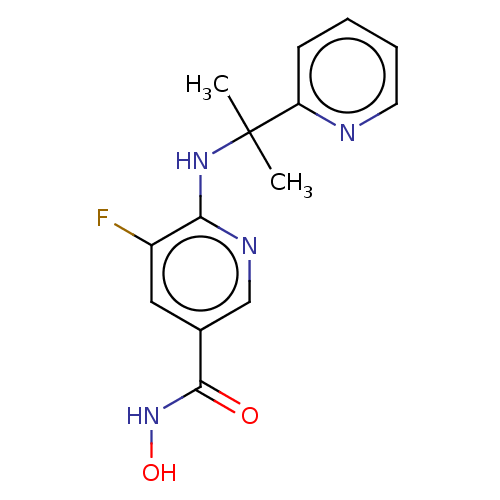
(CHEMBL4777764 | WO2021067859, Compound I-21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.601 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC6 (unknown origin) using luminescent substrate by HDAC-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00282
BindingDB Entry DOI: 10.7270/Q2D2229N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557848

(CHEMBL4777764 | WO2021067859, Compound I-21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.601 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50507260

(CHEMBL4531853)Show SMILES ONC(=O)c1ccc(CNC(=O)c2cc(Cn3c4ccccc4c4ccccc34)on2)cc1 Show InChI InChI=1S/C25H20N4O4/c30-24(27-32)17-11-9-16(10-12-17)14-26-25(31)21-13-18(33-28-21)15-29-22-7-3-1-5-19(22)20-6-2-4-8-23(20)29/h1-13,32H,14-15H2,(H,26,31)(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Deakin University
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 using (Z-(Ac)Lys-AMC) as substrate after 90 mins by fluorescence analysis |
Eur J Med Chem 162: 321-333 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.020
BindingDB Entry DOI: 10.7270/Q2JQ14BB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Mus musculus) | BDBM50048864

(CHEMBL3310505)Show SMILES [H][C@@]12CC(=O)N[C@H](C(C)C)C(=O)N[C@]([H])(CSSCC\C=C\1)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O2 |r,t:20| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17+,19+,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.625 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rajshahi
Curated by ChEMBL
| Assay Description
Inhibition of mouse HDAC6 using fluorescent substrate Ac-KGLGK(Ac)-MCA after 30 mins by fluorescence plate reader in presence of 0.1 mM dithiothreito... |
Bioorg Med Chem 22: 3850-5 (2014)
Article DOI: 10.1016/j.bmc.2014.06.029
BindingDB Entry DOI: 10.7270/Q23F4R9P |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557846

(CHEMBL4762877) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.639 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant HDAC6 (unknown origin) using luminescent substrate by HDAC-Glo assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00282
BindingDB Entry DOI: 10.7270/Q2D2229N |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50557848

(CHEMBL4777764 | WO2021067859, Compound I-21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.639 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50286578
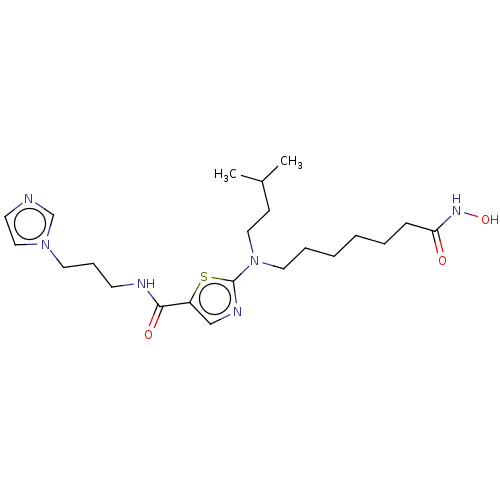
(CHEMBL4170617)Show SMILES CC(C)CCN(CCCCCCC(=O)NO)c1ncc(s1)C(=O)NCCCn1ccnc1 Show InChI InChI=1S/C22H36N6O3S/c1-18(2)9-14-28(13-6-4-3-5-8-20(29)26-31)22-25-16-19(32-22)21(30)24-10-7-12-27-15-11-23-17-27/h11,15-18,31H,3-10,12-14H2,1-2H3,(H,24,30)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length C-terminal flag-tagged HDAC6 expressed in baculovirus infected Sf9 insect cells after 30 mins by fluoresc... |
ACS Med Chem Lett 9: 34-38 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00414
BindingDB Entry DOI: 10.7270/Q2CZ39PH |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50283689
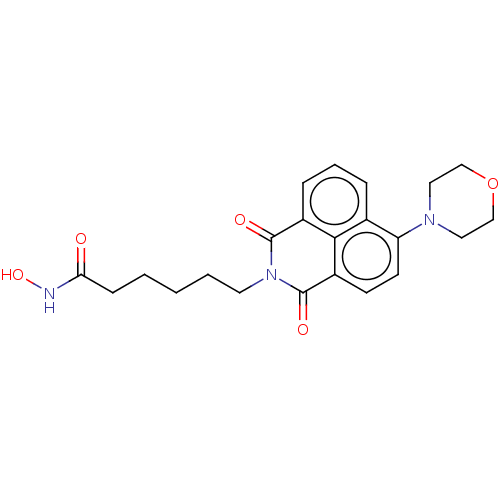
(CHEMBL4160632)Show SMILES ONC(=O)CCCCCN1C(=O)c2cccc3c(ccc(C1=O)c23)N1CCOCC1 Show InChI InChI=1S/C22H25N3O5/c26-19(23-29)7-2-1-3-10-25-21(27)16-6-4-5-15-18(24-11-13-30-14-12-24)9-8-17(20(15)16)22(25)28/h4-6,8-9,29H,1-3,7,10-14H2,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full-length HDAC6 expressed in baculovirus infected Sf9 cells using Boc-Lys(acetyl)-AMC as substrate after 30 mins by... |
Eur J Med Chem 143: 1543-1552 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.022
BindingDB Entry DOI: 10.7270/Q2028V2K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM637051

(WO2021067859, Compound I-24 | WO2021067859, Compou...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.669 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM637061
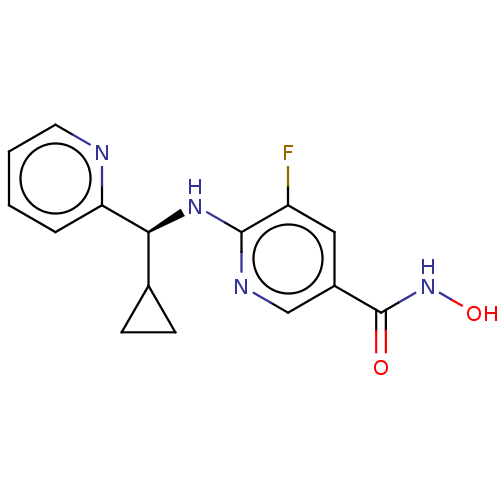
(WO2021067859, Compound I-9B | WO2021067859, Compou...)Show SMILES ONC(=O)c1cnc(N[C@@H](C2CC2)c2ccccn2)c(F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.677 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM119440
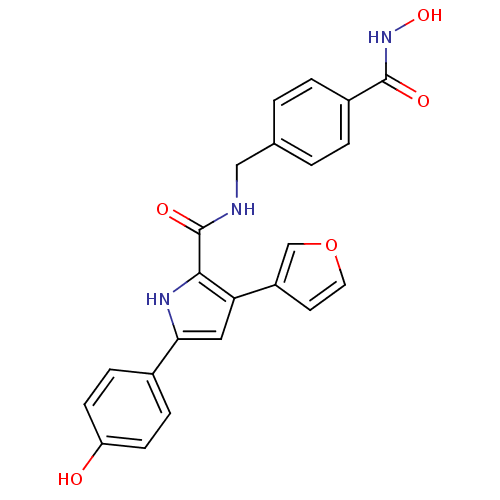
(BDBM119696 | US8685986, 15)Show SMILES ONC(=O)c1ccc(CNC(=O)c2[nH]c(cc2-c2ccoc2)-c2ccc(O)cc2)cc1 Show InChI InChI=1S/C23H19N3O5/c27-18-7-5-15(6-8-18)20-11-19(17-9-10-31-13-17)21(25-20)23(29)24-12-14-1-3-16(4-2-14)22(28)26-30/h1-11,13,25,27,30H,12H2,(H,24,29)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 30 |
Universidad del País Vasco; Ikerschem, S.L.
US Patent
| Assay Description
The reactions were carried out in a 96-well microplate for fluorometry in a 50 μl reaction volume. After the deacetylation reaction, Fluor-de-Ly... |
US Patent US8685992 (2014)
BindingDB Entry DOI: 10.7270/Q2CJ8C4X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50571528
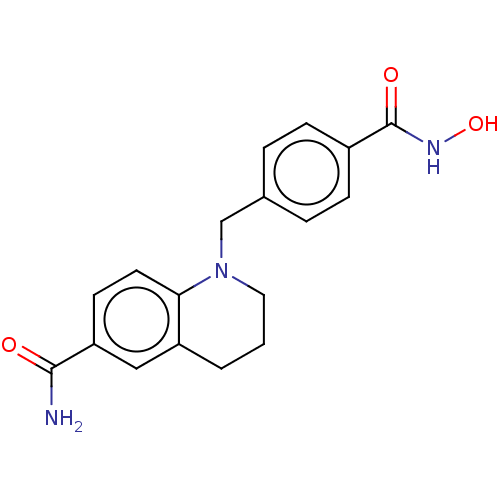
(CHEMBL4846435) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02210
BindingDB Entry DOI: 10.7270/Q2222ZJF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380400
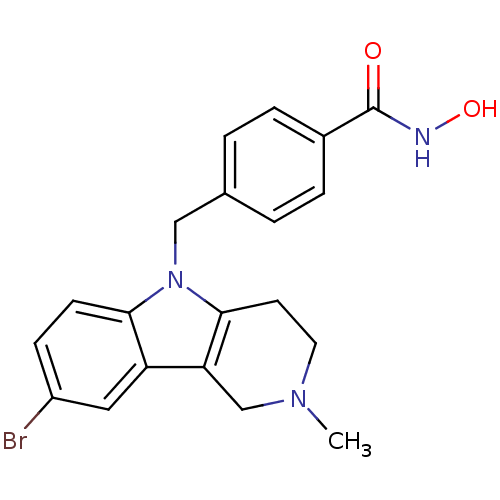
(CHEMBL2018301)Show SMILES CN1CCc2c(C1)c1cc(Br)ccc1n2Cc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C20H20BrN3O2/c1-23-9-8-19-17(12-23)16-10-15(21)6-7-18(16)24(19)11-13-2-4-14(5-3-13)20(25)22-26/h2-7,10,26H,8-9,11-12H2,1H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.704 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 using RHKKAc peptide as substrate incubated for 5 to mins prior to substrate addition measured after 2 hrs |
J Med Chem 55: 639-51 (2012)
Article DOI: 10.1021/jm200773h
BindingDB Entry DOI: 10.7270/Q2CJ8FG7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.721 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Genentech, Inc.
| Assay Description
Biochemical assays of HDAC activity were carried out by Nanosyn in a reaction volume of 10 ul in 384-well microplates. A standard enzymatic reaction ... |
J Biol Chem 288: 26926-43 (2013)
Article DOI: 10.1074/jbc.M113.490706
BindingDB Entry DOI: 10.7270/Q2KK99MZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50526161

(CHEMBL4464421)Show SMILES CCOC(=O)Nc1cccc(c1)-c1csc(NC(=O)CCCCCC(=O)NO)n1 Show InChI InChI=1S/C19H24N4O5S/c1-2-28-19(26)20-14-8-6-7-13(11-14)15-12-29-18(21-15)22-16(24)9-4-3-5-10-17(25)23-27/h6-8,11-12,27H,2-5,9-10H2,1H3,(H,20,26)(H,23,25)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Jadavpur University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) using (FAM)-labeled acetylated peptide as substrate measured after 17 hrs by fluorescence assay |
Eur J Med Chem 166: 369-380 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.077
BindingDB Entry DOI: 10.7270/Q2K93BZT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data