

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24541 (4-{2-[(3Z)-5-(benzylsulfamoyl)-2-oxo-2,3-dihydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24549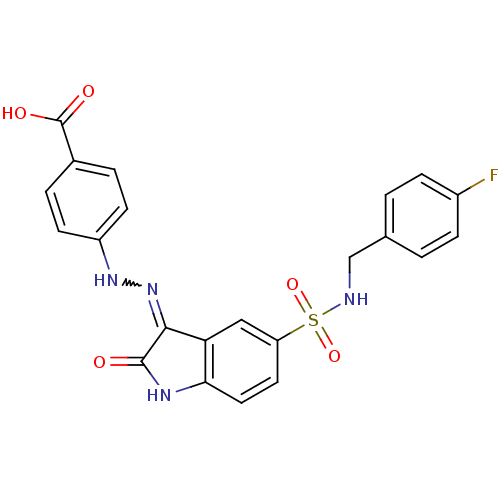 (4-{2-[(3Z)-5-{[(4-fluorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24533 ((3Z)-3-[2-(2-carboxyphenyl)hydrazin-1-ylidene]-2-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24536 (3-{2-[(3Z)-2-oxo-5-(propan-2-ylsulfamoyl)-2,3-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24546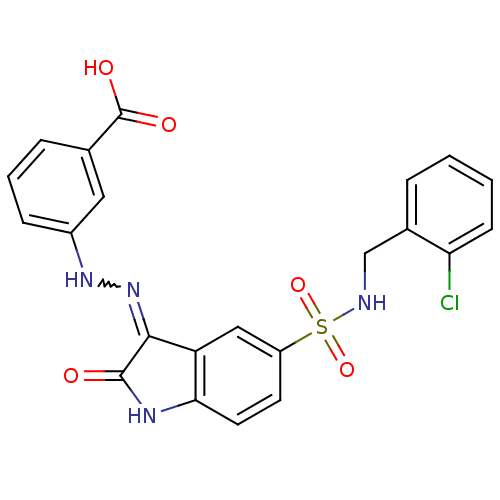 (3-{2-[(3Z)-5-{[(2-chlorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24543 (3-{2-[(3Z)-5-{[(4-chlorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24544 (4-{2-[(3Z)-5-{[(4-chlorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24548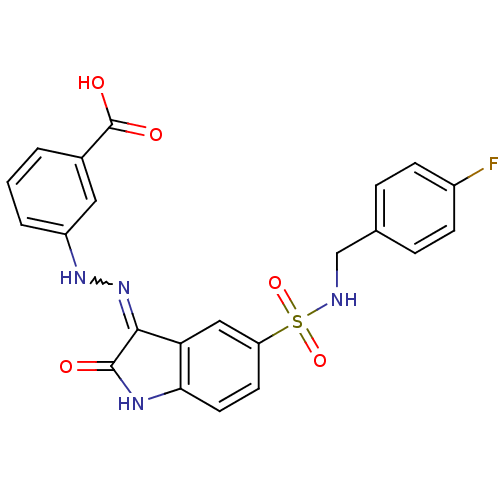 (3-{2-[(3Z)-5-{[(4-fluorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24535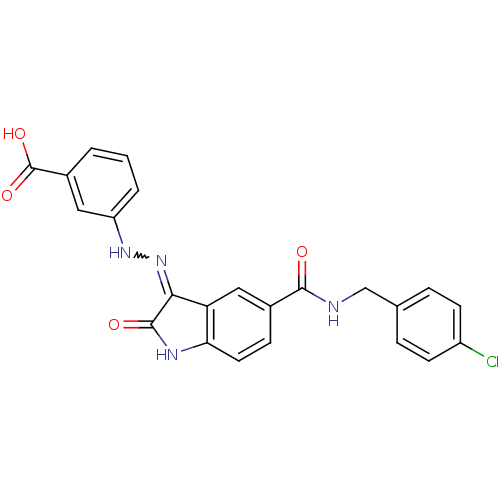 (3-{2-[(3Z)-5-{[(4-chlorophenyl)methyl]carbamoyl}-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24537 (4-{2-[(3Z)-2-oxo-5-(propan-2-ylsulfamoyl)-2,3-dihy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.77E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24547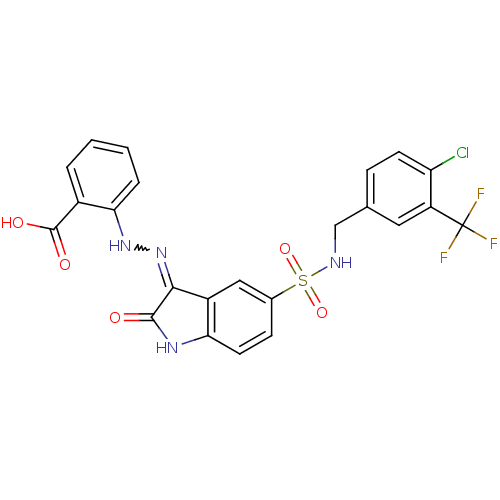 (2-{2-[(3Z)-5-({[4-chloro-3-(trifluoromethyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24540 (3-{2-[(3Z)-5-{[(4-methylphenyl)methyl]sulfamoyl}-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24539 ((3Z)-N-[(4-chlorophenyl)methyl]-3-[2-(2-nitropheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.09E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24542 (3-{2-[(3Z)-5-{[(3-chlorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24552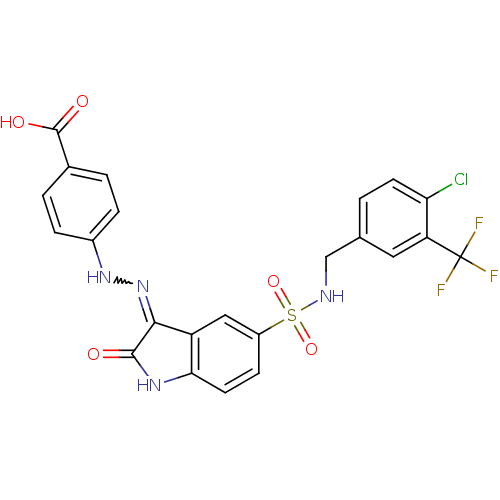 (4-{2-[(3Z)-5-({[4-chloro-3-(trifluoromethyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24545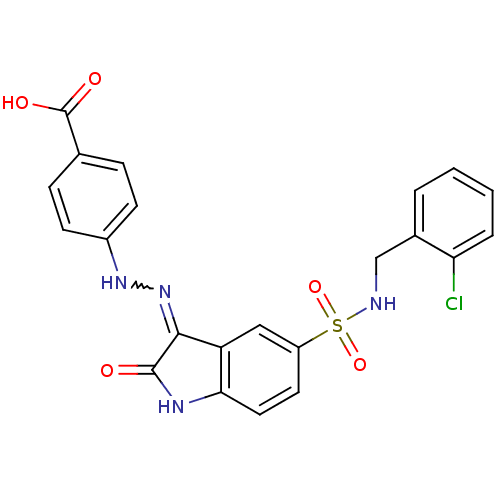 (4-{2-[(3Z)-5-{[(2-chlorophenyl)methyl]sulfamoyl}-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24531 ((3Z)-3-[2-(2-nitrophenyl)hydrazin-1-ylidene]-2-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24534 ((3Z)-3-[2-(3-carboxyphenyl)hydrazin-1-ylidene]-2-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.25E+4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24551 (3-{2-[(3Z)-5-{[2-(2,4-dichlorophenyl)ethyl]sulfamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24553 (3-{2-[(3Z)-5-({[4-chloro-3-(trifluoromethyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24538 ((3Z)-3-[2-(2-nitrophenyl)hydrazin-1-ylidene]-2-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 [205-595] (Homo sapiens (Human)) | BDBM24550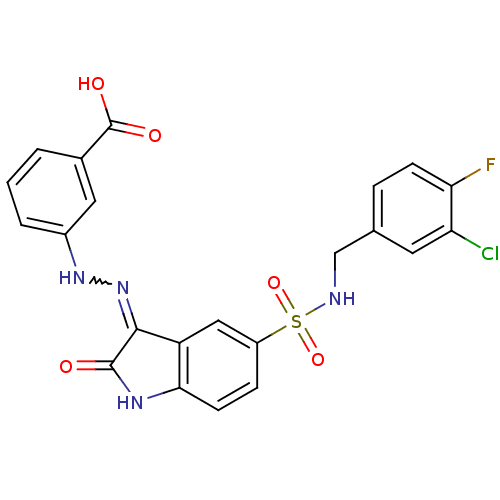 (3-{2-[(3Z)-5-{[(3-chloro-4-fluorophenyl)methyl]sul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center | Assay Description PTP activity was measured in a black 96-well plate. Reaction was initiated by addition of the substrate DiFMUP to enzyme mixtures containing test com... | J Med Chem 51: 4948-56 (2008) Article DOI: 10.1021/jm8002526 BindingDB Entry DOI: 10.7270/Q2XD0ZZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||