 Found 143 hits of ki data for polymerid = 2814,4416
Found 143 hits of ki data for polymerid = 2814,4416 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098378
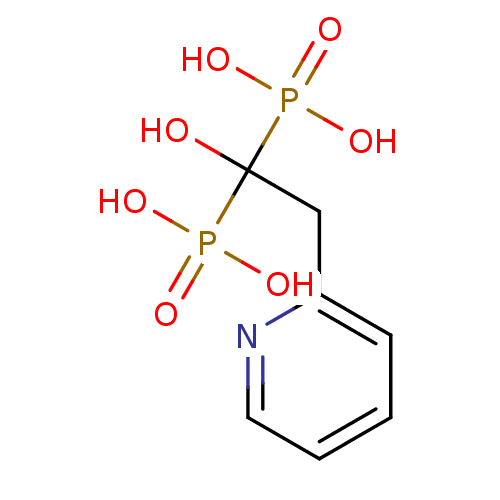
((1-Hydroxy-1-phosphono-2-pyridin-2-yl-ethyl)-phosp...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)5-6-3-1-2-4-8-6/h1-4,9H,5H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373094
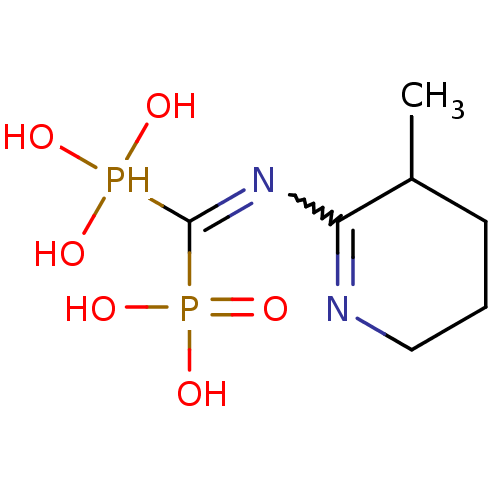
(CHEMBL408608)Show SMILES CC1CCCN=C1N=C(P(O)(O)O)P(O)(O)=O |w:7.7,c:5| Show InChI InChI=1S/C7H16N2O6P2/c1-5-3-2-4-8-6(5)9-7(16(10,11)12)17(13,14)15/h5,10-12,16H,2-4H2,1H3,(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098390
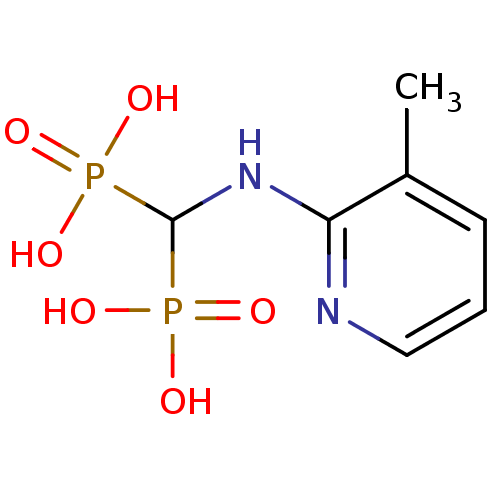
((3-methylpyridin-2-ylamino)methylenediphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c1-5-3-2-4-8-6(5)9-7(16(10,11)12)17(13,14)15/h2-4,7H,1H3,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373098
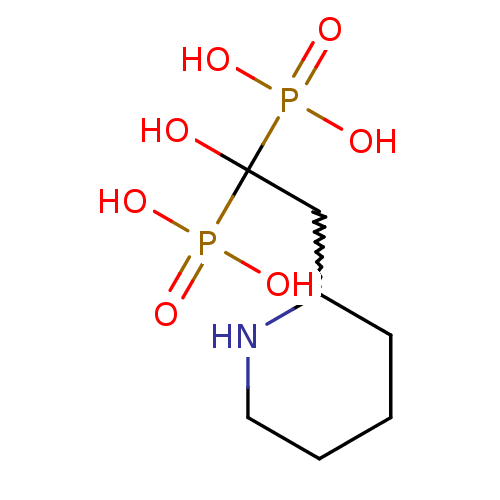
(CHEMBL259729)Show InChI InChI=1S/C7H17NO7P2/c9-7(16(10,11)12,17(13,14)15)5-6-3-1-2-4-8-6/h6,8-9H,1-5H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115104
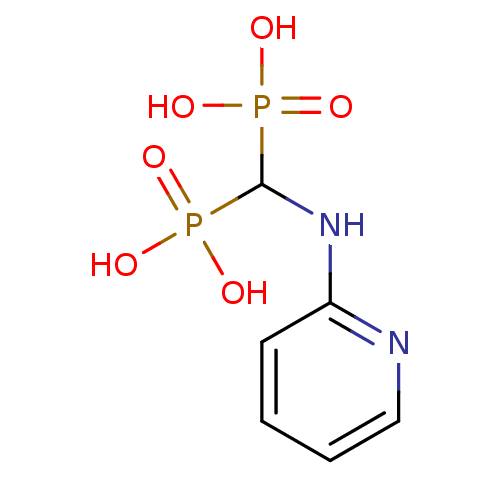
((pyridin-2-ylamino)methylenediphosphonic acid | 2-...)Show InChI InChI=1S/C6H10N2O6P2/c9-15(10,11)6(16(12,13)14)8-5-3-1-2-4-7-5/h1-4,6H,(H,7,8)(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115106
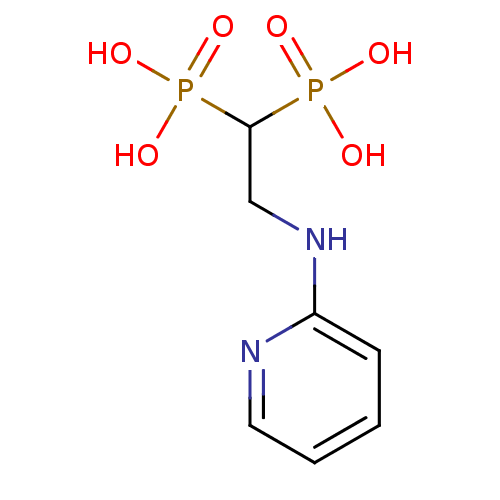
(2-(pyridin-2-ylamino)ethane-1,1-diyldiphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c10-16(11,12)7(17(13,14)15)5-9-6-3-1-2-4-8-6/h1-4,7H,5H2,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373097
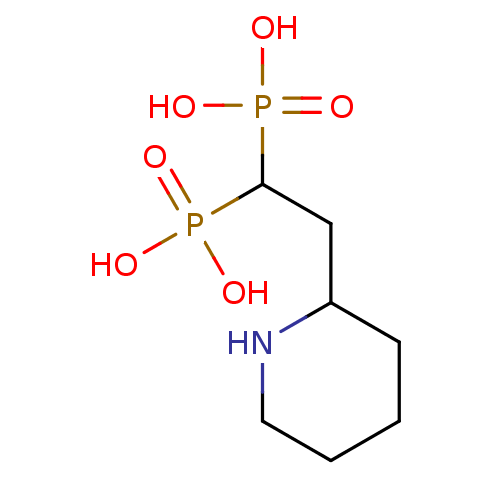
(CHEMBL406820)Show InChI InChI=1S/C7H17NO6P2/c9-15(10,11)7(16(12,13)14)5-6-3-1-2-4-8-6/h6-8H,1-5H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50173792

(2-(2-aminophenyl)-1-hydroxyethane-1,1-diyldiphosph...)Show InChI InChI=1S/C8H13NO7P2/c9-7-4-2-1-3-6(7)5-8(10,17(11,12)13)18(14,15)16/h1-4,10H,5,9H2,(H2,11,12,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12577
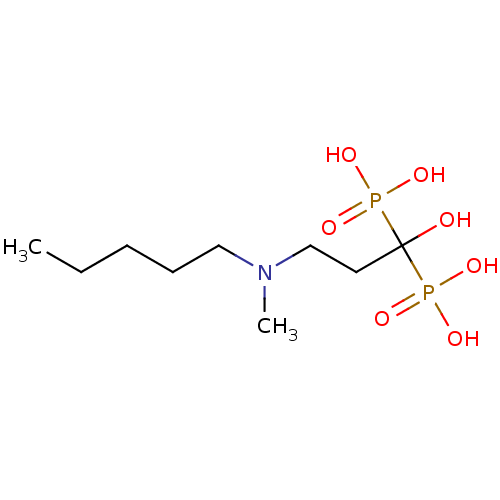
(Bisphosphonate 2 | CHEMBL997 | JMC515594 Compound ...)Show InChI InChI=1S/C9H23NO7P2/c1-3-4-5-7-10(2)8-6-9(11,18(12,13)14)19(15,16)17/h11H,3-8H2,1-2H3,(H2,12,13,14)(H2,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373099

(CHEMBL99553 | NE-10575)Show InChI InChI=1S/C8H13NO7P2/c1-9-4-2-3-7(6-9)5-8(10,17(11,12)13)18(14,15)16/h2-4,6,10H,5H2,1H3,(H3-,11,12,13,14,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373096

(CHEMBL99369 | Piridronic acid)Show InChI InChI=1S/C7H11NO6P2/c9-15(10,11)7(16(12,13)14)5-6-3-1-2-4-8-6/h1-4,7H,5H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50059860
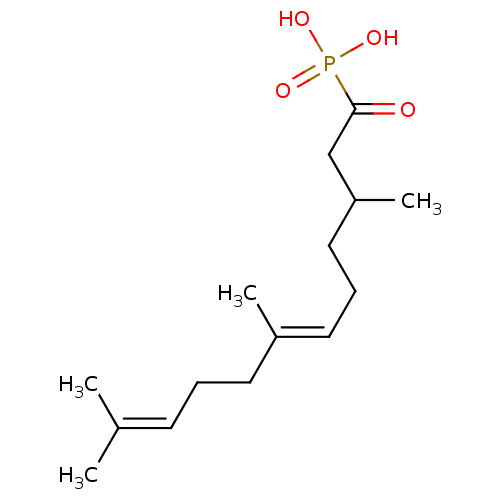
(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Assay using farnesyl-ras-CVLS as the protein acceptor substrate. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50059860

(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
FPTase activity was assayed in the biosynthetically forward direction at 30C. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50138725

((1-phosphono-2-pyridin-3-yl-ethyl)-phosphonic acid...)Show InChI InChI=1S/C7H11NO6P2/c9-15(10,11)7(16(12,13)14)4-6-2-1-3-8-5-6/h1-3,5,7H,4H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135836
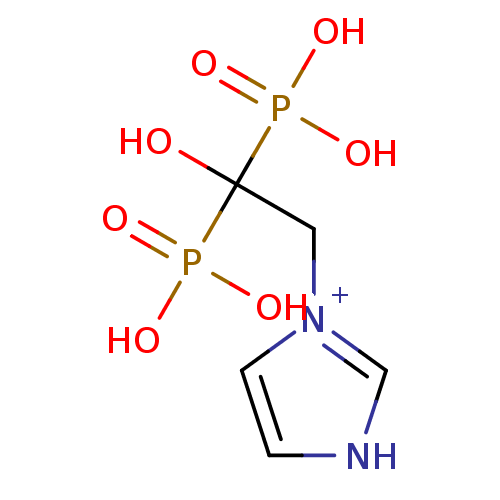
(1-(2-Hydroxy-2,2-bis-phosphono-ethyl)-3H-imidazol-...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H4,9,10,11,12,13,14)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115115
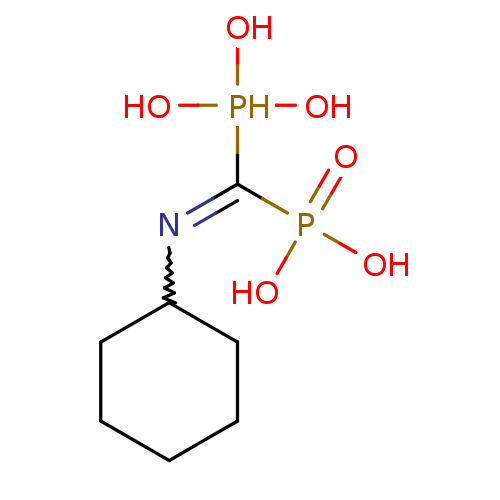
((Cyclohexylamino-phosphono-methyl)-phosphonic acid...)Show InChI InChI=1S/C7H17NO6P2/c9-15(10,11)7(16(12,13)14)8-6-4-2-1-3-5-6/h6,9-11,15H,1-5H2,(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098390

((3-methylpyridin-2-ylamino)methylenediphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c1-5-3-2-4-8-6(5)9-7(16(10,11)12)17(13,14)15/h2-4,7H,1H3,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373093
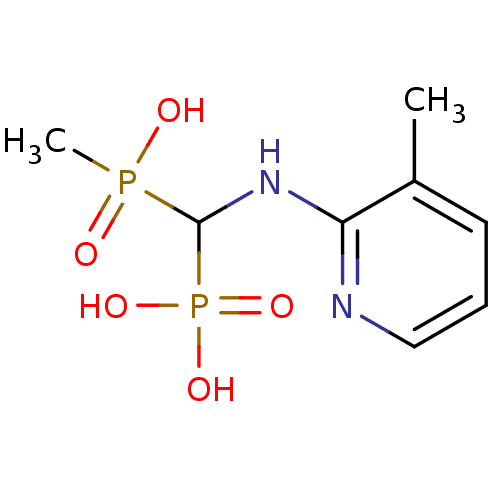
(CHEMBL100154)Show InChI InChI=1S/C8H14N2O5P2/c1-6-4-3-5-9-7(6)10-8(16(2,11)12)17(13,14)15/h3-5,8H,1-2H3,(H,9,10)(H,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098390

((3-methylpyridin-2-ylamino)methylenediphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c1-5-3-2-4-8-6(5)9-7(16(10,11)12)17(13,14)15/h2-4,7H,1H3,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098378

((1-Hydroxy-1-phosphono-2-pyridin-2-yl-ethyl)-phosp...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)5-6-3-1-2-4-8-6/h1-4,9H,5H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135818
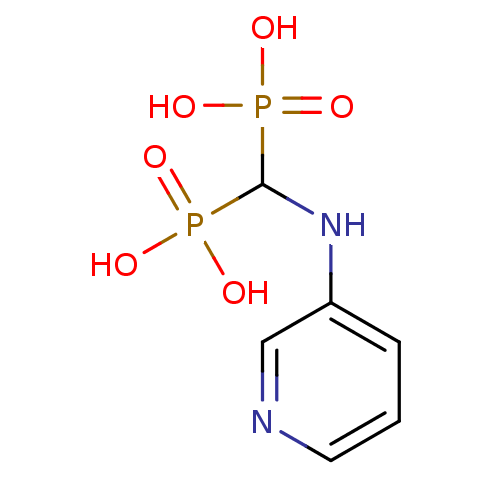
((pyridin-3-ylamino)methylenediphosphonic acid | 3-...)Show InChI InChI=1S/C6H10N2O6P2/c9-15(10,11)6(16(12,13)14)8-5-2-1-3-7-4-5/h1-4,6,8H,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115115

((Cyclohexylamino-phosphono-methyl)-phosphonic acid...)Show InChI InChI=1S/C7H17NO6P2/c9-15(10,11)7(16(12,13)14)8-6-4-2-1-3-5-6/h6,9-11,15H,1-5H2,(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115106

(2-(pyridin-2-ylamino)ethane-1,1-diyldiphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c10-16(11,12)7(17(13,14)15)5-9-6-3-1-2-4-8-6/h1-4,7H,5H2,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50113291
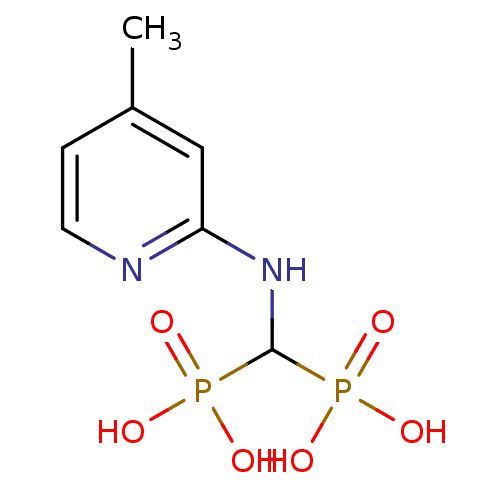
((4-methylpyridin-2-ylamino)methylenediphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c1-5-2-3-8-6(4-5)9-7(16(10,11)12)17(13,14)15/h2-4,7H,1H3,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135817
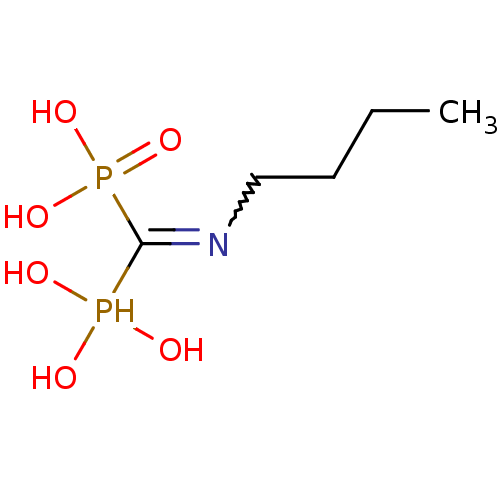
((Bis-phosphono-methyl)-butyl-ammonium | (Butylamin...)Show InChI InChI=1S/C5H15NO6P2/c1-2-3-4-6-5(13(7,8)9)14(10,11)12/h7-9,13H,2-4H2,1H3,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115104

((pyridin-2-ylamino)methylenediphosphonic acid | 2-...)Show InChI InChI=1S/C6H10N2O6P2/c9-15(10,11)6(16(12,13)14)8-5-3-1-2-4-7-5/h1-4,6H,(H,7,8)(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25266
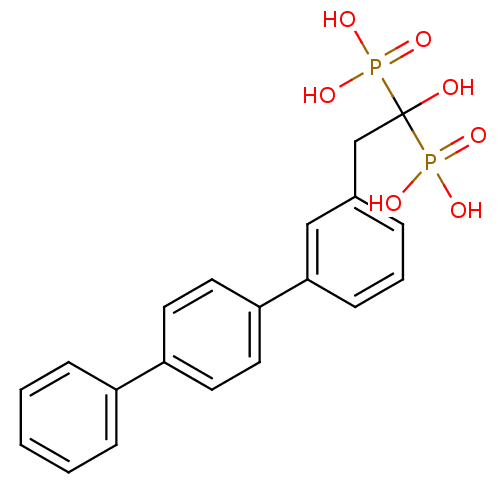
(BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...)Show SMILES OC(Cc1cccc(c1)-c1ccc(cc1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-5-4-8-19(13-15)18-11-9-17(10-12-18)16-6-2-1-3-7-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135821
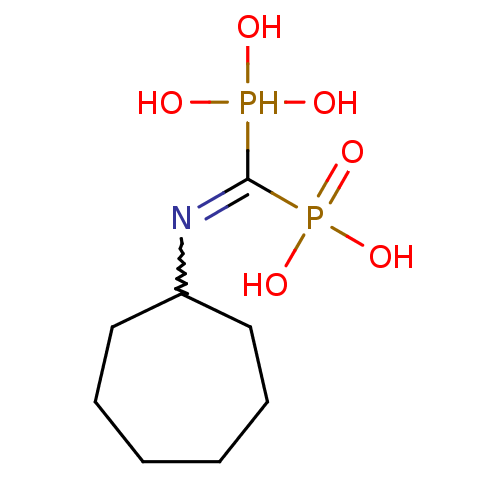
((Bis-phosphono-methyl)-cycloheptyl-ammonium | (Cyc...)Show InChI InChI=1S/C8H19NO6P2/c10-16(11,12)8(17(13,14)15)9-7-5-3-1-2-4-6-7/h7,10-12,16H,1-6H2,(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115109
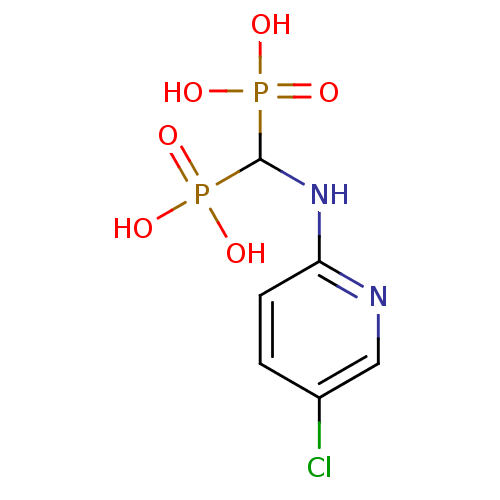
((5-chloropyridin-2-ylamino)methylenediphosphonic a...)Show InChI InChI=1S/C6H9ClN2O6P2/c7-4-1-2-5(8-3-4)9-6(16(10,11)12)17(13,14)15/h1-3,6H,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135839
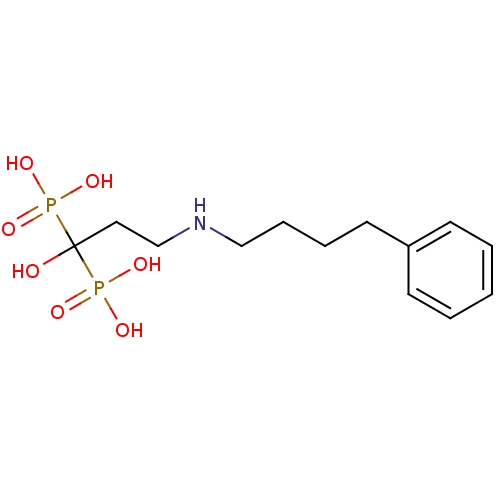
(CHEMBL55464 | [1-Hydroxy-3-(4-phenyl-butylamino)-1...)Show InChI InChI=1S/C13H23NO7P2/c15-13(22(16,17)18,23(19,20)21)9-11-14-10-5-4-8-12-6-2-1-3-7-12/h1-3,6-7,14-15H,4-5,8-11H2,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115104

((pyridin-2-ylamino)methylenediphosphonic acid | 2-...)Show InChI InChI=1S/C6H10N2O6P2/c9-15(10,11)6(16(12,13)14)8-5-3-1-2-4-7-5/h1-4,6H,(H,7,8)(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098389
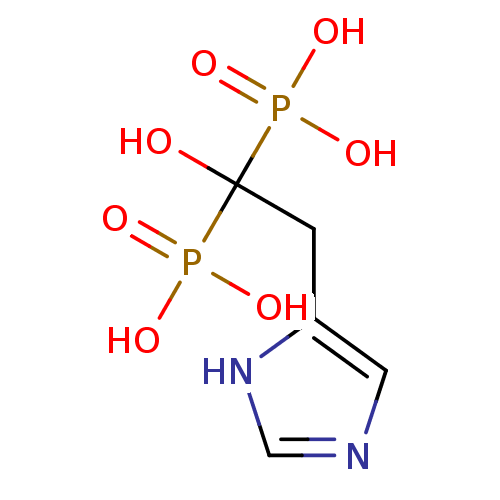
(1-hydroxy-2-(1H-imidazol-5-yl)ethane-1,1-diyldipho...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)1-4-2-6-3-7-4/h2-3,8H,1H2,(H,6,7)(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50117260
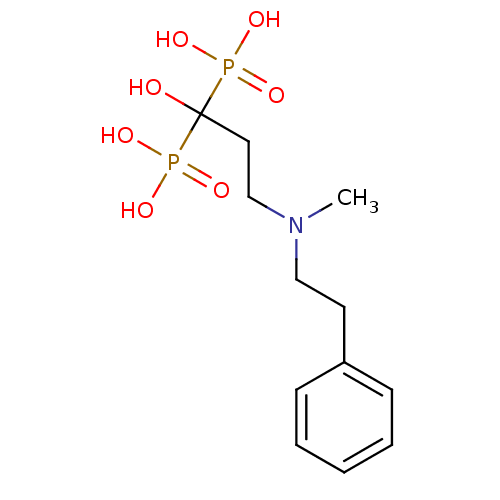
(1-hydroxy-3-(methyl(phenethyl)amino)propane-1,1-di...)Show InChI InChI=1S/C12H21NO7P2/c1-13(9-7-11-5-3-2-4-6-11)10-8-12(14,21(15,16)17)22(18,19)20/h2-6,14H,7-10H2,1H3,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115110
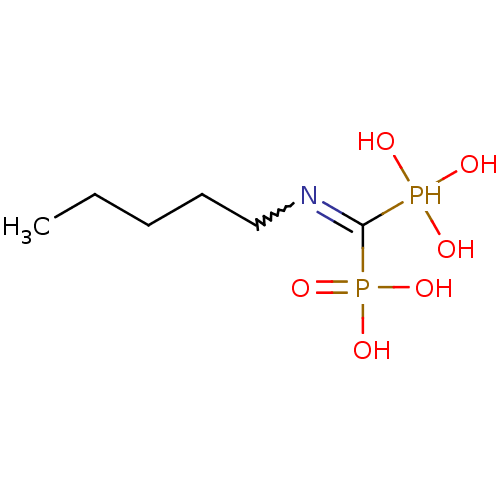
((Pentylamino-phosphono-methyl)-phosphonic acid | (...)Show InChI InChI=1S/C6H17NO6P2/c1-2-3-4-5-7-6(14(8,9)10)15(11,12)13/h8-10,14H,2-5H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135833
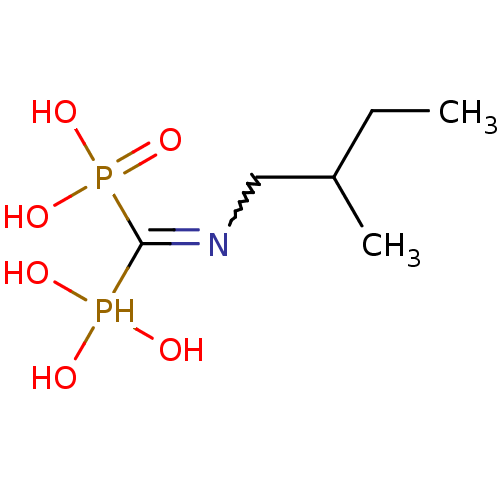
((2-methylbutyl)aminomethylene-1,1-bisphosphonate |...)Show InChI InChI=1S/C6H17NO6P2/c1-3-5(2)4-7-6(14(8,9)10)15(11,12)13/h5,8-10,14H,3-4H2,1-2H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50138725

((1-phosphono-2-pyridin-3-yl-ethyl)-phosphonic acid...)Show InChI InChI=1S/C7H11NO6P2/c9-15(10,11)7(16(12,13)14)4-6-2-1-3-8-5-6/h1-3,5,7H,4H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115106

(2-(pyridin-2-ylamino)ethane-1,1-diyldiphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c10-16(11,12)7(17(13,14)15)5-9-6-3-1-2-4-8-6/h1-4,7H,5H2,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 36.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50373096

(CHEMBL99369 | Piridronic acid)Show InChI InChI=1S/C7H11NO6P2/c9-15(10,11)7(16(12,13)14)5-6-3-1-2-4-8-6/h1-4,7H,5H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 39.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135820
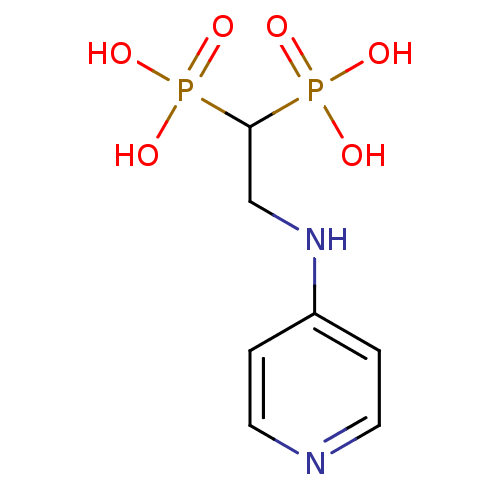
(2-(pyridin-4-ylamino)ethane-1,1-diyldiphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c10-16(11,12)7(17(13,14)15)5-9-6-1-3-8-4-2-6/h1-4,7H,5H2,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135835
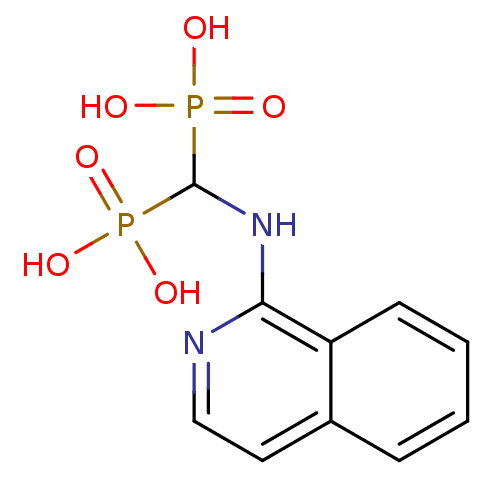
((isoquinolin-1-ylamino)methylenediphosphonic acid ...)Show InChI InChI=1S/C10H12N2O6P2/c13-19(14,15)10(20(16,17)18)12-9-8-4-2-1-3-7(8)5-6-11-9/h1-6,10H,(H,11,12)(H2,13,14,15)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135831
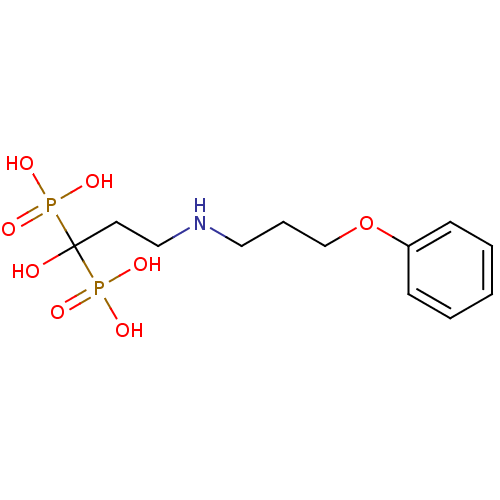
(CHEMBL316844 | [1-Hydroxy-3-(3-phenoxy-propylamino...)Show InChI InChI=1S/C12H21NO8P2/c14-12(22(15,16)17,23(18,19)20)7-9-13-8-4-10-21-11-5-2-1-3-6-11/h1-3,5-6,13-14H,4,7-10H2,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25313

((4-amino-1-hydroxy-1-phosphonobutyl)phosphonic aci...)Show InChI InChI=1S/C4H13NO7P2/c5-3-1-2-4(6,13(7,8)9)14(10,11)12/h6H,1-3,5H2,(H2,7,8,9)(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 44.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25290
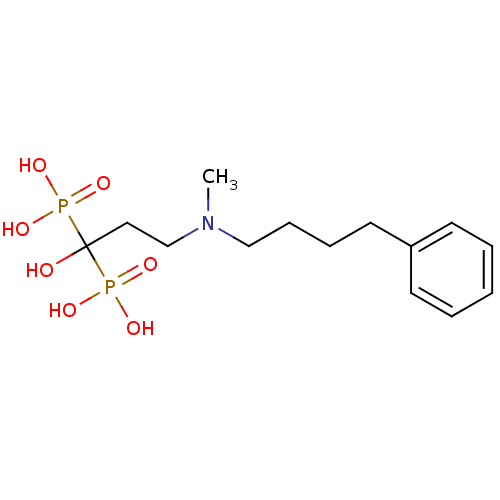
(CHEMBL56073 | bisphosphonate, 39 | {1-hydroxy-3-[m...)Show InChI InChI=1S/C14H25NO7P2/c1-15(11-6-5-9-13-7-3-2-4-8-13)12-10-14(16,23(17,18)19)24(20,21)22/h2-4,7-8,16H,5-6,9-12H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12577

(Bisphosphonate 2 | CHEMBL997 | JMC515594 Compound ...)Show InChI InChI=1S/C9H23NO7P2/c1-3-4-5-7-10(2)8-6-9(11,18(12,13)14)19(15,16)17/h11H,3-8H2,1-2H3,(H2,12,13,14)(H2,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50115112
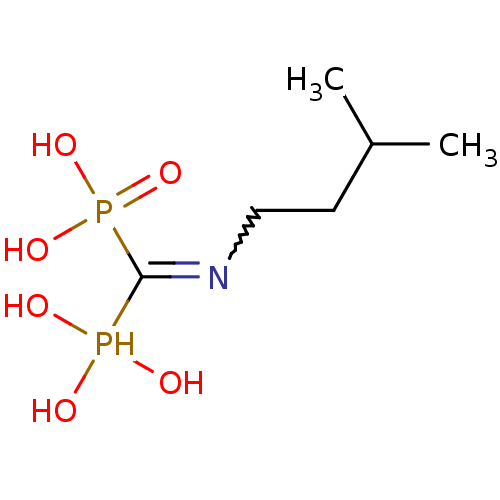
((Bis-phosphono-methyl)-(3-methyl-butyl)-ammonium |...)Show InChI InChI=1S/C6H17NO6P2/c1-5(2)3-4-7-6(14(8,9)10)15(11,12)13/h5,8-10,14H,3-4H2,1-2H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50117257
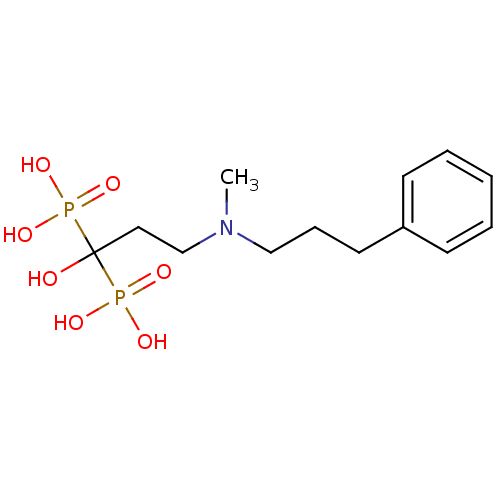
(1-hydroxy-3-(methyl(3-phenylpropyl)amino)propane-1...)Show InChI InChI=1S/C13H23NO7P2/c1-14(10-5-8-12-6-3-2-4-7-12)11-9-13(15,22(16,17)18)23(19,20)21/h2-4,6-7,15H,5,8-11H2,1H3,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098380
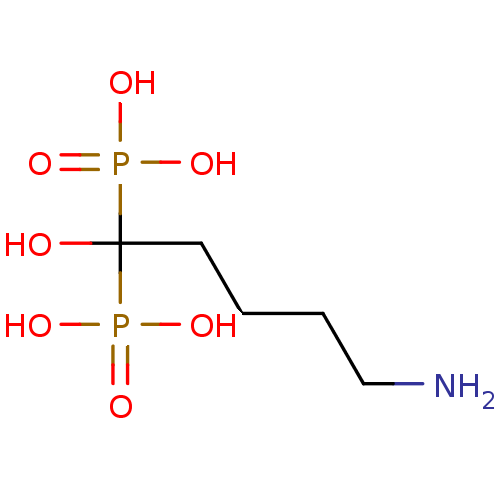
((5-Amino-1-hydroxy-1-phosphono-pentyl)-phosphonic ...)Show InChI InChI=1S/C5H15NO7P2/c6-4-2-1-3-5(7,14(8,9)10)15(11,12)13/h7H,1-4,6H2,(H2,8,9,10)(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data