

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303176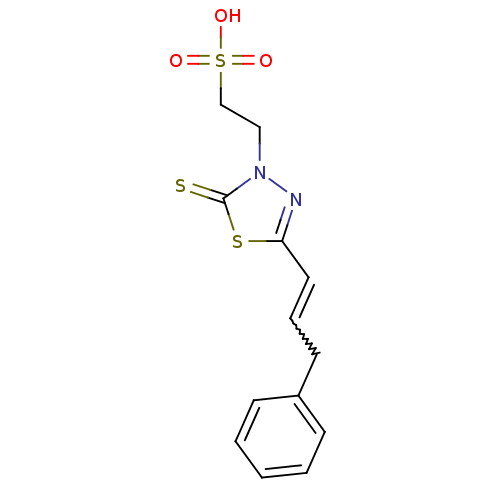 (2-((Z)-4-oxo-5-((E)-3-phenylallylidene)-2-thioxoth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 809 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM199180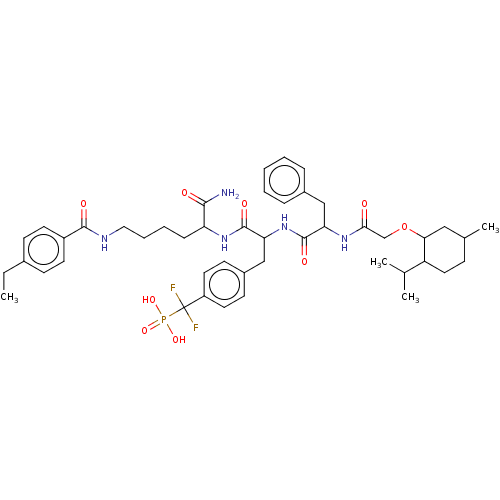 (US9217012, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | >-8.18 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ... | US Patent US9217012 (2015) BindingDB Entry DOI: 10.7270/Q2FX788H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303177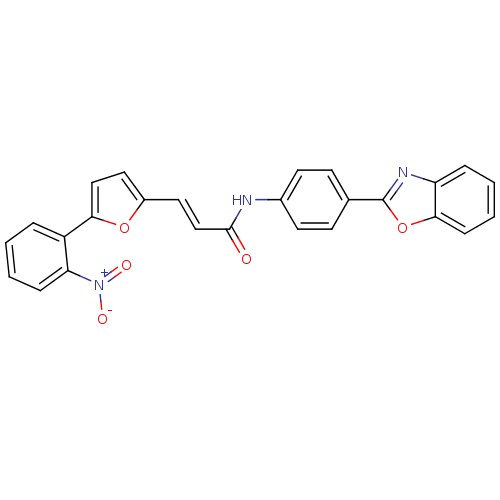 (CHEMBL565369 | N-(4-(benzo[d]oxazol-2-yl)phenyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303178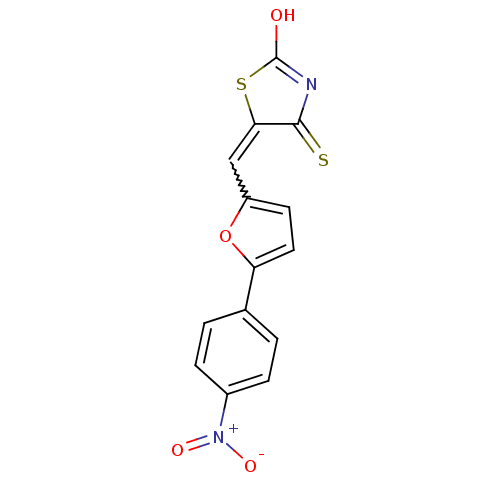 (5-((5-(4-nitrophenyl)furan-2-yl)methylene)-4-thiox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303179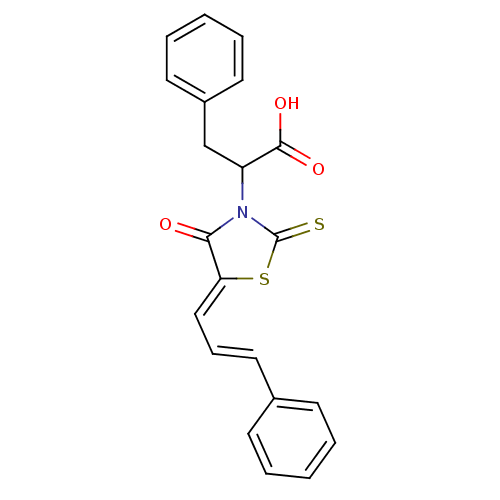 (2-(4-oxo-5-(3-phenylallylidene)-2-thioxothiazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM11994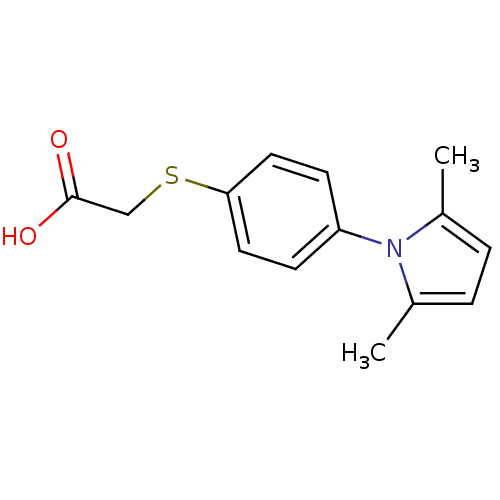 (2-{[4-(2,5-dimethyl-1H-pyrrol-1-yl)phenyl]sulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303180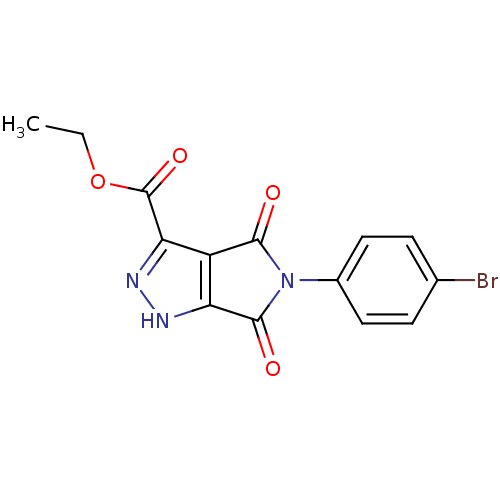 (CHEMBL577311 | ethyl 5-(4-bromophenyl)-4,6-dioxo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303181 (1-(dibenzo[b,d]furan-3-yl)-3-(naphthalen-1-yl)thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303182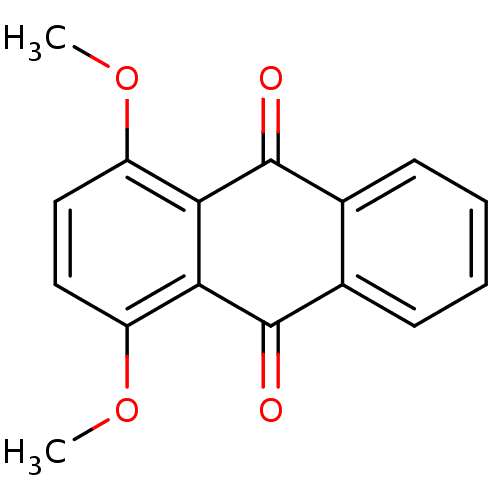 (1,4-dimethoxyanthracene-9,10-dione | CHEMBL570408 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM28851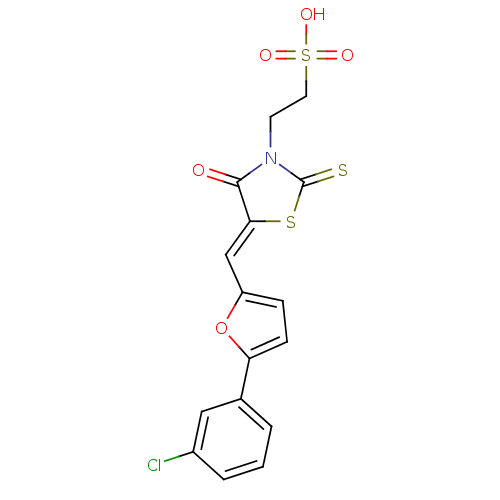 (2-[(5Z)-5-{[5-(3-chlorophenyl)furan-2-yl]methylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303184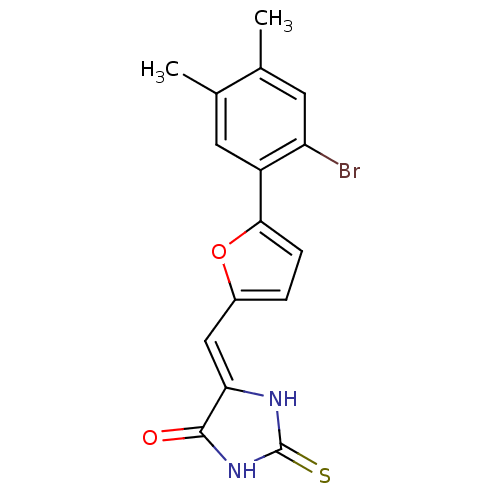 (5-((5-(2-bromo-4,5-dimethylphenyl)furan-2-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303185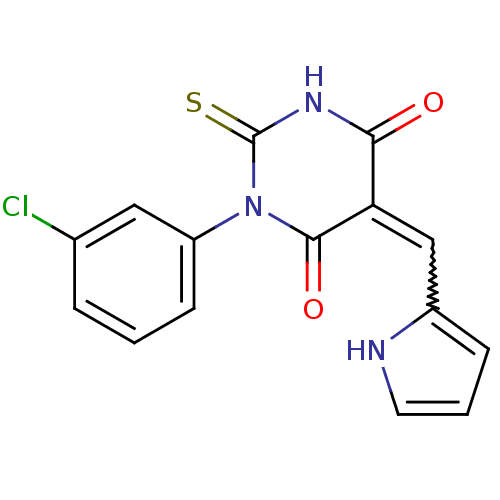 ((E/Z)-5-((1H-pyrrol-2-yl)methylene)-1-(3-chlorophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM15186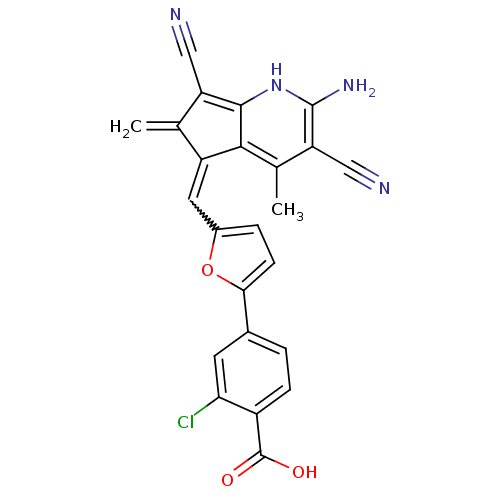 (4-(5-{[(5E)-2-amino-3,7-dicyano-4,6-dimethyl-5H-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303186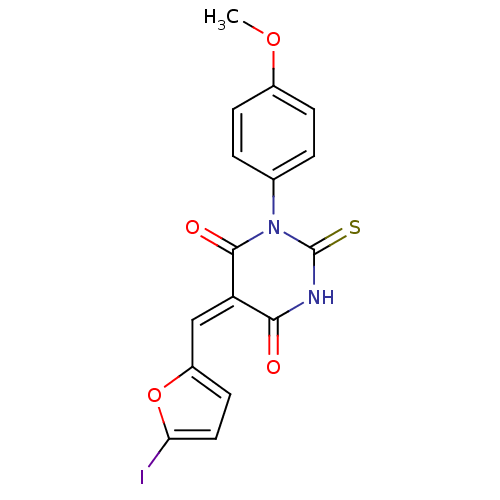 ((E/Z)-5-((5-iodofuran-2-yl)methylene)-1-(4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303187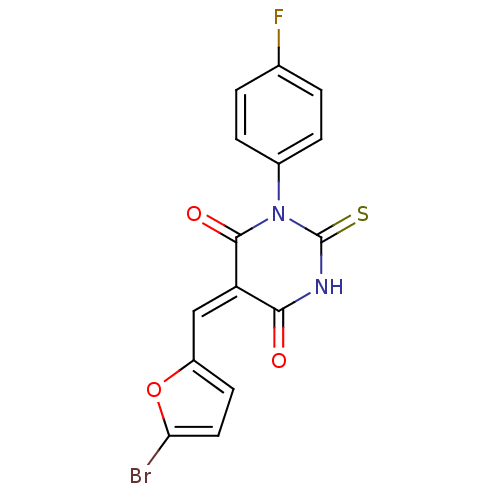 (5-((5-bromofuran-2-yl)methylene)-1-(4-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM11985 (4-(2,5-dimethyl-1H-pyrrol-1-yl)-2-hydroxybenzoic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303188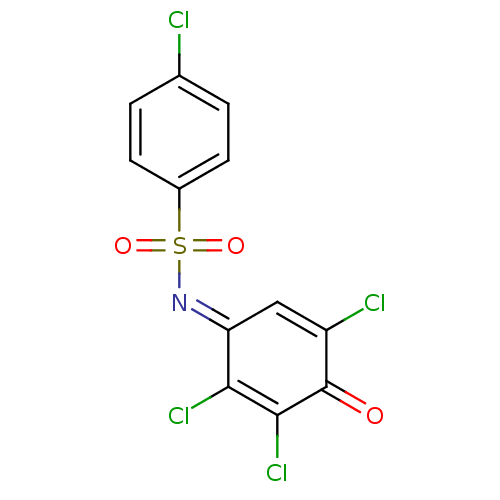 (4-chloro-N-(2,3,5-trichloro-4-oxocyclohexa-2,5-die...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303190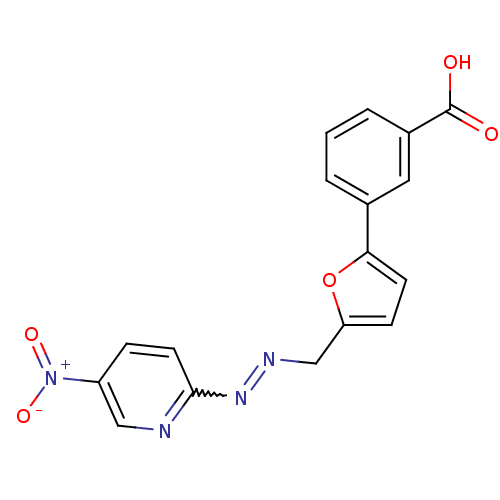 (3-(5-((2-(5-nitropyridin-2-yl)hydrazono)methyl)fur...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303189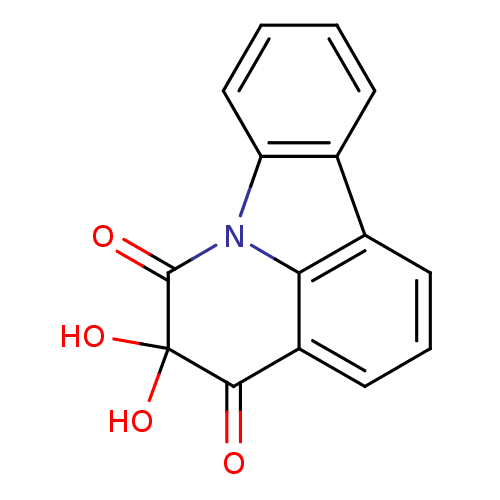 (5,5-dihydroxy-4H-pyrido[3,2,1-jk]carbazole-4,6(5H)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303191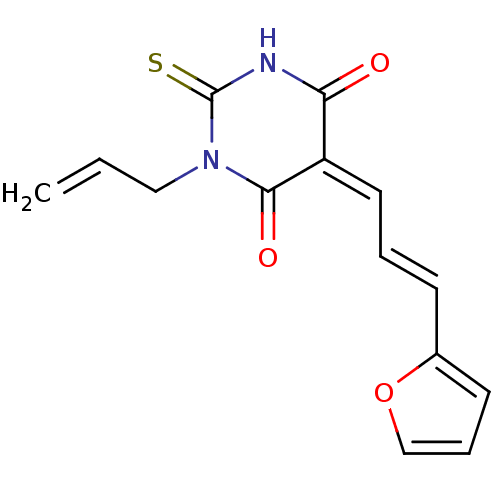 ((E/Z)-1-allyl-5-(3-(furan-2-yl)allylidene)-2-thiox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303192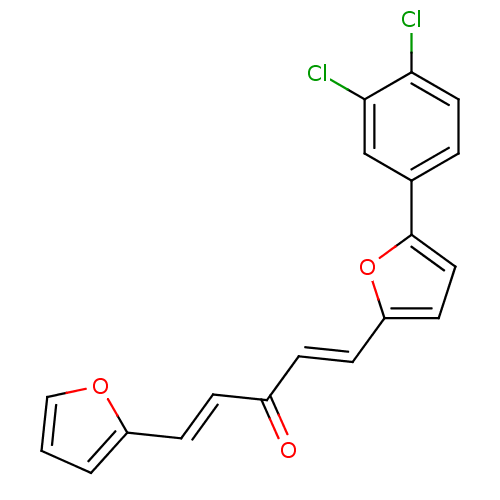 (1-(5-(3,4-dichlorophenyl)furan-2-yl)-5-(furan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50303193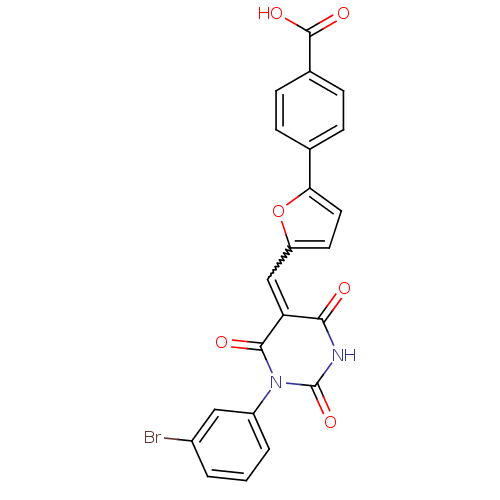 ((E/Z)-4-(5-((1-(3-bromophenyl)-2,4,6-trioxotetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of recombinant vaccina H1-related phosphatase expressed in Escherichia coli by Michaelis-Menton kinetic studies | J Med Chem 52: 6716-23 (2009) Article DOI: 10.1021/jm901016k BindingDB Entry DOI: 10.7270/Q2VX0GK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM26104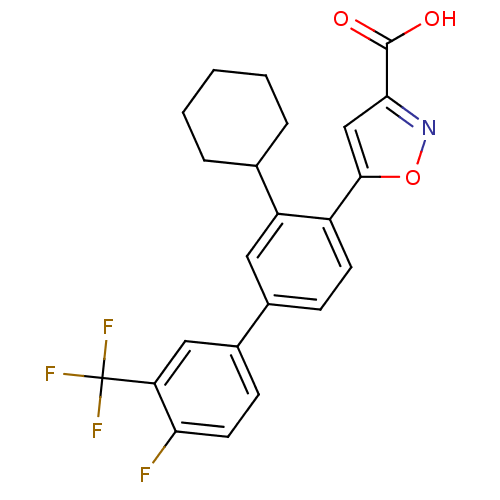 (5-{2-cyclohexyl-4-[4-fluoro-3-(trifluoromethyl)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | >-5.80 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California at Berkeley | Assay Description The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d... | J Am Chem Soc 129: 9613-5 (2007) Article DOI: 10.1021/ja0727520 BindingDB Entry DOI: 10.7270/Q2PG1Q2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50312261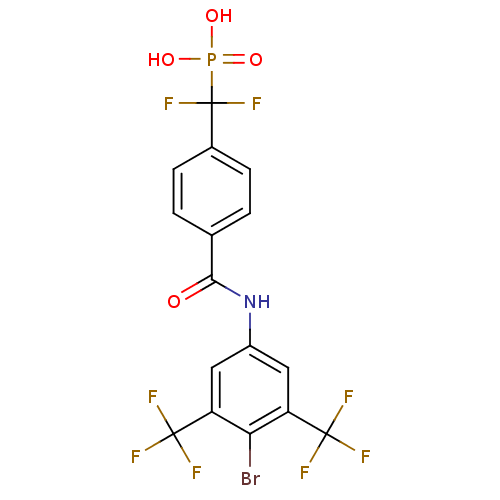 ((4-(4-bromo-3,5-bis(trifluoromethyl)phenylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human VHR | Bioorg Med Chem Lett 19: 6851-4 (2009) Article DOI: 10.1016/j.bmcl.2009.10.090 BindingDB Entry DOI: 10.7270/Q21G0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50139372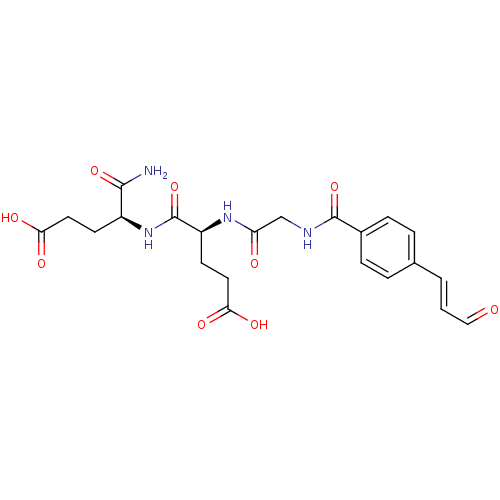 (4-((S)-(S)-1-Carbamoyl-3-carboxy-propylcarbamoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Tested for effect of the compound on inhibition of Dual specificity protein phosphatase 3 and the value reported is overall equilibrium constant | Bioorg Med Chem Lett 14: 685-7 (2004) BindingDB Entry DOI: 10.7270/Q2416WHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50086647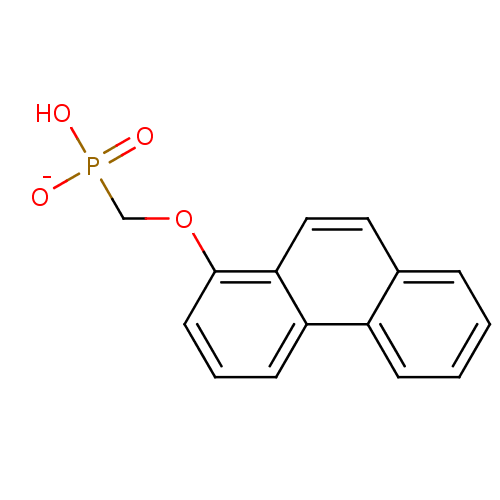 (hydrogen (1-phenanthryloxy)methylphosphonate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition constant against dual specificity phosphatase VHR | Bioorg Med Chem Lett 10: 457-60 (2000) BindingDB Entry DOI: 10.7270/Q2J966W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50086645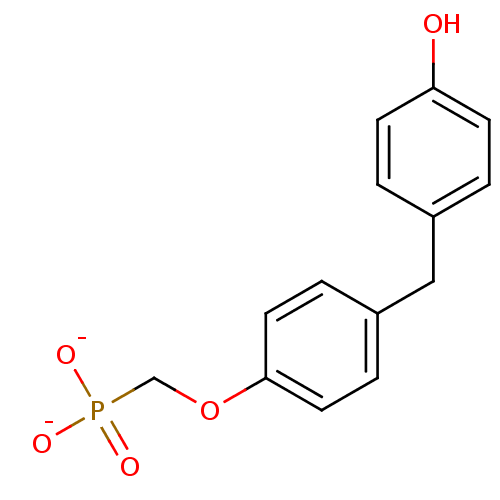 ([4-(4-hydroxybenzyl)phenoxy]methylphosphonate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition constant against dual specificity phosphatase VHR | Bioorg Med Chem Lett 10: 457-60 (2000) BindingDB Entry DOI: 10.7270/Q2J966W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50086644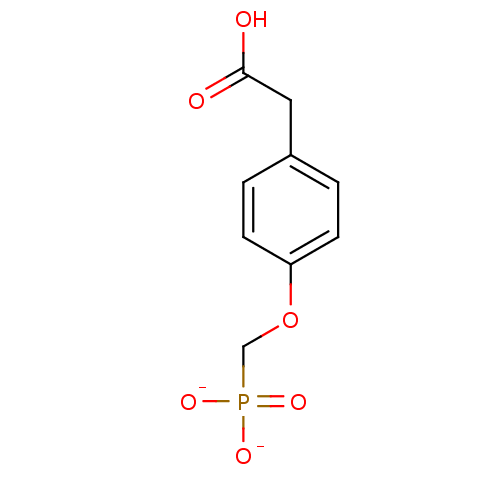 ([4-(carboxymethyl)phenoxy]methylphosphonate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | PubMed | 2.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition constant against dual specificity phosphatase VHR | Bioorg Med Chem Lett 10: 457-60 (2000) BindingDB Entry DOI: 10.7270/Q2J966W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50086649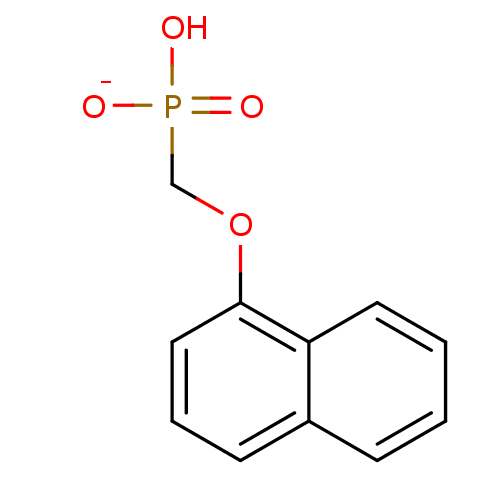 (hydrogen (1-naphthyloxy)methylphosphonate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 4.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition constant against dual specificity phosphatase VHR | Bioorg Med Chem Lett 10: 457-60 (2000) BindingDB Entry DOI: 10.7270/Q2J966W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50086646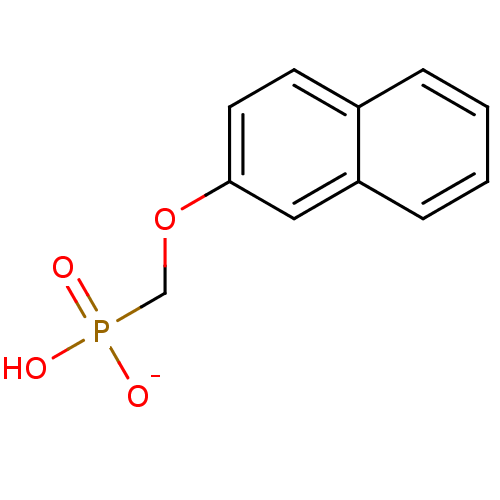 (hydrogen (2-naphthyloxy)methylphosphonate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition constant against dual specificity phosphatase VHR | Bioorg Med Chem Lett 10: 457-60 (2000) BindingDB Entry DOI: 10.7270/Q2J966W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 3 (Homo sapiens (Human)) | BDBM50086648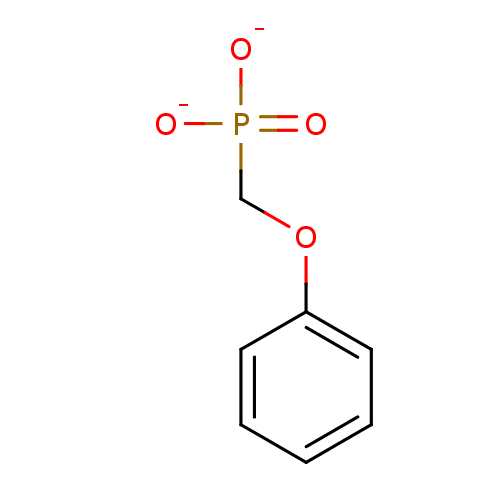 (phenoxymethylphosphonate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.60E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition constant against dual specificity phosphatase VHR | Bioorg Med Chem Lett 10: 457-60 (2000) BindingDB Entry DOI: 10.7270/Q2J966W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||