

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50574654 (CHEMBL4870906) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MAGL assessed as dissociation constant using 4-NPA as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113679 BindingDB Entry DOI: 10.7270/Q2NV9P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50574655 (CHEMBL4852896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MAGL assessed as dissociation constant using 4-NPA as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113679 BindingDB Entry DOI: 10.7270/Q2NV9P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50574656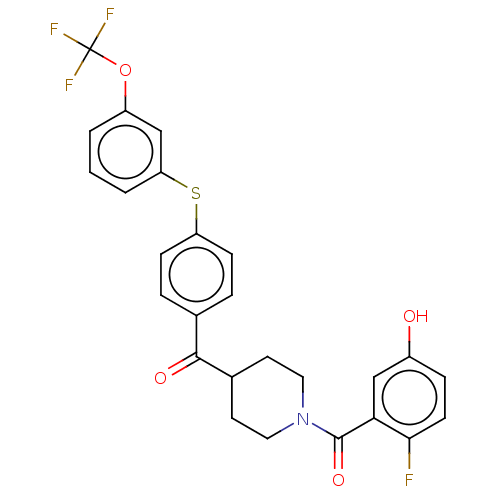 (CHEMBL4866490) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MAGL assessed as dissociation constant using 4-NPA as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113679 BindingDB Entry DOI: 10.7270/Q2NV9P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50605682 (CHEMBL5176915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01806 BindingDB Entry DOI: 10.7270/Q2Q81J4P | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562160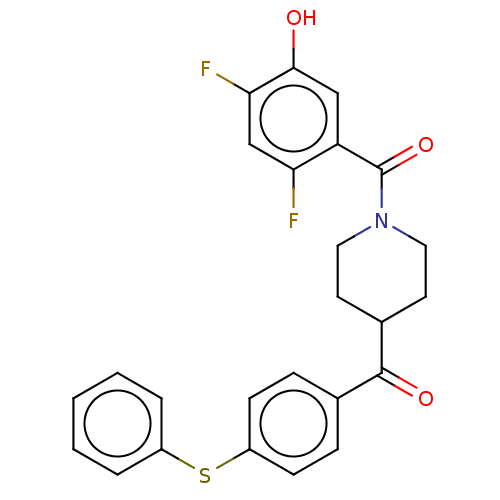 (CHEMBL4757403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50540526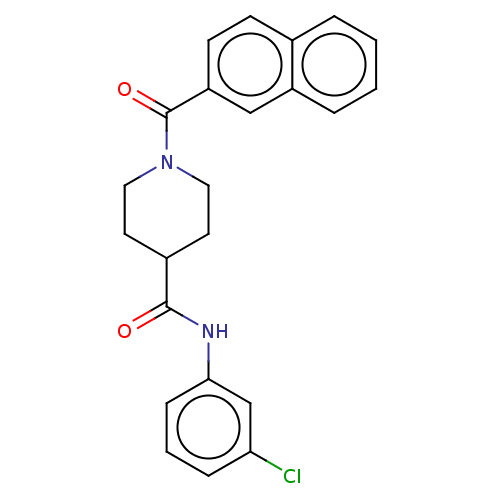 (CHEMBL4637658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAGL at 31.25 nM to 125 nM pre-incubated for 5 mins before MAGL substrate addition and further incubated ... | J Med Chem 63: 5783-5796 (2020) Article DOI: 10.1021/acs.jmedchem.9b02137 BindingDB Entry DOI: 10.7270/Q2GQ7288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562158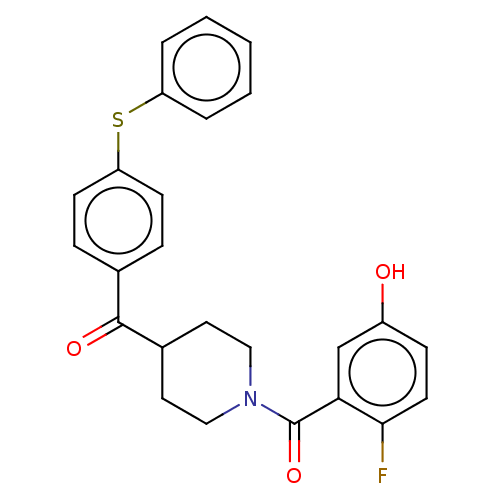 (CHEMBL4747585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562161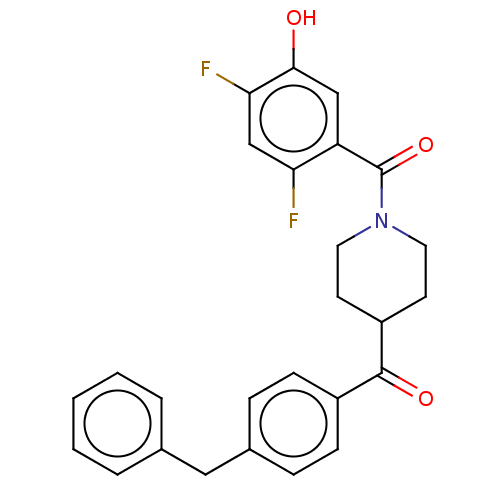 (CHEMBL4755606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50562159 (CHEMBL4798508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAGL using varying concentration of 4-nitrophenylacetate as substrate incubated for 30 mins by microplate... | Bioorg Med Chem Lett 13: 1119-23 (2003) Article DOI: 10.1016/j.ejmech.2020.112857 BindingDB Entry DOI: 10.7270/Q2D21X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50531972 (CHEMBL4536045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant MAGL using 4-NPA as substrate after 30 mins by Michaelis-Menten-based plot analysis | J Med Chem 62: 1932-1958 (2019) Article DOI: 10.1021/acs.jmedchem.8b01483 BindingDB Entry DOI: 10.7270/Q2GM8BSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50600140 (CHEMBL5174588) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116698 BindingDB Entry DOI: 10.7270/Q22B933P | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50394369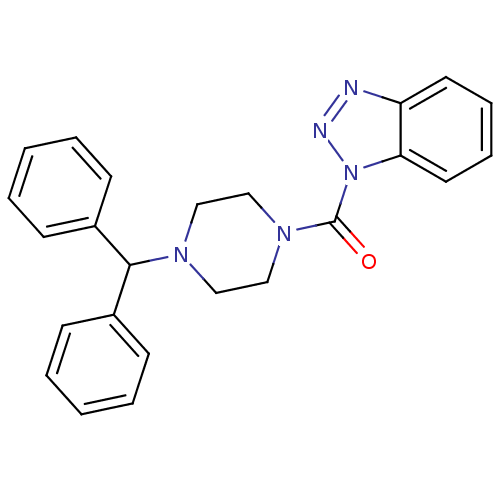 (CHEMBL2159781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Irreversible inhibition of human MAGL using p-nitrophenylpropionate as substrate | Bioorg Med Chem 20: 6260-75 (2012) Article DOI: 10.1016/j.bmc.2012.09.011 BindingDB Entry DOI: 10.7270/Q2W37XDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50255235 (CHEMBL4078924) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 412 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Chemical and Pharmaceutical Sciences, University of Ferrara , 44121 Ferrara, Italy. Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human MAGL using 4-NPA as substrate after 30 mins by Michaelis-Menten plot analysis | J Med Chem 61: 1340-1354 (2018) Article DOI: 10.1021/acs.jmedchem.7b01845 BindingDB Entry DOI: 10.7270/Q2JM2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50470454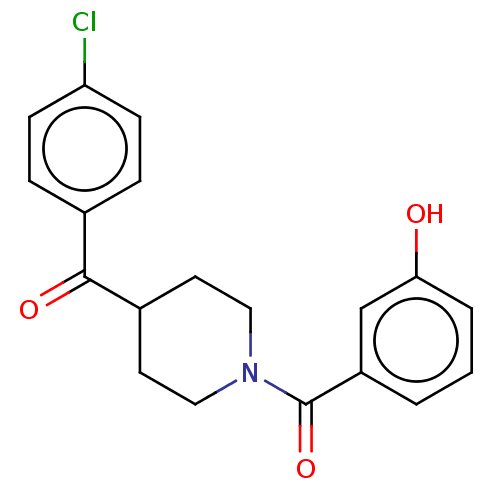 (CHEMBL4282040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human recombinant MAGL using 4-NPA substrate incubated for 30 mins by Michaelis-Menten kinetics analysis | Citation and Details BindingDB Entry DOI: 10.7270/Q20868X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50379241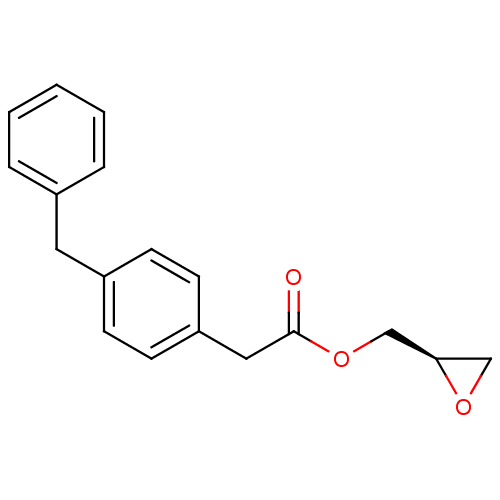 (CHEMBL2011479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Non-competitive inhibition of human recombinant MAGL assessed as 7-hydroxycoumarin level using umbelliferyl arachidonate as substrate by luminescence... | J Med Chem 55: 824-36 (2012) Article DOI: 10.1021/jm201327p BindingDB Entry DOI: 10.7270/Q2B27W9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50479142 (Guineensine | Guineesine | Pipyahyine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Inhibition of MAGL in human U937 cell homogenates using [3H]-2OG as substrate preincubated for 15 mins followed by substrate addition and measured af... | J Nat Prod 82: 636-646 (2019) Article DOI: 10.1021/acs.jnatprod.8b00874 BindingDB Entry DOI: 10.7270/Q2P55RZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||