

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50125842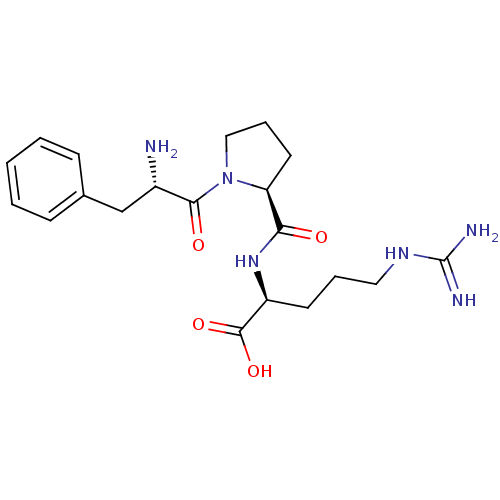 (5-amino(imino)methylamino-2-[1-[2-amino-3-phenyl-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00170 | n/a | n/a | n/a | n/a | n/a | n/a |
Link£ping University Curated by ChEMBL | Assay Description In vitro inhibitory concentration of compound against human thrombin | J Med Chem 46: 1165-79 (2003) Article DOI: 10.1021/jm021065a BindingDB Entry DOI: 10.7270/Q2NG4RCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254888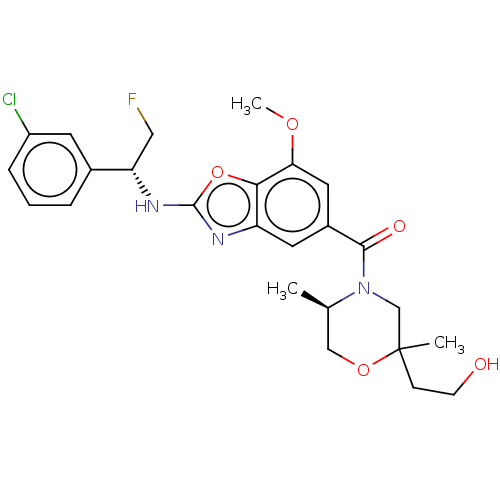 (US9493472, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50063555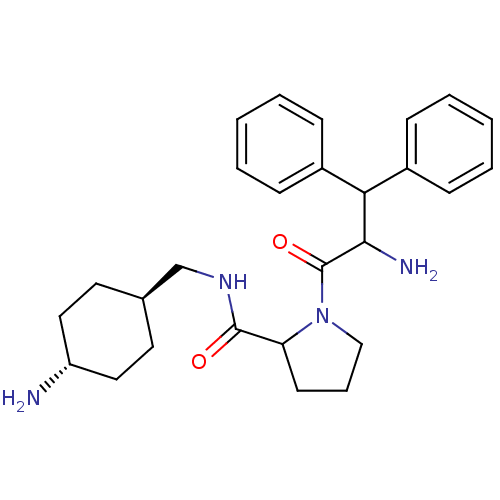 (1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 41: 1011-3 (1998) Article DOI: 10.1021/jm9706933 BindingDB Entry DOI: 10.7270/Q2ZG6RCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254889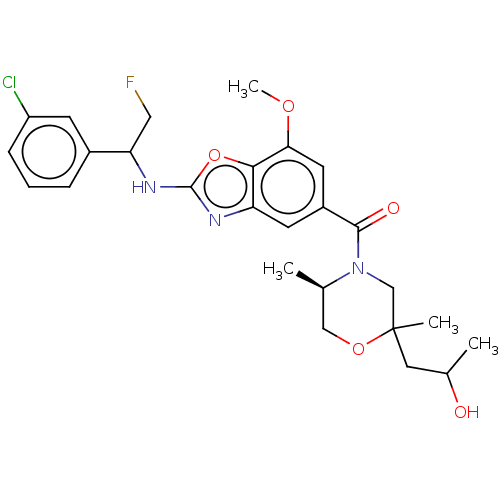 (US9493472, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096624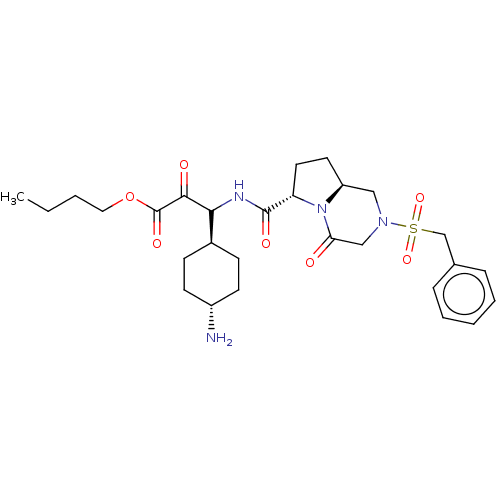 (3-(4-Amino-cyclohexyl)-2-oxo-3-[((6S,8aS)-4-oxo-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50520683 (CHEMBL4476621) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.103 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain (UCLouvain) Curated by ChEMBL | Assay Description Inhibition of human thrombin using Z-GPR-AMC as substrate after 30 mins by fluorimetric analysis | Eur J Med Chem 159: 324-338 (2018) Article DOI: 10.1016/j.ejmech.2018.09.067 BindingDB Entry DOI: 10.7270/Q2VM4GND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254905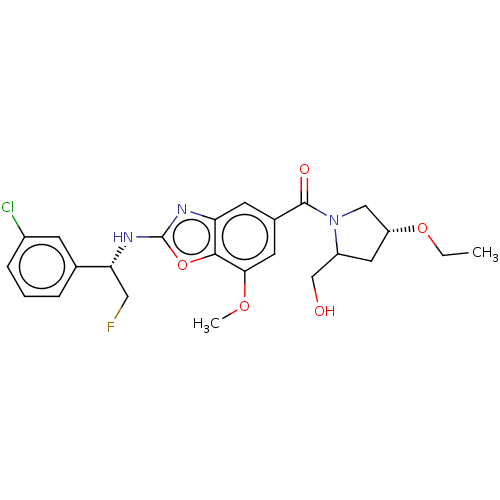 (US9493472, 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50443853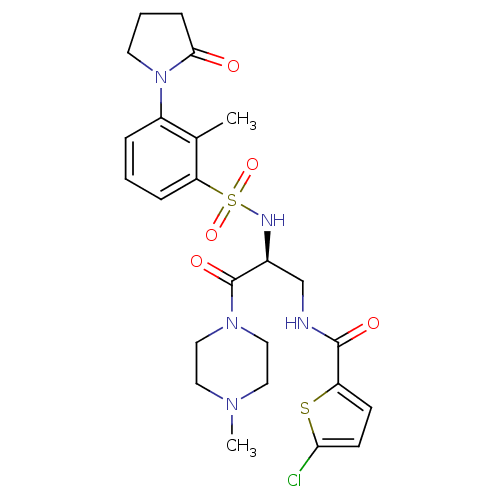 (CHEMBL3091519 | US20230391761, Reference 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254904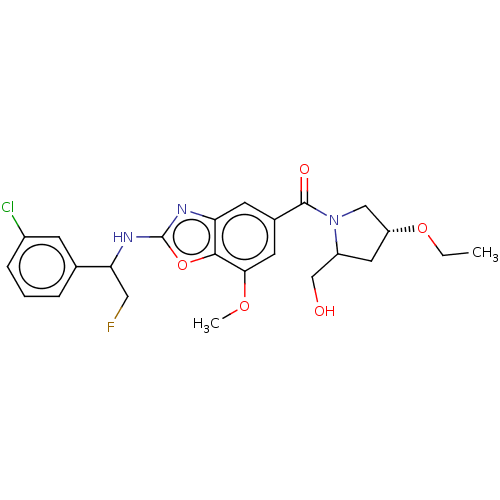 (US9493472, 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254886 (US9493472, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254896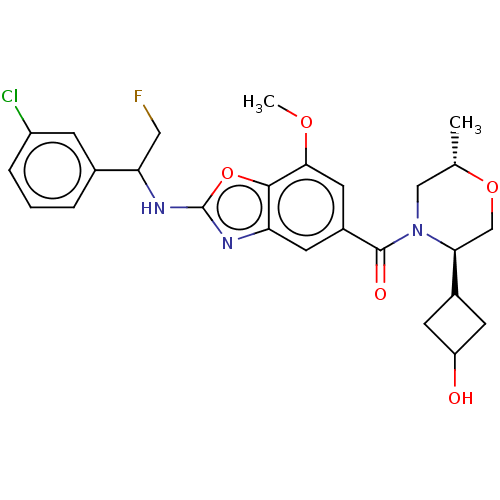 (US9493472, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254895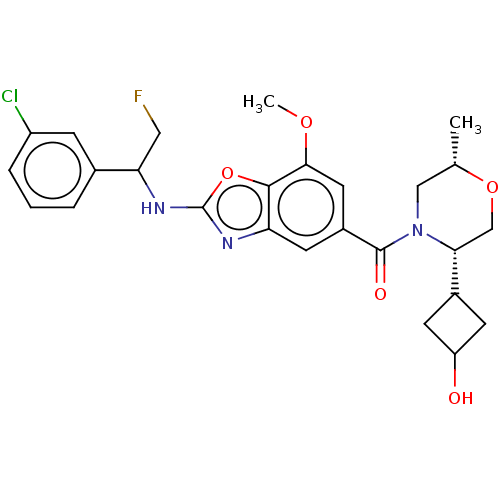 (US9493472, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254911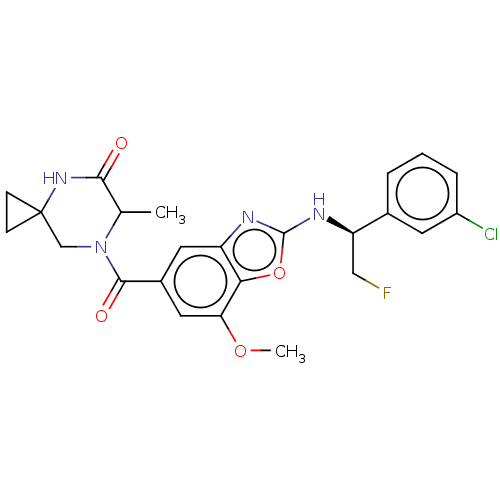 (US9493472, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50289560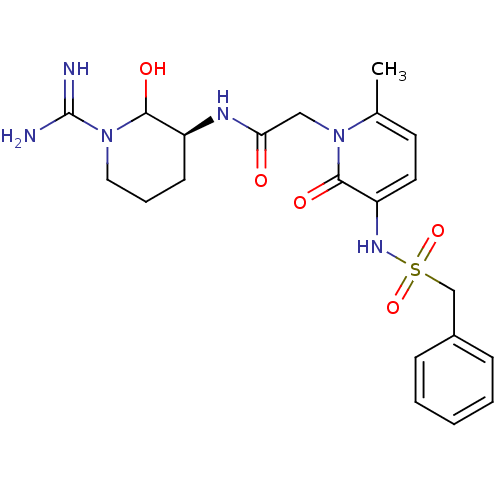 (CHEMBL289389 | N-((S)-1-Carbamimidoyl-2-hydroxy-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.467 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required to inhibit human thrombin enzyme was determined | Bioorg Med Chem Lett 7: 1543-1548 (1997) Article DOI: 10.1016/S0960-894X(97)00258-8 BindingDB Entry DOI: 10.7270/Q2HD7VNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254894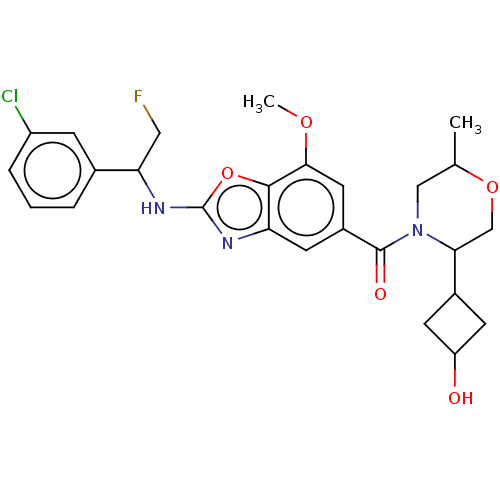 (US9493472, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076074 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.505 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description Concentration required for the in vitro inhibitory activity against human enzyme, thrombin cleavage of the chromogenic substrate | Bioorg Med Chem Lett 9: 895-900 (1999) BindingDB Entry DOI: 10.7270/Q2NS0T3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076074 (1N-[1-amino(imino)methyl-6-hydroxy-(5S)-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.505 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required to inhibit human thrombin enzyme was determined | Bioorg Med Chem Lett 7: 1543-1548 (1997) Article DOI: 10.1016/S0960-894X(97)00258-8 BindingDB Entry DOI: 10.7270/Q2HD7VNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254907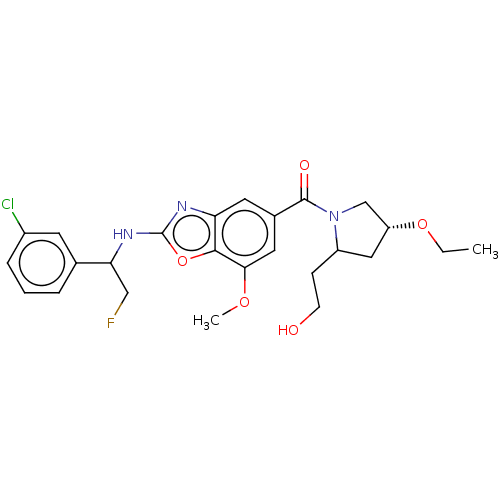 (US9493472, 29 | US9493472, 30 | US9493472, 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054486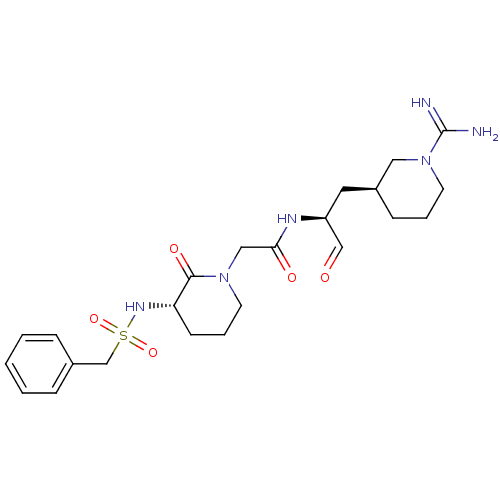 (CHEMBL141424 | N-[(S)-1-((S)-1-Carbamimidoyl-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International Inc. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate thrombin F11a. | J Med Chem 39: 4527-30 (1996) Article DOI: 10.1021/jm960607j BindingDB Entry DOI: 10.7270/Q2CR5SFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254910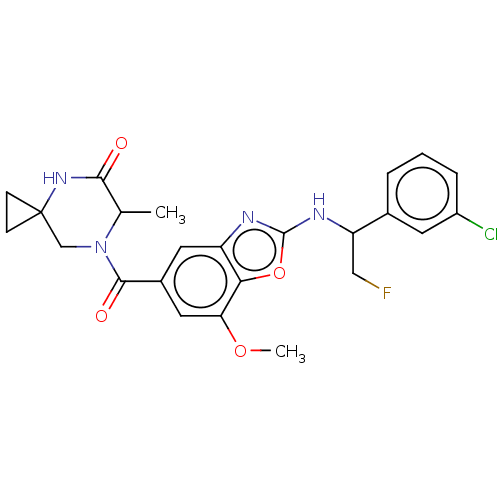 (US9493472, 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054498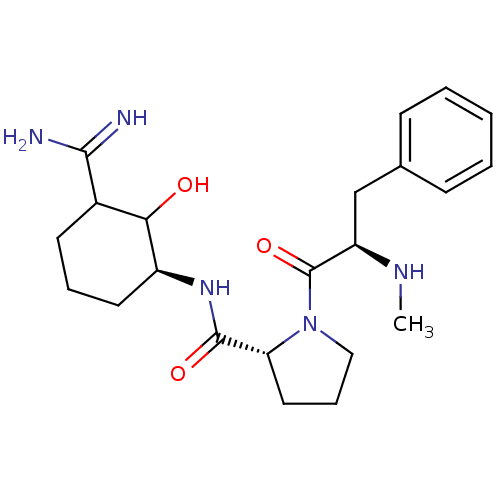 ((R)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073429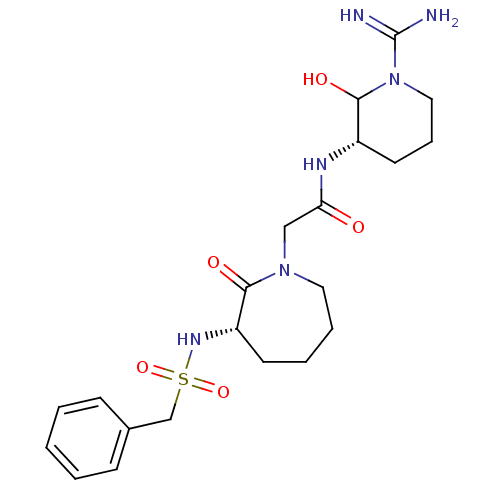 (CHEMBL82032 | CHEMBL84575 | N-((S)-1-Carbamimidoyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibitory activity against Coagulation factor II | Bioorg Med Chem Lett 7: 2421-2426 (1997) Article DOI: 10.1016/S0960-894X(97)00446-0 BindingDB Entry DOI: 10.7270/Q2X92B91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50073429 (CHEMBL82032 | CHEMBL84575 | N-((S)-1-Carbamimidoyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound against human enzyme thrombin | Bioorg Med Chem Lett 9: 2573-8 (1999) BindingDB Entry DOI: 10.7270/Q26M361R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054493 (CHEMBL343805 | N-((S)-3-Carbamimidoyl-2-hydroxy-cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054484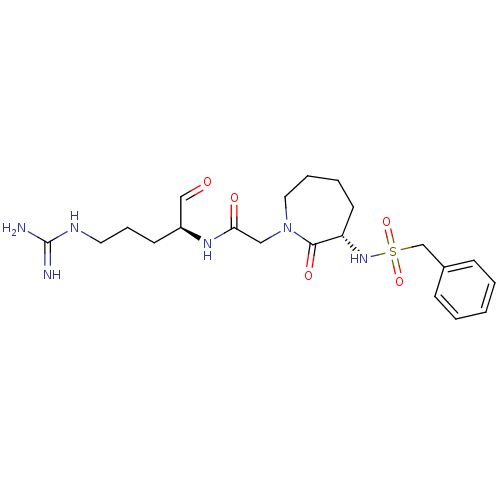 (CHEMBL344204 | CVS-1778 | N-((S)-1-Formyl-4-guanid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International Inc. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate thrombin F11a. | J Med Chem 39: 4527-30 (1996) Article DOI: 10.1021/jm960607j BindingDB Entry DOI: 10.7270/Q2CR5SFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254892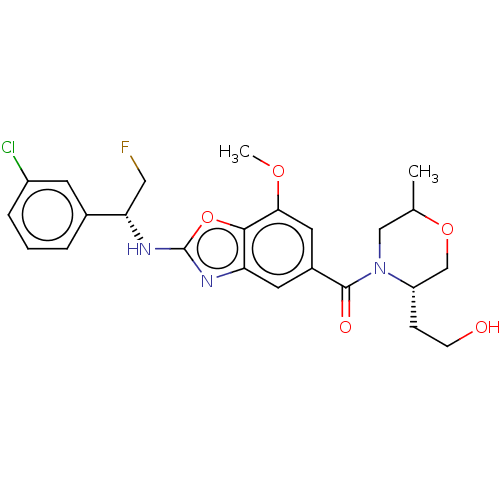 (US9493472, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM639342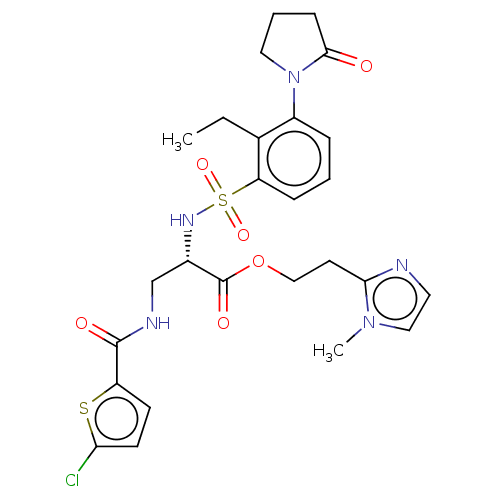 (2-(1-Methyl-1H-imidazol-2-yl)ethyl 3-{[(5-chloro-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM639341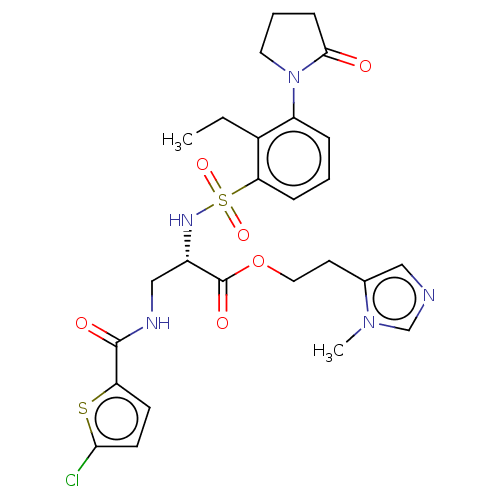 (2-(1-Methyl-1H-imidazol-5-yl)ethyl 3-[(5-chlorothi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50234836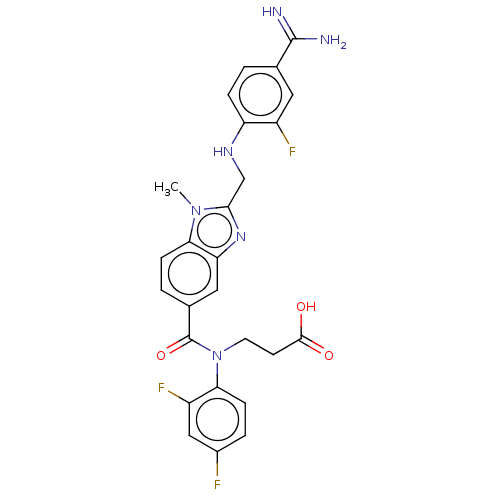 (CHEMBL4086267) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human thrombin preincubated for 10 mins followed by Ac-FVR-AMC substrate addition measured every 20s for 10 mins by fluorescence assay | Eur J Med Chem 126: 799-809 (2017) Article DOI: 10.1016/j.ejmech.2016.12.015 BindingDB Entry DOI: 10.7270/Q2HT2RK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50287790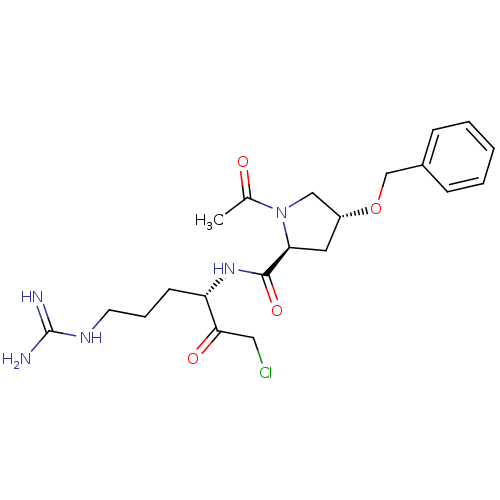 ((2S,4R)-1-Acetyl-4-benzyloxy-pyrrolidine-2-carboxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 6: 2225-2230 (1996) Article DOI: 10.1016/0960-894X(96)00404-0 BindingDB Entry DOI: 10.7270/Q2W95963 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50191108 (CHEMBL3974458) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human thrombin pre-incubated for 10 mins before Ac-FVR-AMC substrate addition and measured after 10 mins by fluorescence based assay | Eur J Med Chem 120: 148-59 (2016) Article DOI: 10.1016/j.ejmech.2016.05.020 BindingDB Entry DOI: 10.7270/Q2QV3PF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176047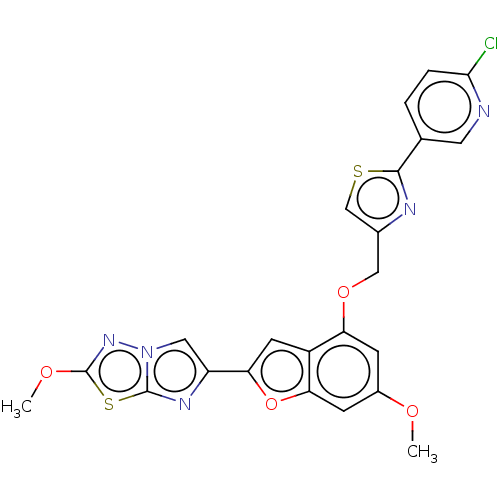 (US10047103, 80 | US9688695, 80) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176041 (US10047103, 74 | US9688695, 74) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50114263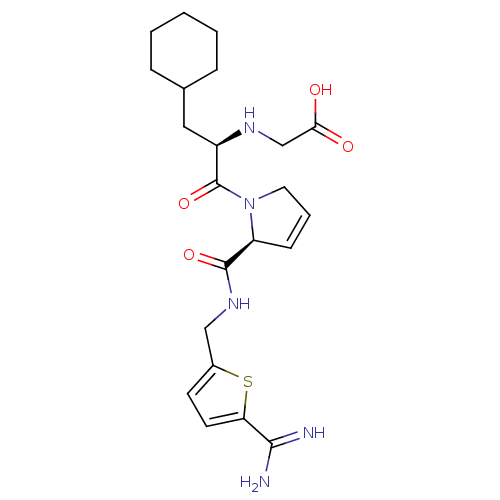 (((R)-2-{(S)-2-[(5-Carbamimidoyl-thiophen-2-ylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 16: 2641-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.040 BindingDB Entry DOI: 10.7270/Q2MC90T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254897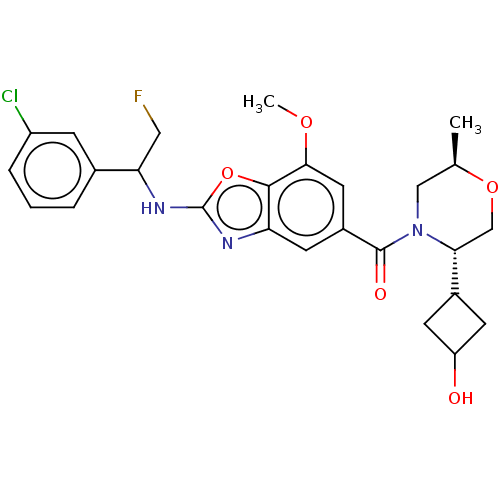 (US9493472, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076017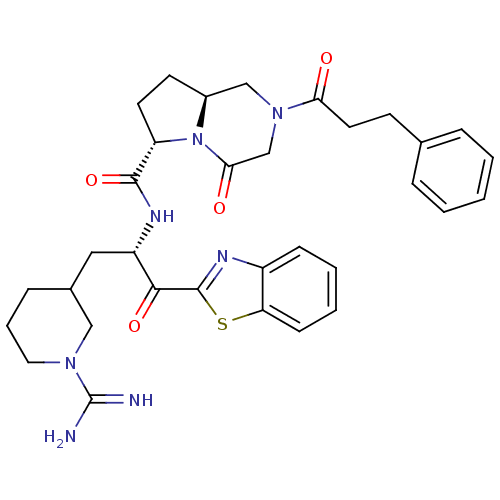 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of human Thrombin | Bioorg Med Chem Lett 9: 835-40 (1999) BindingDB Entry DOI: 10.7270/Q25Q4V8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072741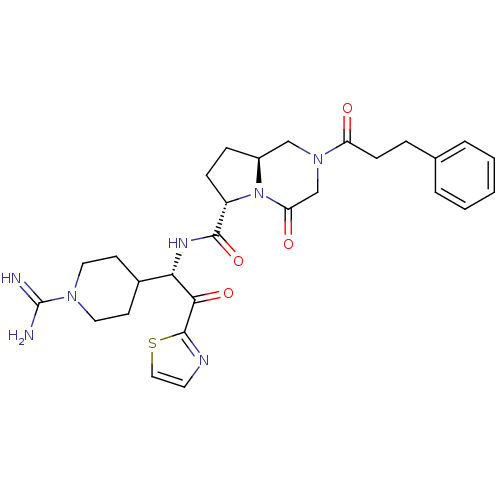 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50217503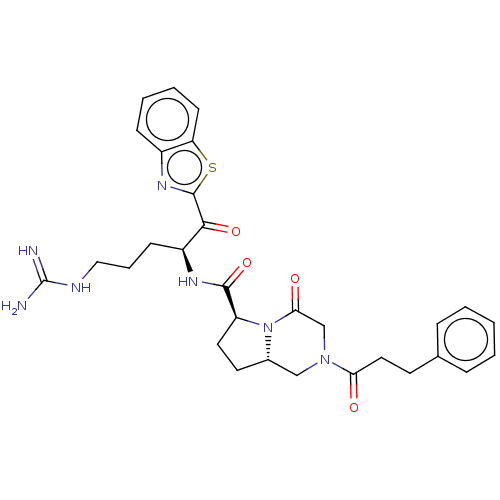 (CHEMBL352928) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of human Thrombin | Bioorg Med Chem Lett 9: 835-40 (1999) BindingDB Entry DOI: 10.7270/Q25Q4V8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50182198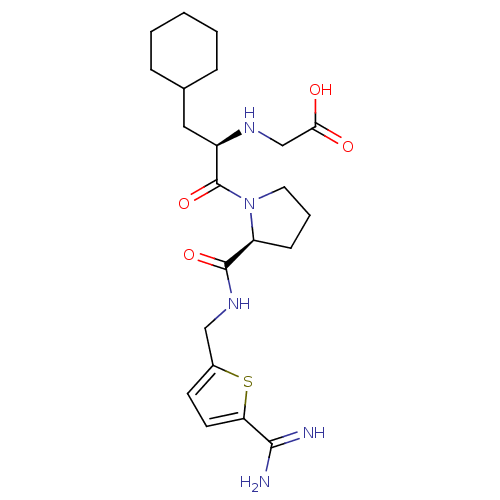 (((R)-2-{(S)-2-[(5-carbamimidoyl-thiophen-2-ylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of thrombin by chromogenic assay | Bioorg Med Chem Lett 16: 2648-53 (2006) Article DOI: 10.1016/j.bmcl.2006.01.046 BindingDB Entry DOI: 10.7270/Q2GM883V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50182201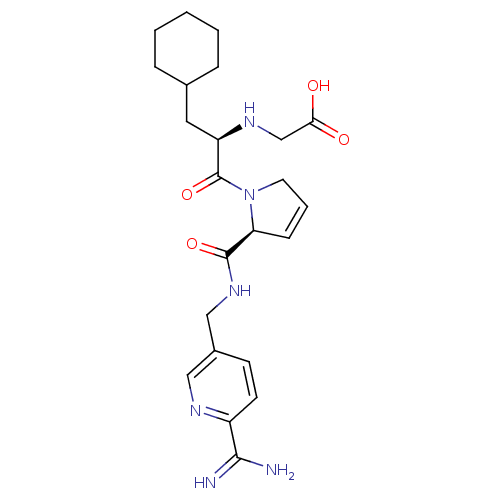 (((R)-2-{(S)-2-[(6-carbamimidoyl-pyridin-3-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 16: 2641-7 (2006) Article DOI: 10.1016/j.bmcl.2006.02.040 BindingDB Entry DOI: 10.7270/Q2MC90T5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM32881 (MLS000706208 | SMR000288260 | [3-(2-furanyl)-5-(me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 23 |
PCMD Curated by PubChem BioAssay | Assay Description HTS was performed using 217,350 compounds of the MLSCN library individually plated into 10ul 1536 compound plates at a concentration of 2.5 mM each, ... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2K64GDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM33812 (1-[5-[(4-fluorobenzyl)amino]-3-(3-pyridyl)-1,2,4-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 23 |
PCMD Curated by PubChem BioAssay | Assay Description HTS was performed using 217,350 compounds of the MLSCN library individually plated into 10ul 1536 compound plates at a concentration of 2.5 mM each, ... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2K64GDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50543723 (CHEMBL4640512) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127072 BindingDB Entry DOI: 10.7270/Q20P13M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM33836 (3-(2-furyl)-1-(3-pyridinylcarbonyl)-N-(2-thienylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 23 |
PCMD Curated by PubChem BioAssay | Assay Description HTS was performed using 217,350 compounds of the MLSCN library individually plated into 10ul 1536 compound plates at a concentration of 2.5 mM each, ... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2K64GDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072741 ((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against thrombin(FIIa) | Bioorg Med Chem Lett 8: 3409-14 (1999) BindingDB Entry DOI: 10.7270/Q27943VV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50114263 (((R)-2-{(S)-2-[(5-Carbamimidoyl-thiophen-2-ylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH& Co. KG Curated by ChEMBL | Assay Description Inhibition of thrombin by chromogenic assay | Bioorg Med Chem Lett 16: 2648-53 (2006) Article DOI: 10.1016/j.bmcl.2006.01.046 BindingDB Entry DOI: 10.7270/Q2GM883V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM176255 (US10047103, 288 | US9688695, 288) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite de Montreal US Patent | Assay Description The ability of the compounds of the current invention to inhibit platelet aggregation induced by gamma-thrombin was tested in a 96-well microplate ag... | US Patent US10047103 (2018) BindingDB Entry DOI: 10.7270/Q2QF8VWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50054492 ((S)-4-[(R)-2-((S)-3-Carbamimidoyl-2-hydroxy-cycloh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description In vitro for inhibition of serine protease thrombin(FIIa). | J Med Chem 39: 4531-6 (1996) Article DOI: 10.1021/jm960572n BindingDB Entry DOI: 10.7270/Q28051QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3716 total ) | Next | Last >> |