

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Agonist activity at human THRbeta expressed in green monkey CV1 cells after 24 hrs by luciferase assay | J Med Chem 63: 6727-6740 (2020) Article DOI: 10.1021/acs.jmedchem.9b02150 BindingDB Entry DOI: 10.7270/Q23N26ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Agonist activity at GST-labelled THRbeta ( 202 to 461 residues) (unknown origin) incubated for 2 hrs in presence SRC1-2 co-activator peptide by Alpha... | J Med Chem 63: 6727-6740 (2020) Article DOI: 10.1021/acs.jmedchem.9b02150 BindingDB Entry DOI: 10.7270/Q23N26ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM18860 ((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Agonist activity at human THRbeta expressed in CHO cells after 24 hrs by luciferase assay | J Med Chem 63: 6727-6740 (2020) Article DOI: 10.1021/acs.jmedchem.9b02150 BindingDB Entry DOI: 10.7270/Q23N26ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046345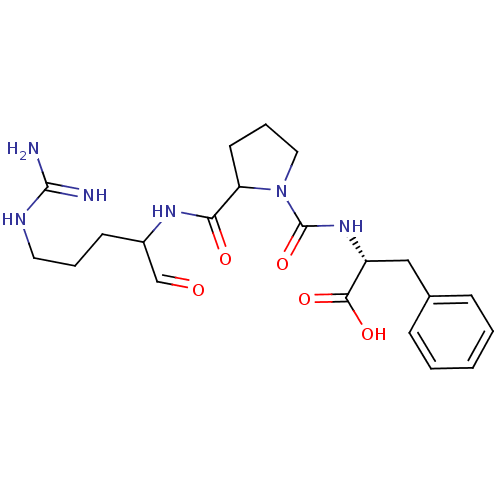 (2-{[2-(1-Formyl-4-guanidino-butylcarbamoyl)-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit thrombin hydrolysis of the chromogenic substrate | J Med Chem 36: 300-3 (1993) BindingDB Entry DOI: 10.7270/Q2JM28Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046345 (2-{[2-(1-Formyl-4-guanidino-butylcarbamoyl)-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit thrombin hydrolysis of the chromogenic substrate | J Med Chem 36: 300-3 (1993) BindingDB Entry DOI: 10.7270/Q2JM28Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046350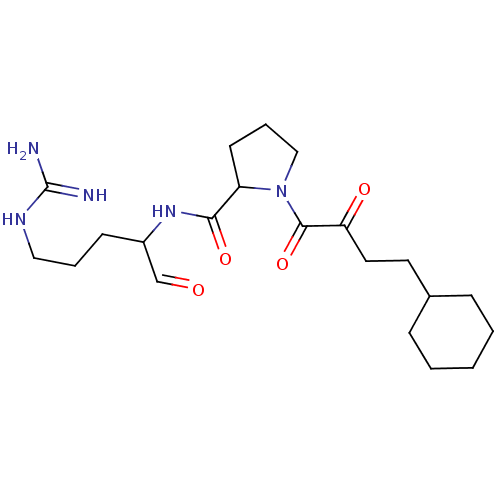 (1-(4-Cyclohexyl-4-oxo-butyryl)-pyrrolidine-2-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit thrombin hydrolysis of the chromogenic substrate | J Med Chem 36: 300-3 (1993) BindingDB Entry DOI: 10.7270/Q2JM28Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046343 (1-(4-Oxo-4-phenyl-butyryl)-pyrrolidine-2-carboxyli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin was determined | J Med Chem 36: 300-3 (1993) BindingDB Entry DOI: 10.7270/Q2JM28Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046343 (1-(4-Oxo-4-phenyl-butyryl)-pyrrolidine-2-carboxyli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human thrombin was determined | J Med Chem 36: 300-3 (1993) BindingDB Entry DOI: 10.7270/Q2JM28Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046344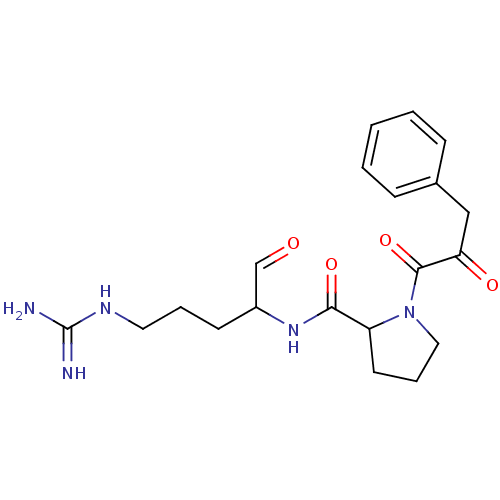 (1-(3-Oxo-3-phenyl-propionyl)-pyrrolidine-2-carboxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 590 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit thrombin hydrolysis of the chromogenic substrate | J Med Chem 36: 300-3 (1993) BindingDB Entry DOI: 10.7270/Q2JM28Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046347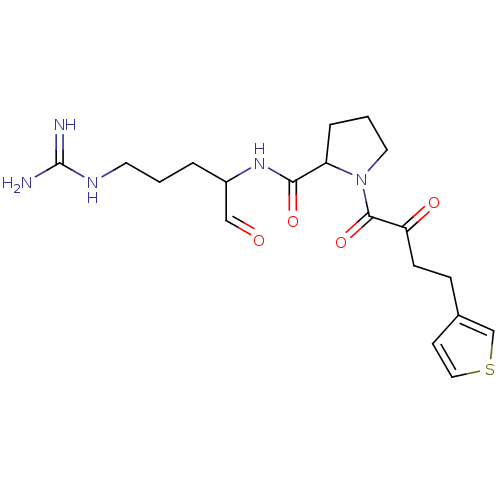 (1-(4-Oxo-4-thiophen-2-yl-butyryl)-pyrrolidine-2-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 590 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit thrombin hydrolysis of the chromogenic substrate | J Med Chem 36: 300-3 (1993) BindingDB Entry DOI: 10.7270/Q2JM28Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50037996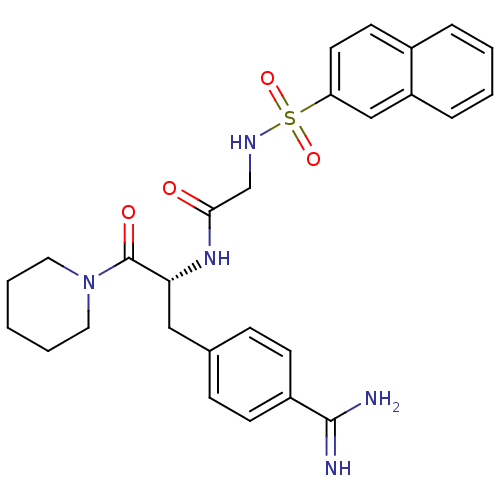 (1-[N-(naphthalen-2-ylsulfonyl)glycyl-4-carbamimido...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Effective concentration against thrombin was determined | Bioorg Med Chem Lett 9: 2013-8 (1999) BindingDB Entry DOI: 10.7270/Q26H4HXG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046342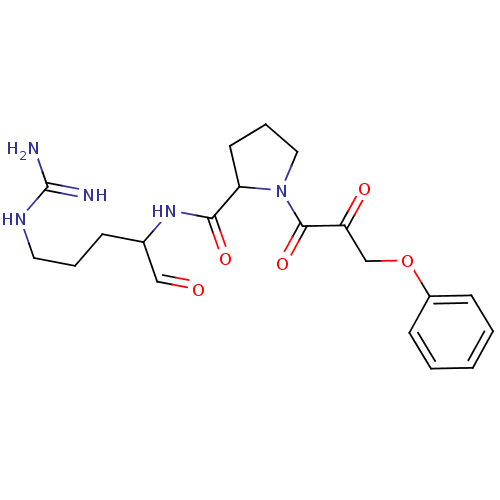 (2-(1-Formyl-4-guanidino-butylcarbamoyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 840 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit thrombin hydrolysis of the chromogenic substrate | J Med Chem 36: 300-3 (1993) BindingDB Entry DOI: 10.7270/Q2JM28Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046346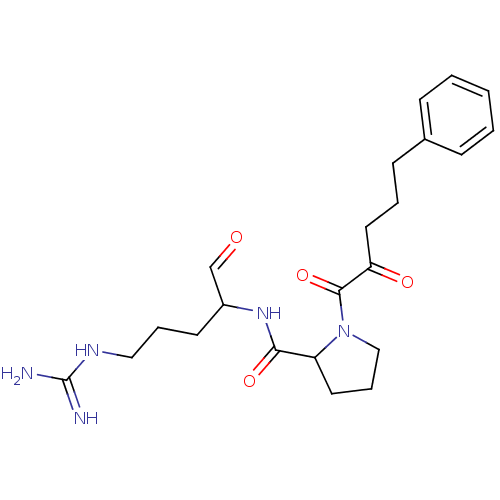 (1-(2-Oxo-5-phenyl-pentanoyl)-pyrrolidine-2-carboxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 880 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit thrombin hydrolysis of the chromogenic substrate | J Med Chem 36: 300-3 (1993) BindingDB Entry DOI: 10.7270/Q2JM28Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046348 (1-(4-Oxo-4-pyridin-3-yl-butyryl)-pyrrolidine-2-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit thrombin hydrolysis of the chromogenic substrate | J Med Chem 36: 300-3 (1993) BindingDB Entry DOI: 10.7270/Q2JM28Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50391540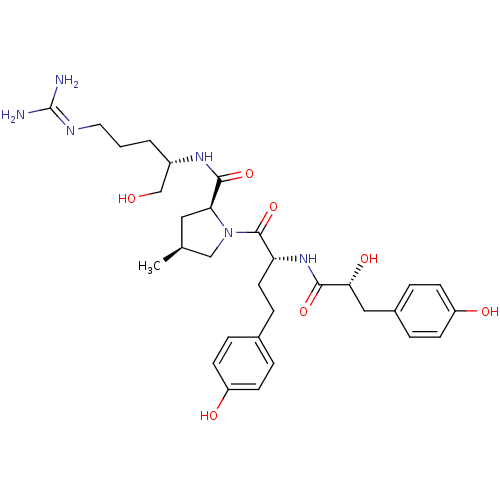 (CHEMBL2147469) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of thrombin | J Nat Prod 75: 1546-52 (2012) Article DOI: 10.1021/np300282a BindingDB Entry DOI: 10.7270/Q2Z03979 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046349 (1-(4-Cyclohexyl-butyryl)-pyrrolidine-2-carboxylic ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit thrombin hydrolysis of the chromogenic substrate | J Med Chem 36: 300-3 (1993) BindingDB Entry DOI: 10.7270/Q2JM28Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||