

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50125842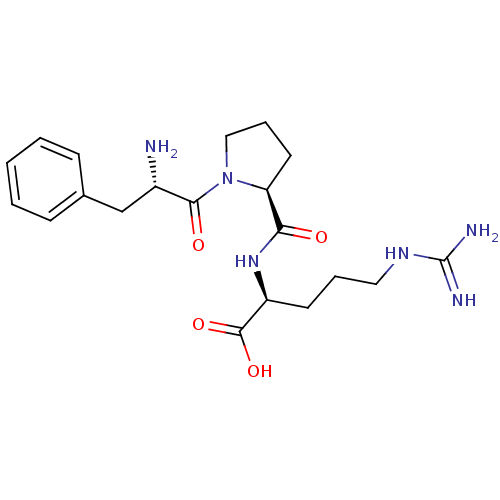 (5-amino(imino)methylamino-2-[1-[2-amino-3-phenyl-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00170 | n/a | n/a | n/a | n/a | n/a | n/a |
Link£ping University Curated by ChEMBL | Assay Description In vitro inhibitory concentration of compound against human thrombin | J Med Chem 46: 1165-79 (2003) Article DOI: 10.1021/jm021065a BindingDB Entry DOI: 10.7270/Q2NG4RCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254888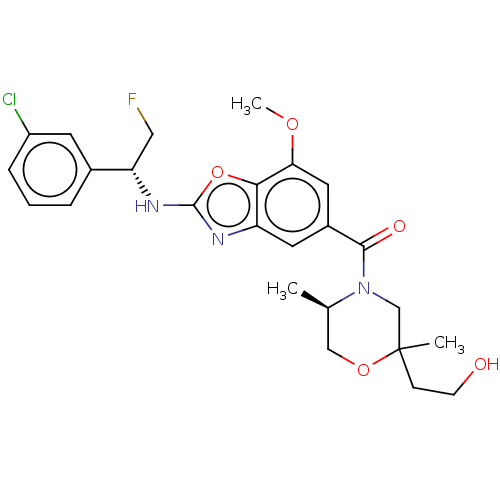 (US9493472, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50583309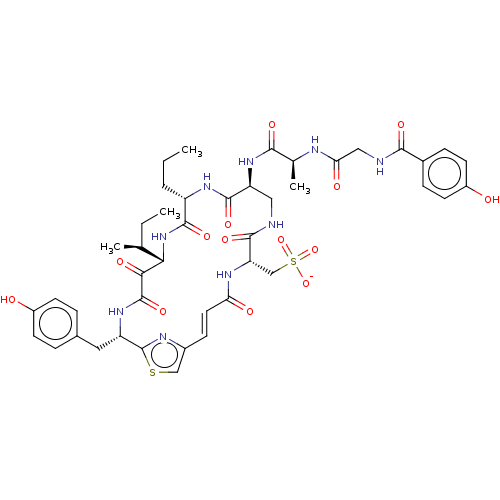 (CHEMBL4204851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human neutrophil elastase | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01583 BindingDB Entry DOI: 10.7270/Q2R78K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50096484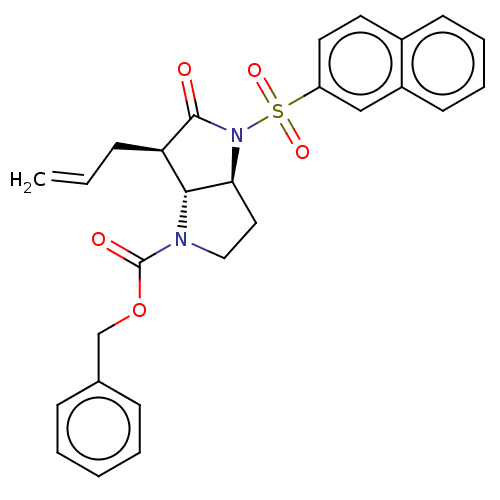 ((4S,6R)-6-Allyl-4-(naphthalene-2-sulfonyl)-5-oxo-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066997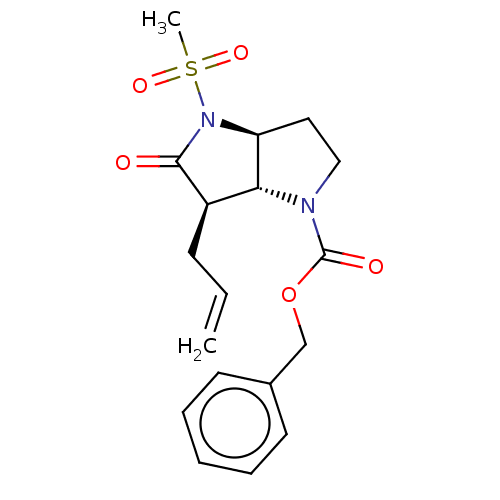 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) | Bioorg Med Chem Lett 11: 243-6 (2001) BindingDB Entry DOI: 10.7270/Q2K074SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120437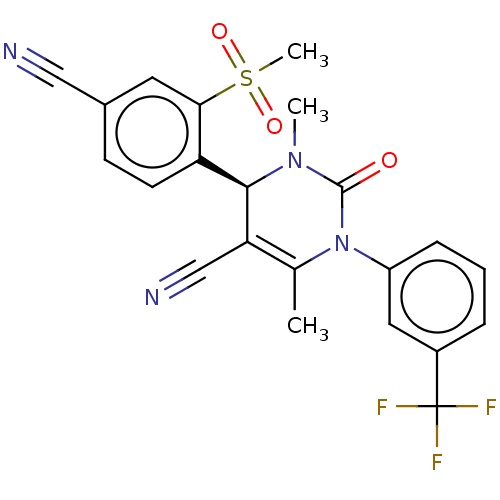 (CHEMBL3617973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-AMC as substrate measured after 60 mins by fluorescence method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00960 BindingDB Entry DOI: 10.7270/Q29W0K46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120437 (CHEMBL3617973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase | Bioorg Med Chem 26: 5583-5595 (2018) Article DOI: 10.1016/j.bmc.2018.09.034 BindingDB Entry DOI: 10.7270/Q2M32ZGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50063555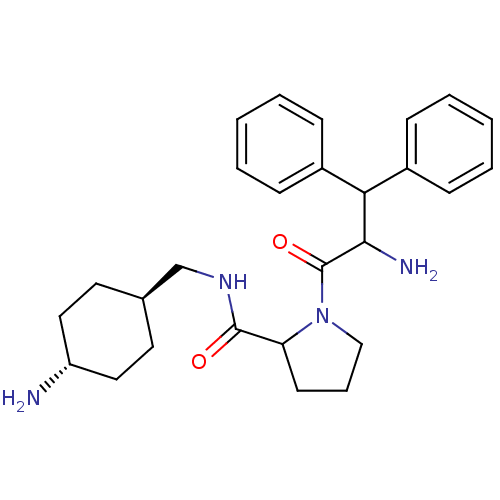 (1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 41: 1011-3 (1998) Article DOI: 10.1021/jm9706933 BindingDB Entry DOI: 10.7270/Q2ZG6RCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254889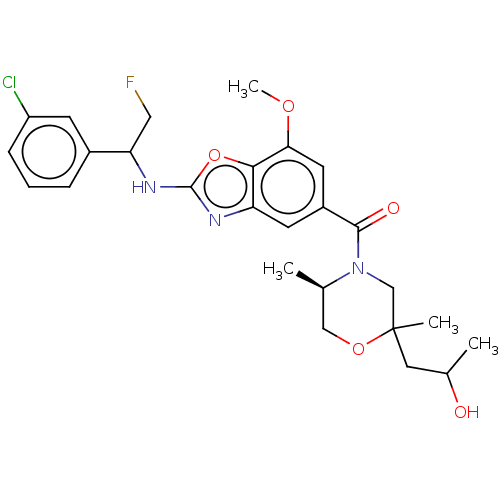 (US9493472, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50096624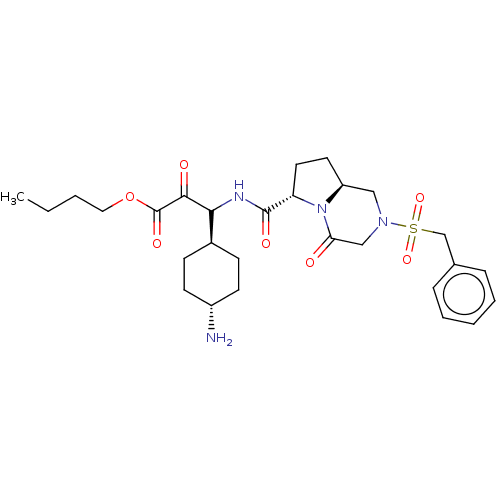 (3-(4-Amino-cyclohexyl)-2-oxo-3-[((6S,8aS)-4-oxo-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc. Curated by ChEMBL | Assay Description In vitro activity of the compound against human alpha thrombin was determined | Bioorg Med Chem Lett 11: 287-90 (2001) BindingDB Entry DOI: 10.7270/Q2P55MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50058495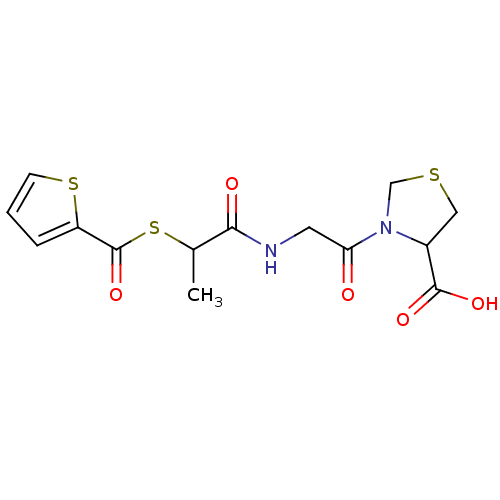 (3-{2-[2-(Thiophene-2-carbonylsulfanyl)-propionylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory concentration against Human Leukocyte Elastase from human sputum | J Med Chem 40: 1906-18 (1997) Article DOI: 10.1021/jm960772z BindingDB Entry DOI: 10.7270/Q2MS3RW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50520683 (CHEMBL4476621) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.103 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Catholique de Louvain (UCLouvain) Curated by ChEMBL | Assay Description Inhibition of human thrombin using Z-GPR-AMC as substrate after 30 mins by fluorimetric analysis | Eur J Med Chem 159: 324-338 (2018) Article DOI: 10.1016/j.ejmech.2018.09.067 BindingDB Entry DOI: 10.7270/Q2VM4GND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254905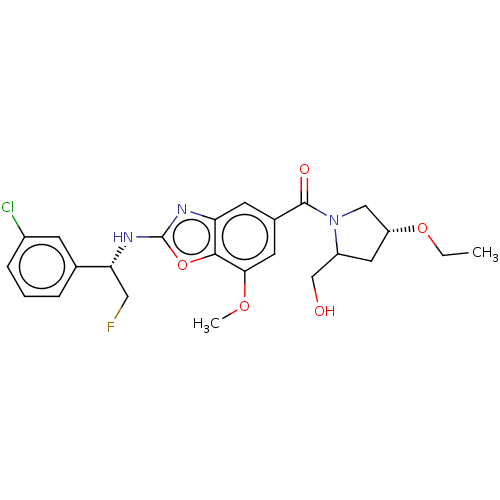 (US9493472, 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254904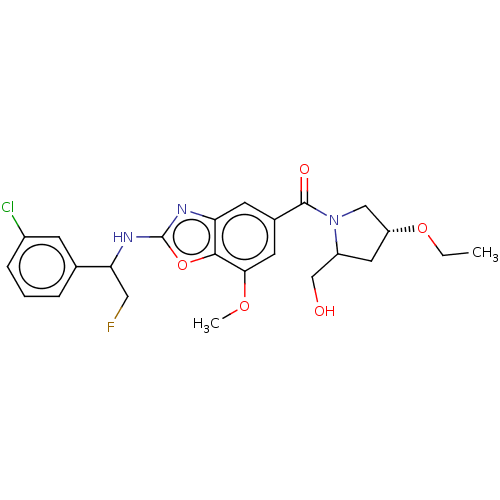 (US9493472, 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50443853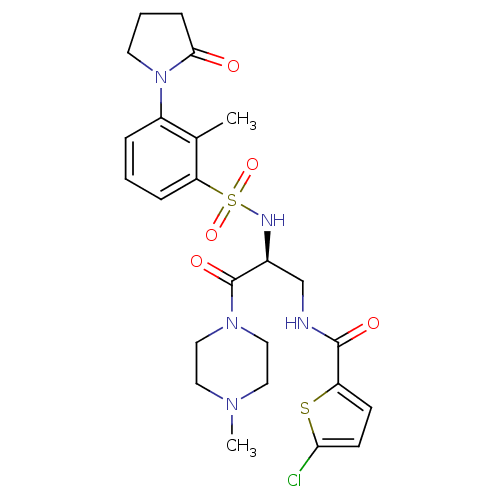 (CHEMBL3091519 | US20230391761, Reference 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254886 (US9493472, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120428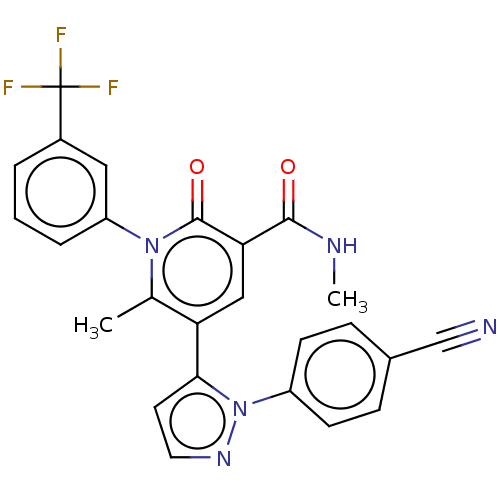 (CHEMBL3617966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase | Bioorg Med Chem Lett 25: 4370-81 (2015) Article DOI: 10.1016/j.bmcl.2015.08.049 BindingDB Entry DOI: 10.7270/Q29Z96PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50556037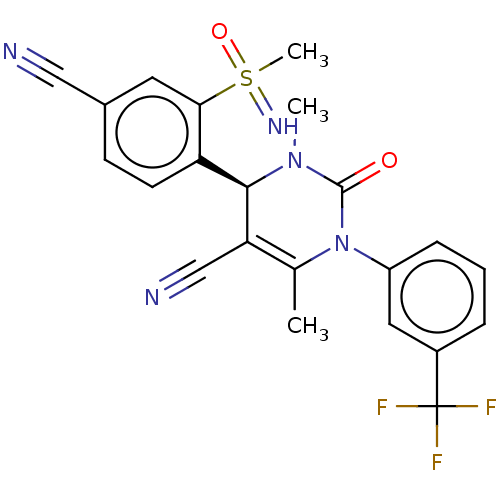 (CHEMBL4744163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-AMC as substrate measured after 60 mins by fluorescence method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00960 BindingDB Entry DOI: 10.7270/Q29W0K46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189813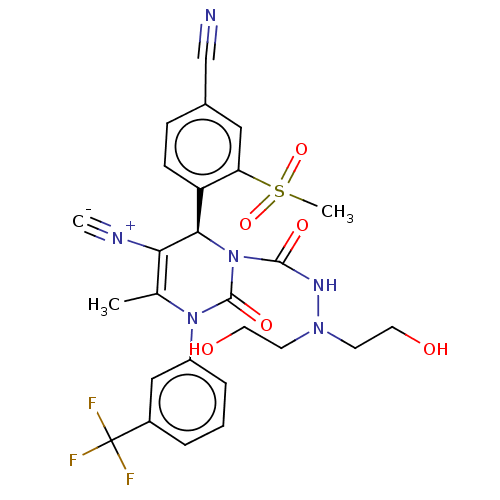 (US9174997, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189818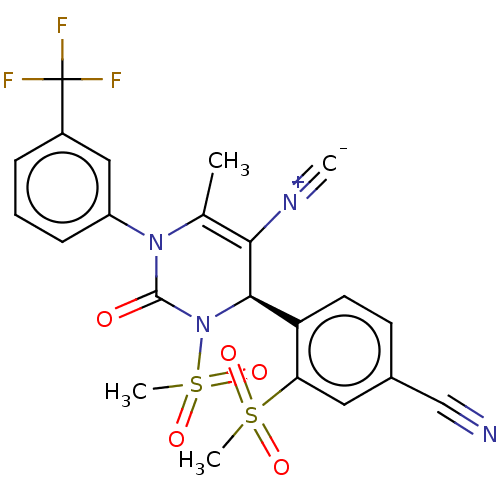 (US9174997, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189819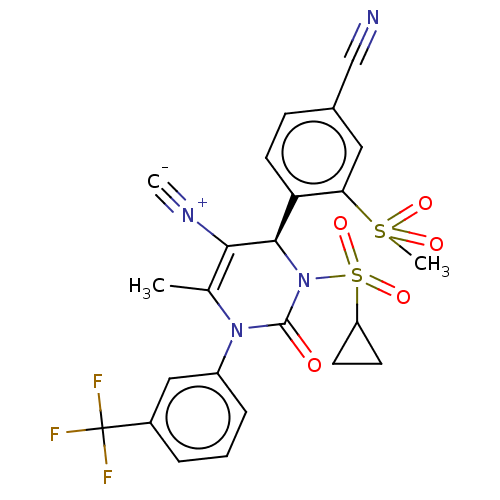 (US9174997, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189820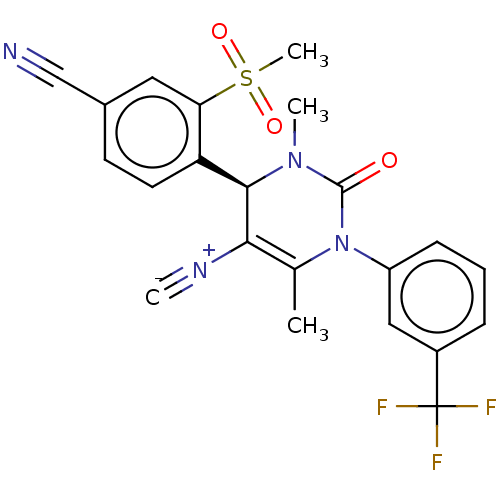 (US9174997, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189821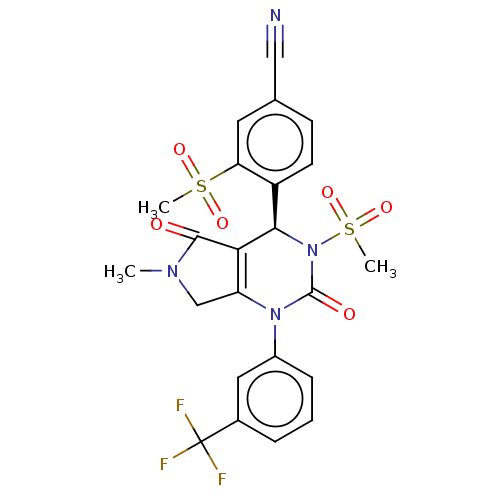 (US9174997, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189822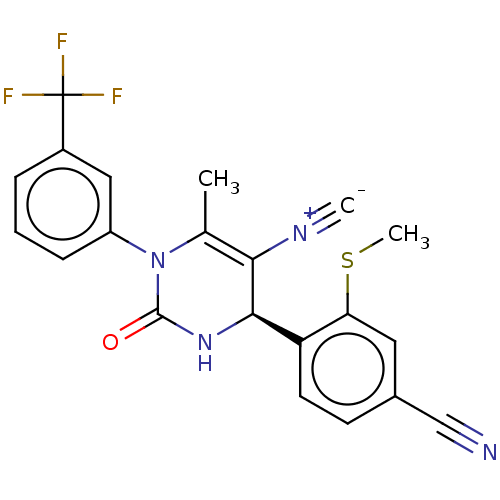 (US9174997, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189823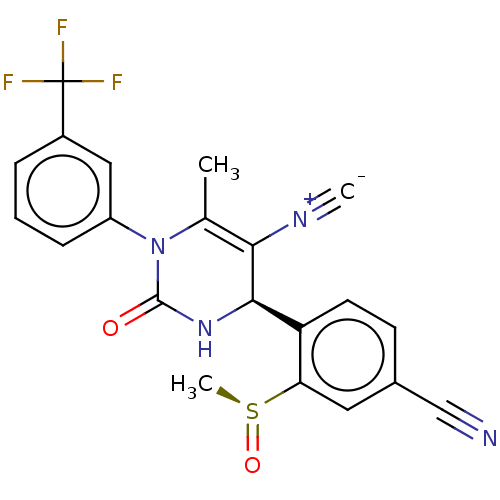 (US9174997, 43 (Diastereomer 1)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189831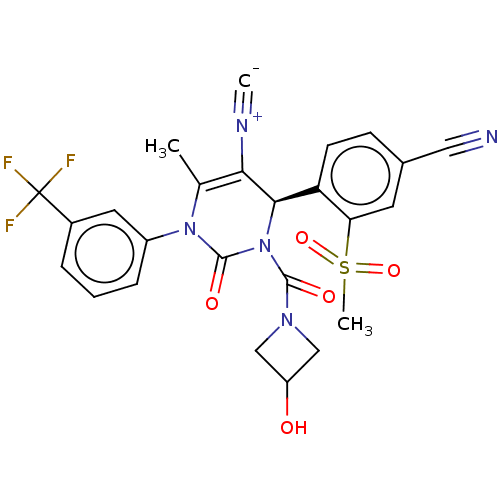 (US9174997, 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189835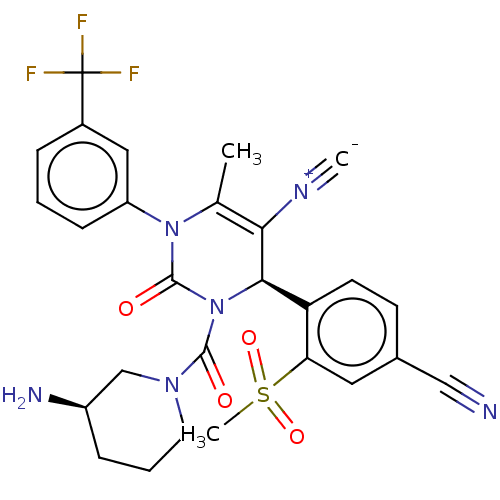 (US9174997, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189860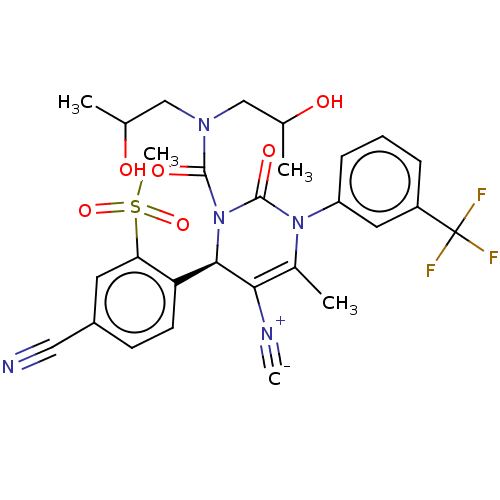 (US9174997, 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189865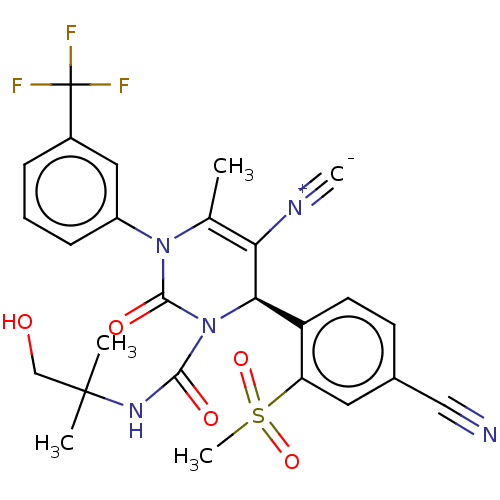 (US9174997, 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189871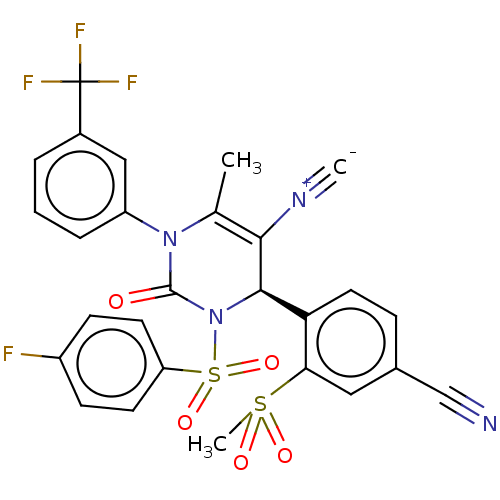 (US9174997, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189876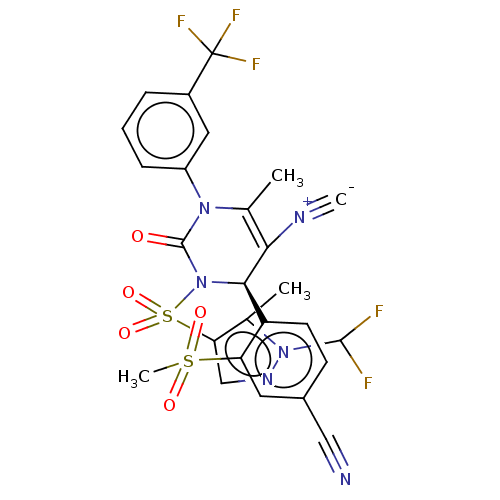 (US9174997, 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189883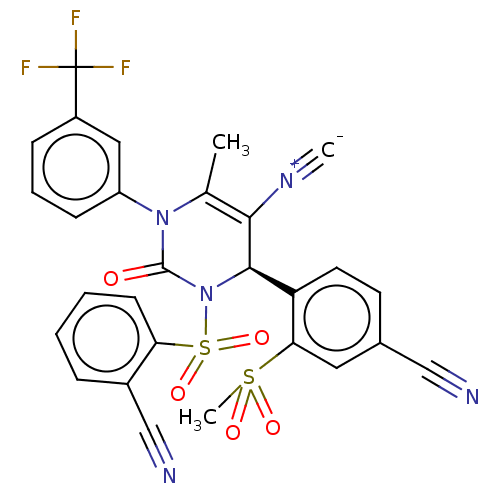 (US9174997, 103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189896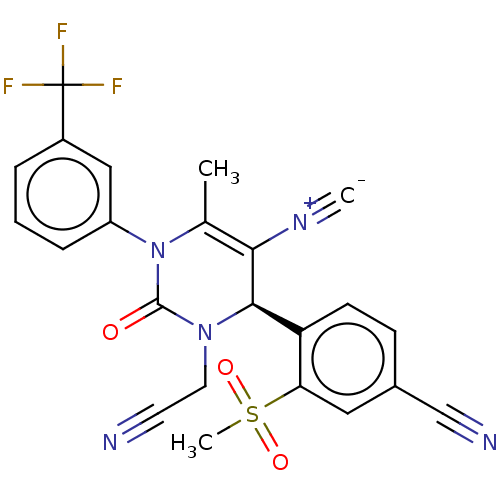 (US9174997, 116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189900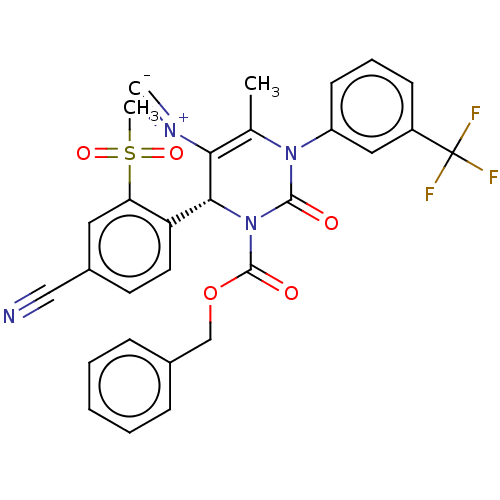 (US9174997, 120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189908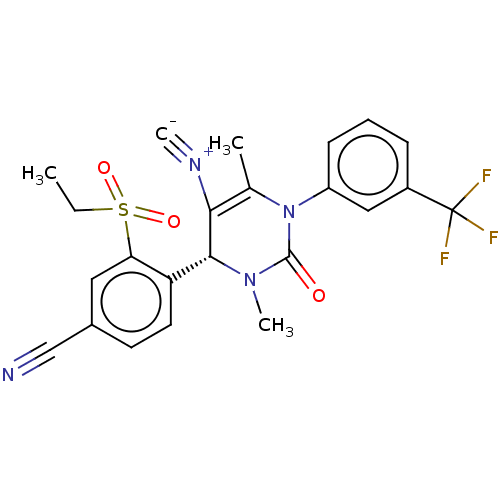 (US9174997, 128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189921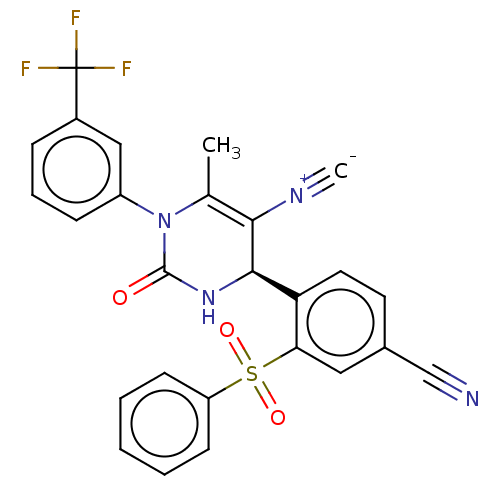 (US9174997, 141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189924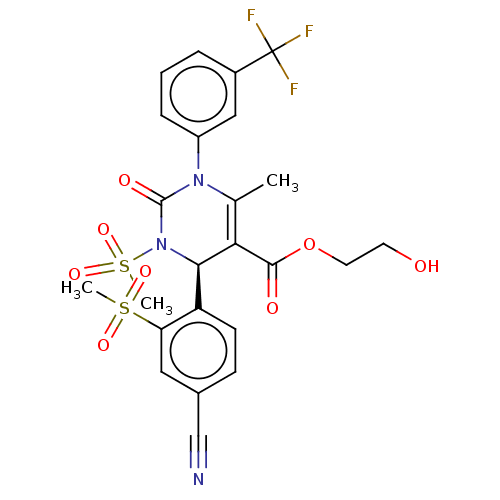 (US9174997, 144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM211514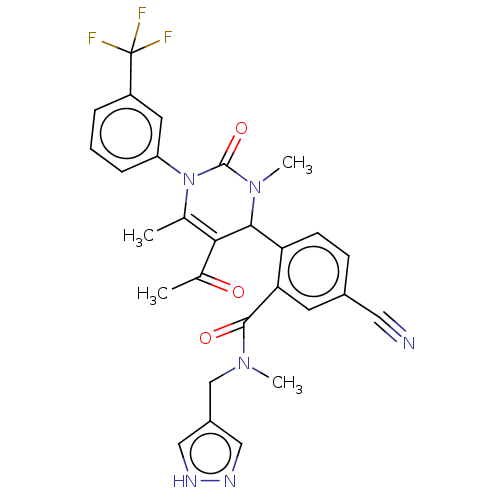 (US9290457, Example 1.56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description Materials: Human neutrophil elastase was purchased from Calbiochem (Cat. No.: 324681) and the elastase substrate MeOSuc-Ala-Ala-Pro-Val-AMC from Bach... | US Patent US9290457 (2016) BindingDB Entry DOI: 10.7270/Q2QJ7G4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189812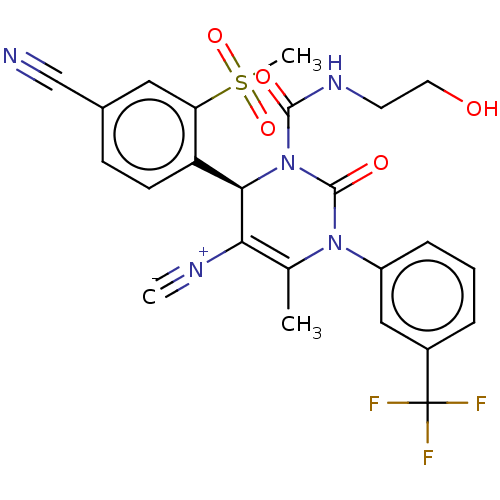 (US9174997, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104826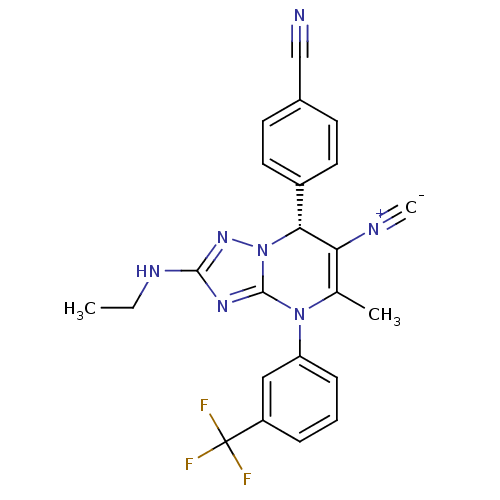 (US8569314, 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104825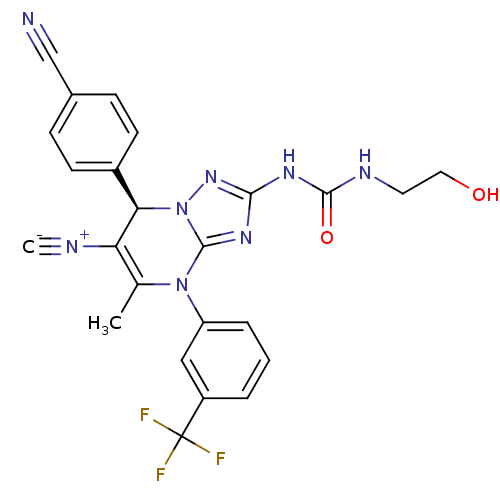 (US8569314, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189814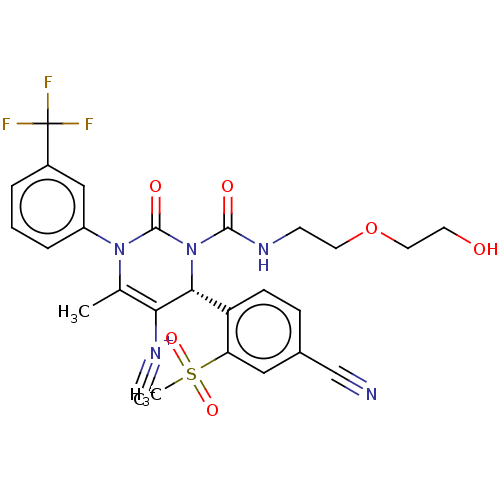 (US9174997, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189815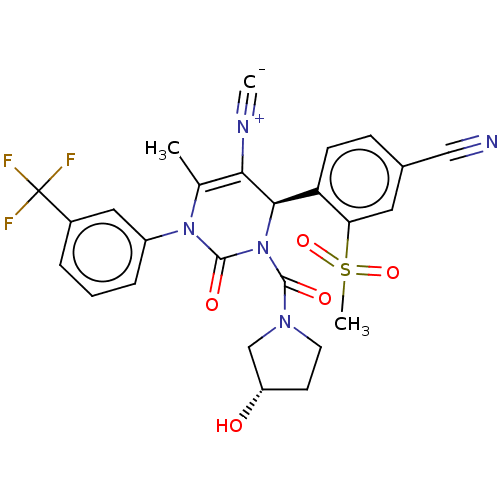 (US9174997, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50120436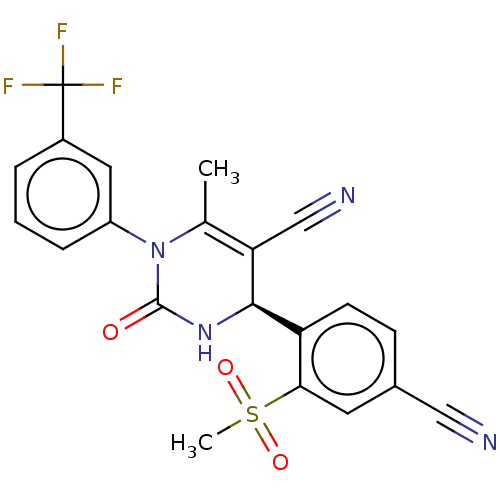 (CHEMBL3617972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase using MeOSuc-AAPV-AMC as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 25: 4370-81 (2015) Article DOI: 10.1016/j.bmcl.2015.08.049 BindingDB Entry DOI: 10.7270/Q29Z96PX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104820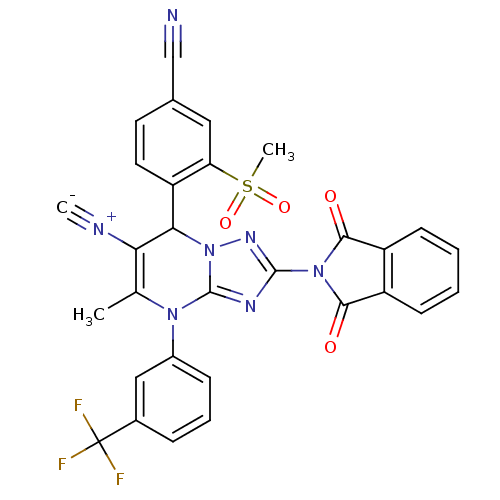 (US8569314, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104821 (US8569314, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM254896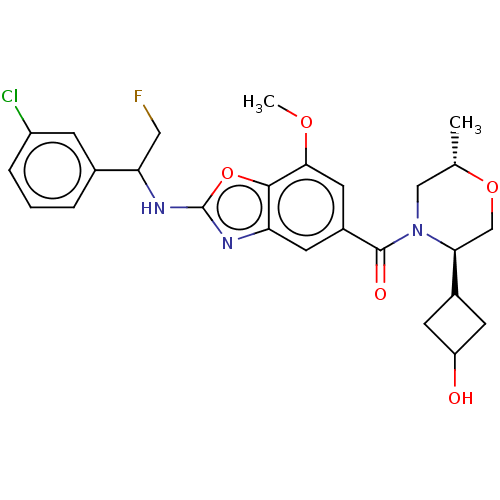 (US9493472, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the thrombin inhibition of the substances listed above, a biochemical test system is constructed in which the conversion of a thrombin s... | US Patent US9493472 (2016) BindingDB Entry DOI: 10.7270/Q2B56HPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104824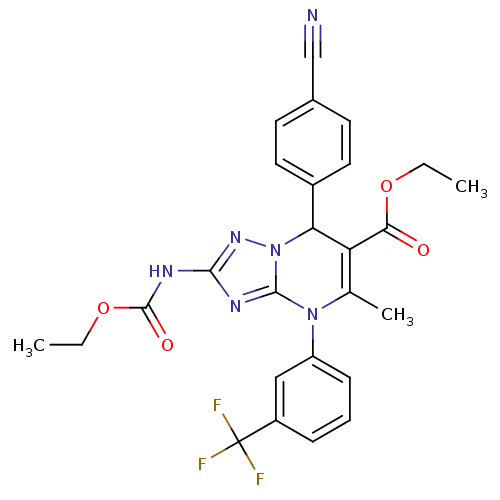 (US8569314, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7122 total ) | Next | Last >> |