

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | 0.00000207 | 6.63E+3 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406217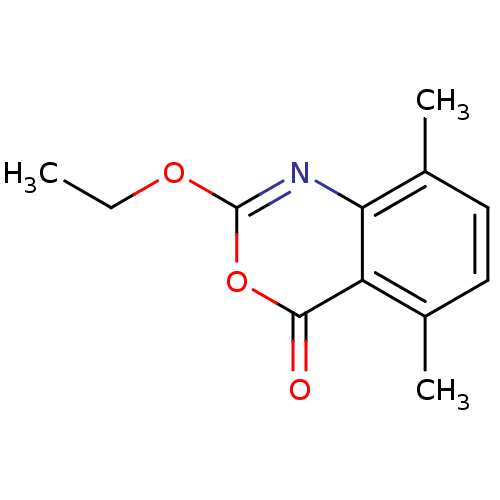 (CHEMBL145002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | 0.00000617 | 6.46E+4 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406216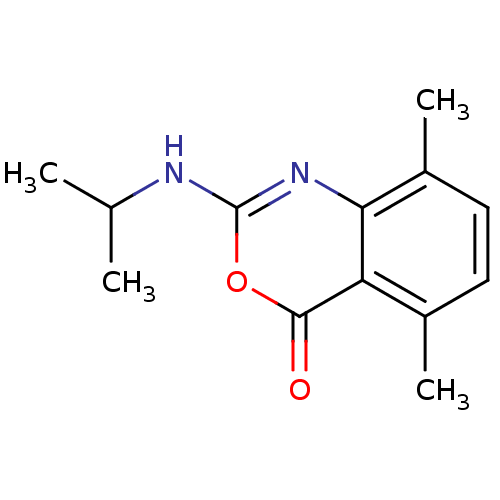 (CHEMBL146003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.31 | n/a | n/a | n/a | n/a | 0.00000871 | 1.32E+3 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118027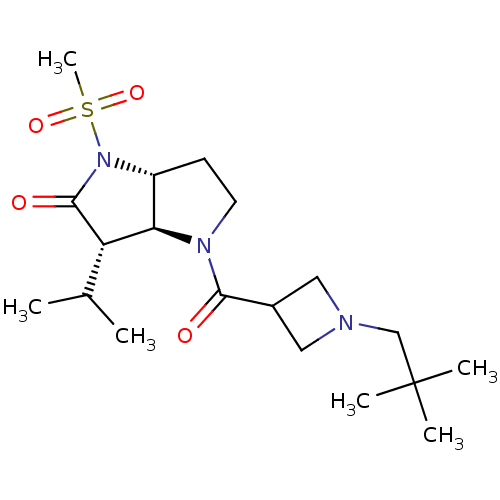 (4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.73 | n/a | n/a | n/a | n/a | 0.0000109 | 6.31E+3 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406221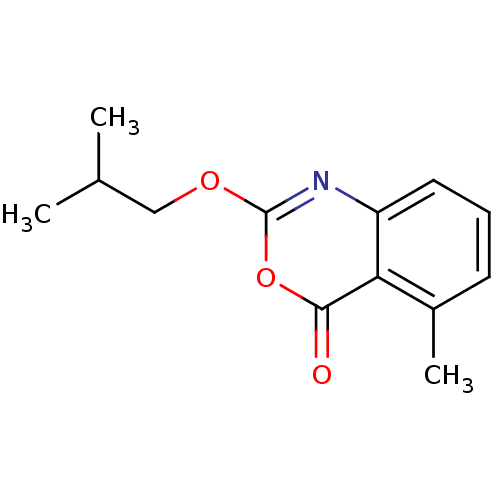 (CHEMBL145729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.58 | n/a | n/a | n/a | n/a | 0.0000112 | 7.08E+3 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50288087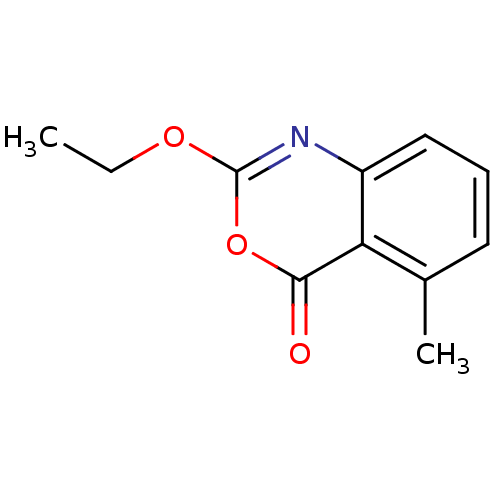 (2-Ethoxy-5-methyl-benzo[d][1,3]oxazin-4-one | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.126 | n/a | n/a | n/a | n/a | 0.0000117 | 1.02E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029699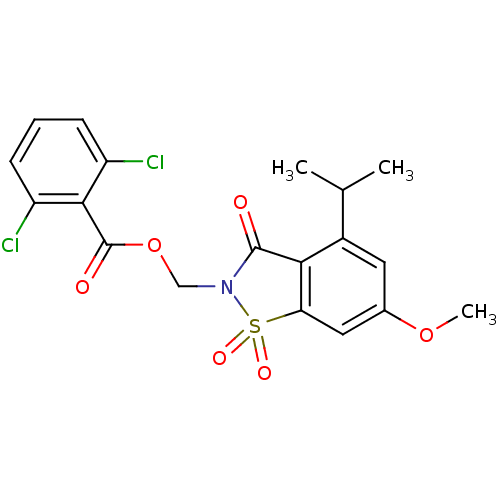 (2,6-Dichloro-benzoic acid 4-isopropyl-6-methoxy-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000120 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50029703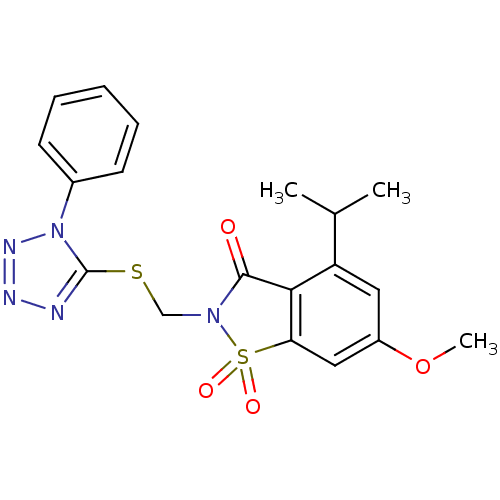 (4-Isopropyl-6-methoxy-1,1-dioxo-2-(1-phenyl-1H-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000120 | 4.45E+4 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406306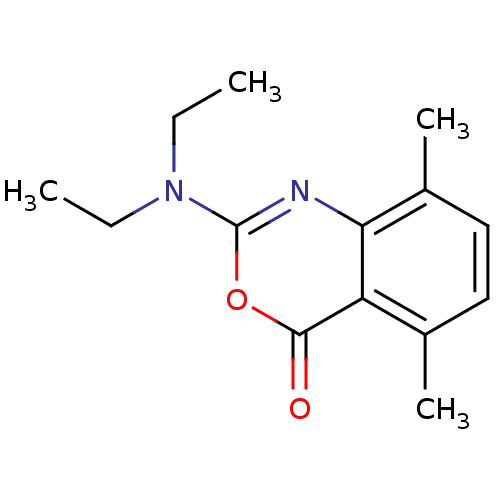 (CHEMBL145107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | 0.0000138 | 132 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406226 (CHEMBL342522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.01 | n/a | n/a | n/a | n/a | 0.0000141 | 2.88E+3 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50288098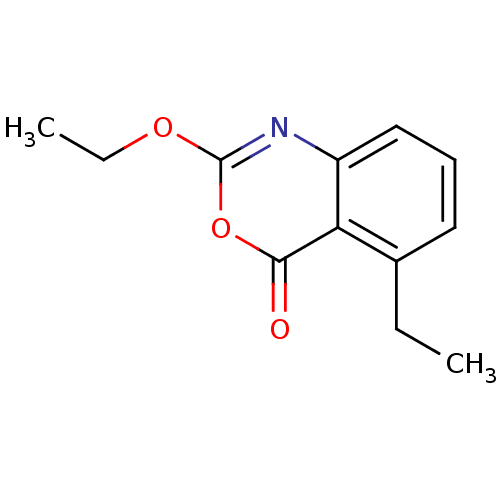 (2-Ethoxy-5-ethyl-benzo[d][1,3]oxazin-4-one | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0430 | n/a | n/a | n/a | n/a | 0.0000145 | 3.39E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406208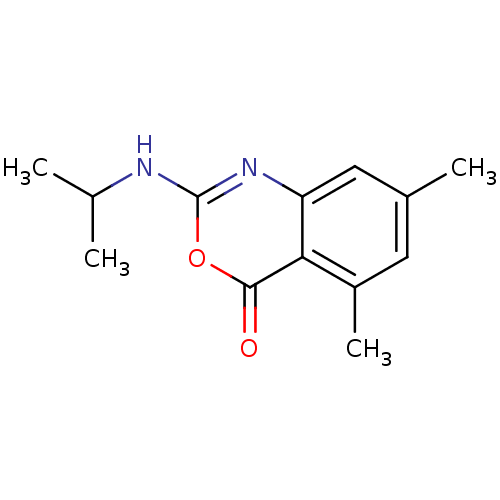 (CHEMBL146004) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.31 | n/a | n/a | n/a | n/a | 0.0000151 | 2.57E+3 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286331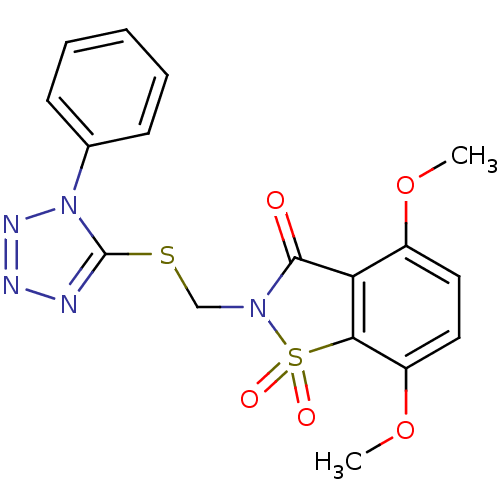 (4,7-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000160 | 1.10E+3 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50063719 (2-Isopropylamino-5-methyl-benzo[d][1,3]oxazin-4-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.58 | n/a | n/a | n/a | n/a | 0.0000178 | 1.18E+4 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406206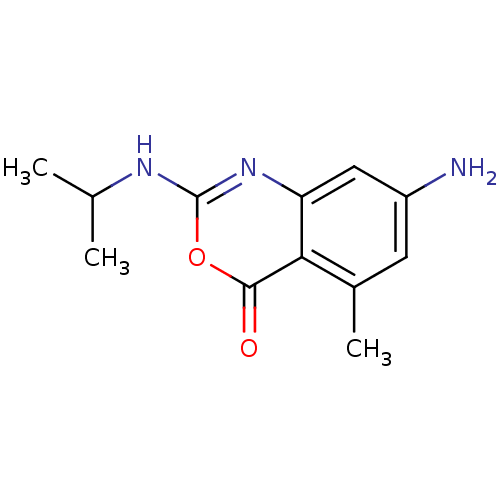 (CHEMBL145152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79.4 | n/a | n/a | n/a | n/a | 0.0000178 | 229 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50063719 (2-Isopropylamino-5-methyl-benzo[d][1,3]oxazin-4-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.0000180 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Rate of deacylation by human leukocyte elastase | J Med Chem 30: 589-591 (1987) Article DOI: 10.1021/jm00387a001 BindingDB Entry DOI: 10.7270/Q2GQ6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406240 (CHEMBL347760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.398 | n/a | n/a | n/a | n/a | 0.0000182 | 4.57E+4 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406244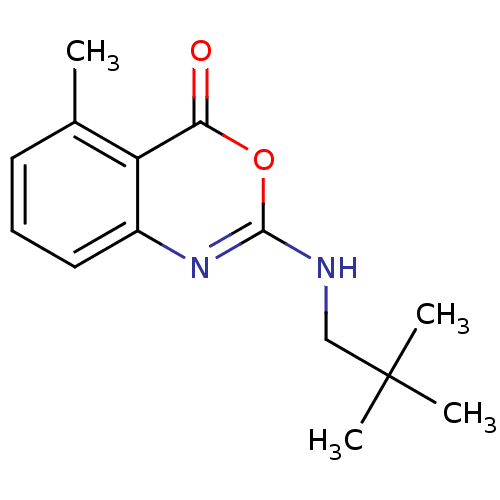 (CHEMBL444082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 251 | n/a | n/a | n/a | n/a | 0.0000182 | 75.9 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406212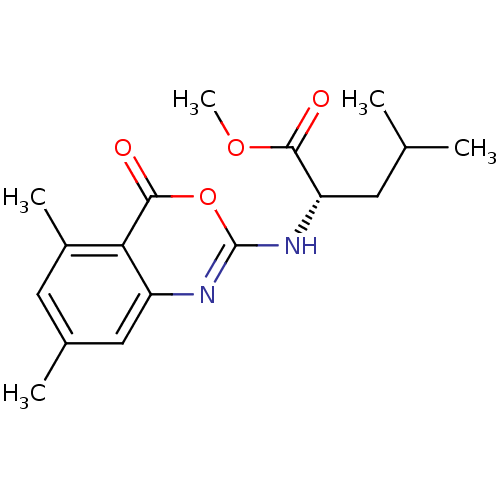 (CHEMBL357238) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | 0.0000200 | 15.8 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406303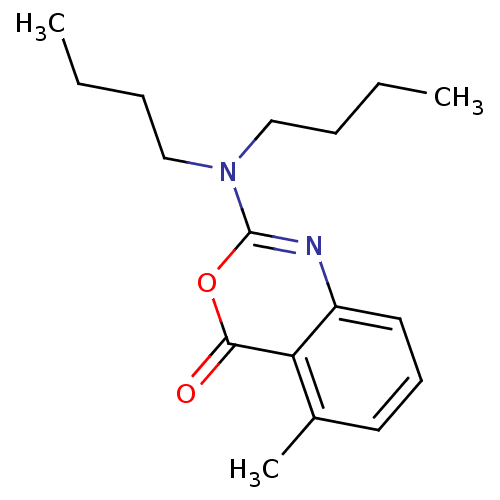 (CHEMBL145690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 398 | n/a | n/a | n/a | n/a | 0.0000240 | 66.1 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286329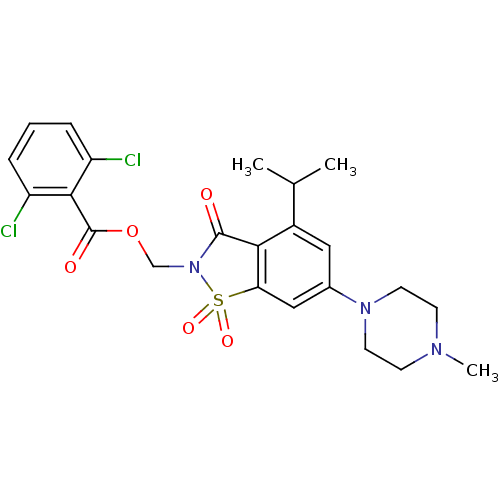 (2,6-Dichloro-benzoic acid 4-isopropyl-6-(4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000240 | 2.15E+4 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286329 (2,6-Dichloro-benzoic acid 4-isopropyl-6-(4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000240 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50039631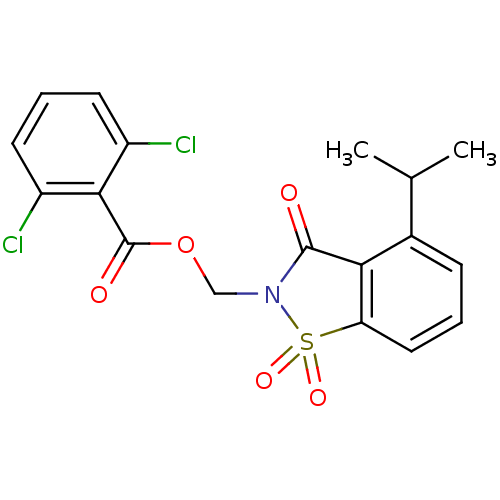 (2,6-Dichloro-benzoic acid 4-isopropyl-1,1,3-trioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000270 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286328 (2,6-Dichloro-benzoic acid 6-dimethylamino-4-isopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000270 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50282874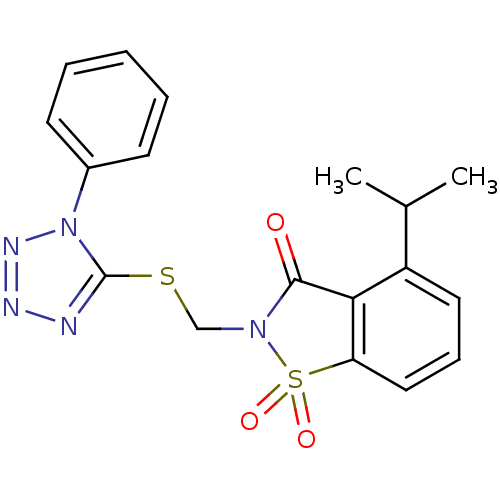 (4-Isopropyl-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000280 | 9.40E+4 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50282874 (4-Isopropyl-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000280 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406273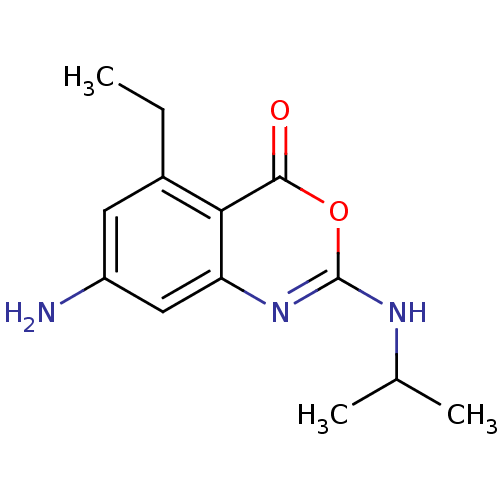 (CHEMBL145471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15.8 | n/a | n/a | n/a | n/a | 0.0000282 | 1.66E+3 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286327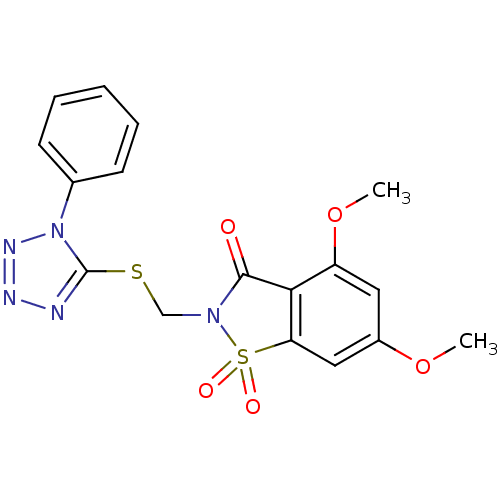 (4,6-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000290 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286327 (4,6-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000290 | 4.90E+4 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50064322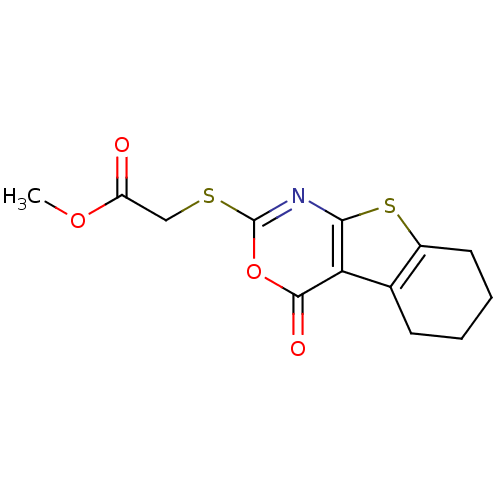 ((4-Oxo-5,6,7,8-tetrahydro-4H-benzo[4,5]thieno[2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17.9 | n/a | n/a | n/a | n/a | 0.0000292 | 1.63E+3 | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Steady state inhibition constant for Human Leukocyte Elastase inhibition | J Med Chem 41: 1729-40 (1998) Article DOI: 10.1021/jm9708341 BindingDB Entry DOI: 10.7270/Q2QV3KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50064309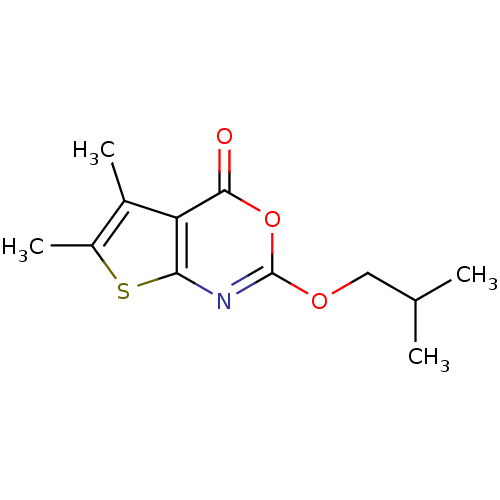 (2-Isobutoxy-5,6-dimethyl-thieno[2,3-d][1,3]oxazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 133 | n/a | n/a | n/a | n/a | 0.0000292 | 219 | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Steady state inhibition constant for Human Leukocyte Elastase inhibition | J Med Chem 41: 1729-40 (1998) Article DOI: 10.1021/jm9708341 BindingDB Entry DOI: 10.7270/Q2QV3KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286326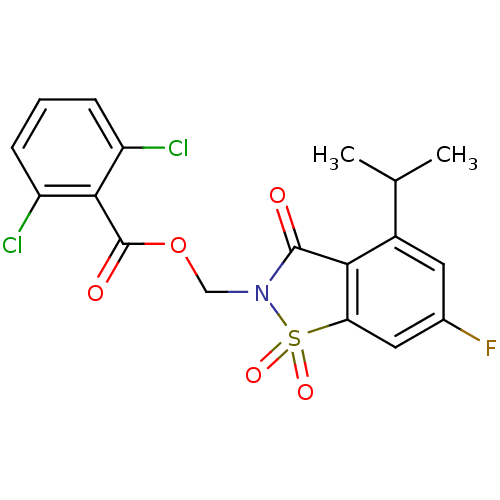 (2,6-Dichloro-benzoic acid 6-fluoro-4-isopropyl-1,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000300 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406258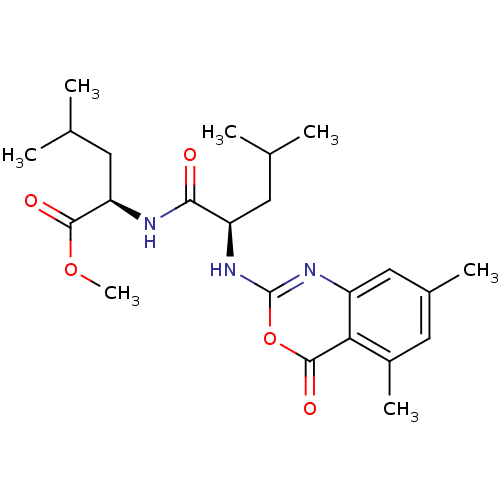 (CHEMBL145568) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | 0.0000372 | 363 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406196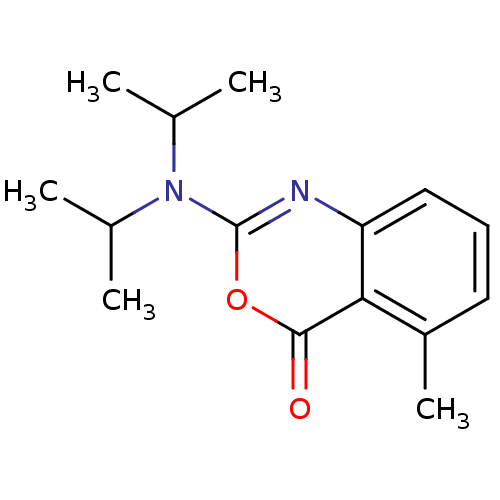 (CHEMBL335798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 398 | n/a | n/a | n/a | n/a | 0.0000389 | 97.7 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406290 (CHEMBL141707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.31 | n/a | n/a | n/a | n/a | 0.0000436 | 7.59E+3 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286333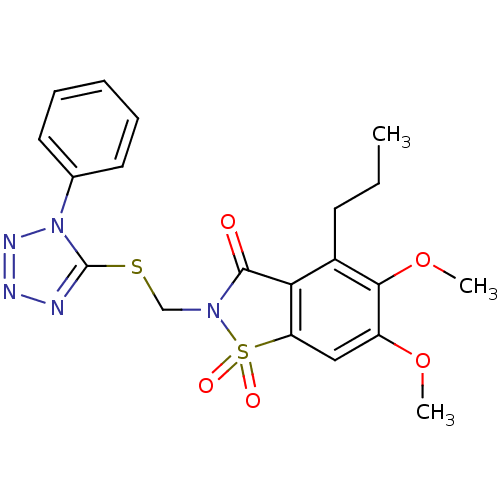 (5,6-Dimethoxy-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000470 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50064317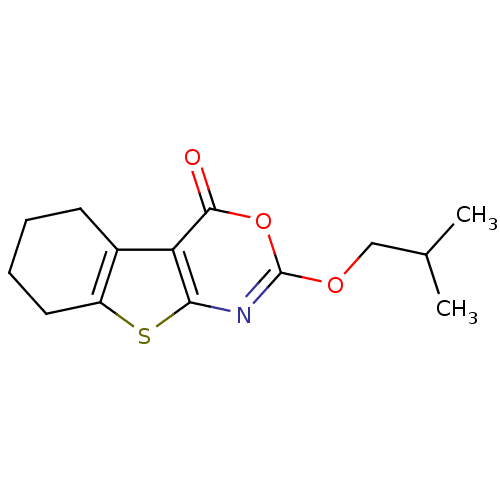 (2-Isobutoxy-5,6,7,8-tetrahydro-benzo[4,5]thieno[2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | 0.0000515 | 1.01E+3 | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Steady state inhibition constant for Human Leukocyte Elastase inhibition | J Med Chem 41: 1729-40 (1998) Article DOI: 10.1021/jm9708341 BindingDB Entry DOI: 10.7270/Q2QV3KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406293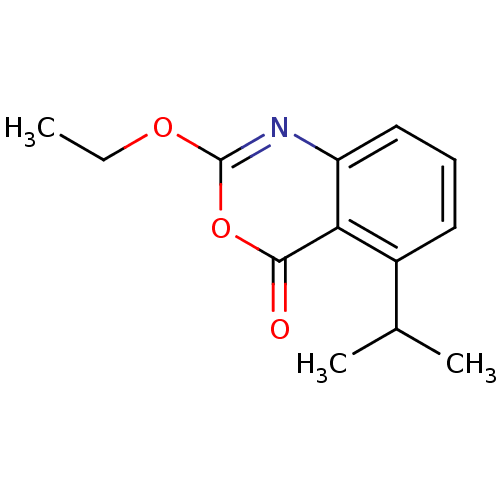 (CHEMBL356120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0710 | n/a | n/a | n/a | n/a | 0.0000550 | 7.76E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50282873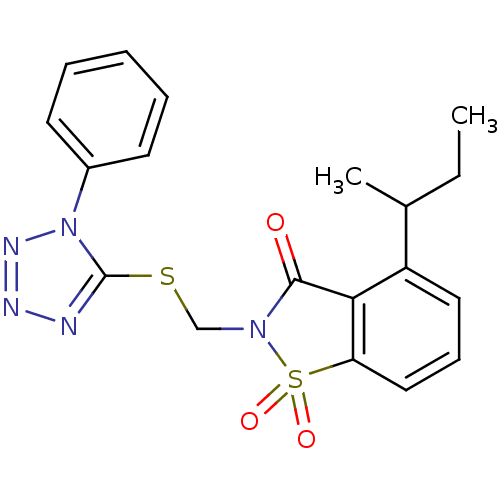 (4-sec-Butyl-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000560 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50282873 (4-sec-Butyl-1,1-dioxo-2-(1-phenyl-1H-tetrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000560 | 9.40E+4 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406285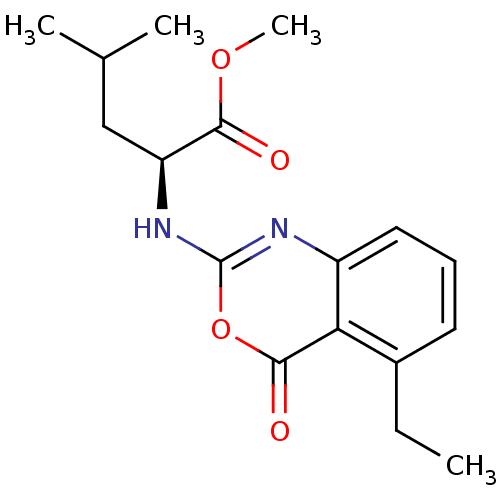 (CHEMBL145583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12.6 | n/a | n/a | n/a | n/a | 0.0000562 | 4.68E+3 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286332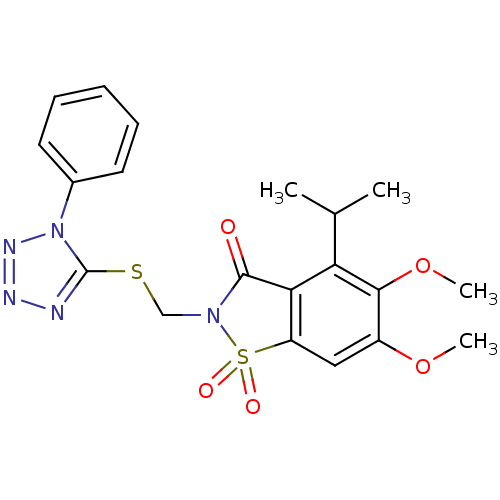 (4-Isopropyl-5,6-dimethoxy-1,1-dioxo-2-(1-phenyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000610 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50064308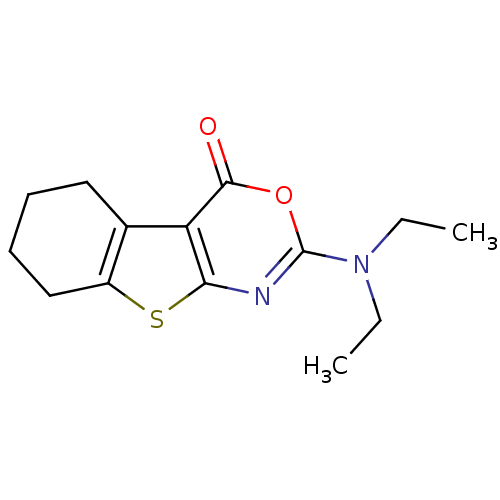 (2-Diethylamino-5,6,7,8-tetrahydro-benzo[4,5]thieno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16.3 | n/a | n/a | n/a | n/a | 0.0000612 | 3.75E+3 | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Steady state inhibition constant for Human Leukocyte Elastase inhibition | J Med Chem 41: 1729-40 (1998) Article DOI: 10.1021/jm9708341 BindingDB Entry DOI: 10.7270/Q2QV3KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50064314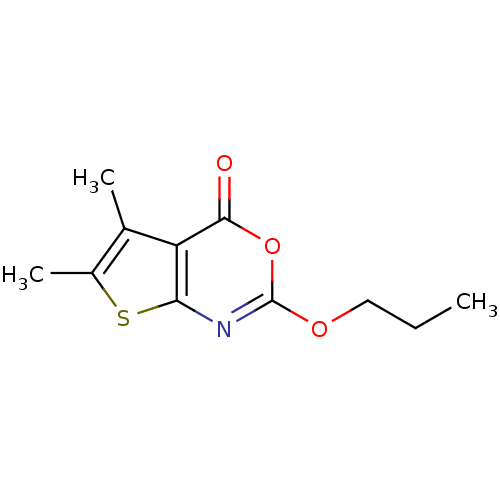 (5,6-Dimethyl-2-propoxy-thieno[2,3-d][1,3]oxazin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.02 | n/a | n/a | n/a | n/a | 0.0000628 | 1.56E+4 | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Steady state inhibition constant for Human Leukocyte Elastase inhibition | J Med Chem 41: 1729-40 (1998) Article DOI: 10.1021/jm9708341 BindingDB Entry DOI: 10.7270/Q2QV3KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291760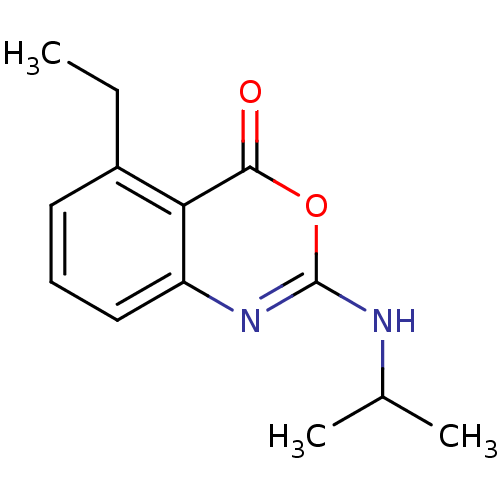 (5-Ethyl-2-isopropylamino-benzo[d][1,3]oxazin-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | 0.0000660 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Rate of deacylation by human leukocyte elastase | J Med Chem 30: 589-591 (1987) Article DOI: 10.1021/jm00387a001 BindingDB Entry DOI: 10.7270/Q2GQ6XRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406234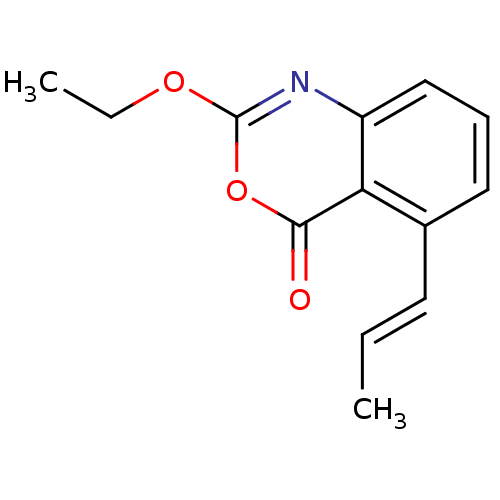 (CHEMBL145774) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | 0.0000661 | 3.47E+5 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291760 (5-Ethyl-2-isopropylamino-benzo[d][1,3]oxazin-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | 0.0000661 | 7.08E+4 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50064323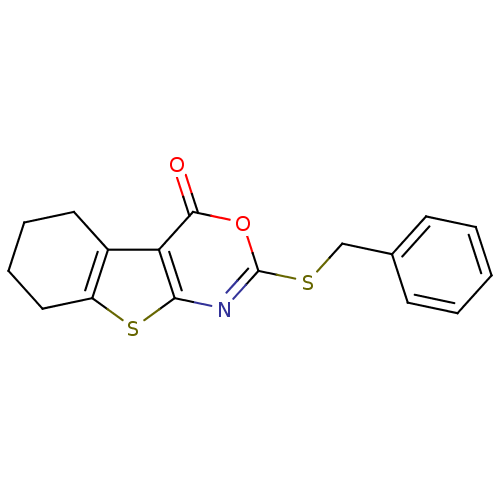 (2-Benzylsulfanyl-5,6,7,8-tetrahydro-benzo[4,5]thie...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | 0.0000692 | 239 | n/a | n/a |
University of Leipzig Curated by ChEMBL | Assay Description Steady state inhibition constant for Human Leukocyte Elastase inhibition | J Med Chem 41: 1729-40 (1998) Article DOI: 10.1021/jm9708341 BindingDB Entry DOI: 10.7270/Q2QV3KNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50282868 (1,1-Dioxo-2-(1-phenyl-1H-tetrazol-5-ylsulfanylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000700 | 1.00E+4 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50282868 (1,1-Dioxo-2-(1-phenyl-1H-tetrazol-5-ylsulfanylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | 0.0000700 | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for inhibitory activity against Human leukocyte elastase (HLE) | Bioorg Med Chem Lett 5: 331-336 (1995) Article DOI: 10.1016/0960-894X(95)00030-W BindingDB Entry DOI: 10.7270/Q2SX6D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 207 total ) | Next | Last >> |