

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291924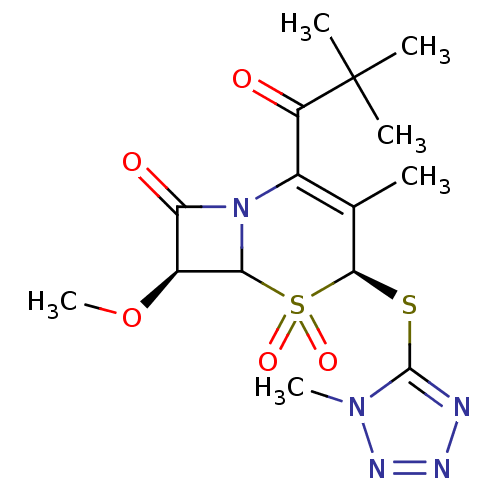 ((4S,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291939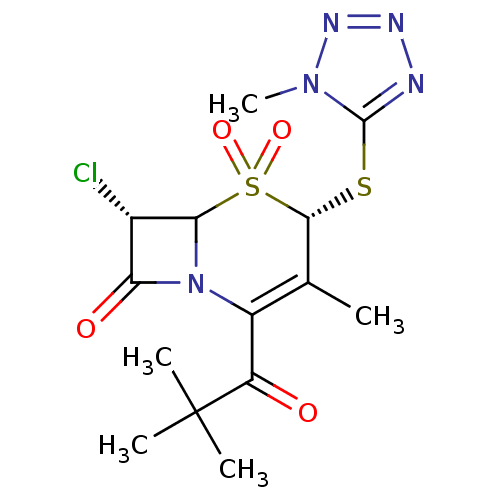 ((4S,7S)-7-Chloro-2-(2,2-dimethyl-propionyl)-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291916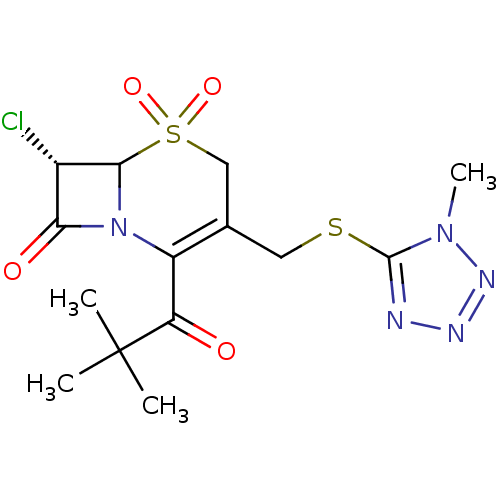 ((S)-7-Chloro-2-(2,2-dimethyl-propionyl)-3-(1-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291921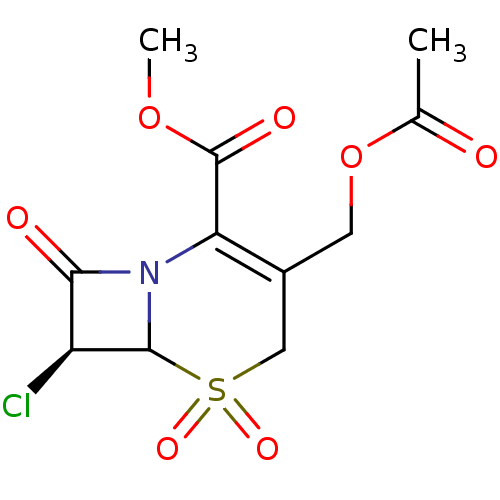 ((S)-3-Acetoxymethyl-7-chloro-5,5,8-trioxo-5lambda*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291928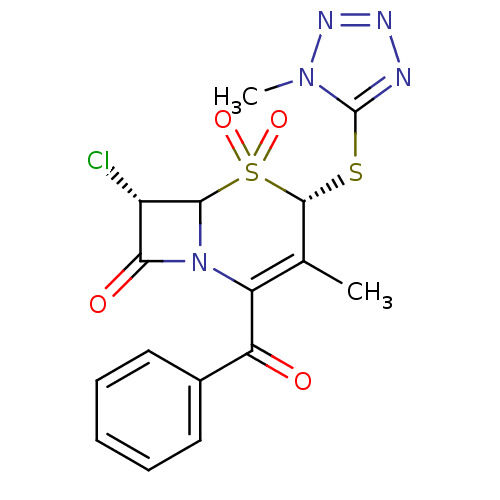 ((4S,7S)-2-Benzoyl-7-chloro-3-methyl-4-(1-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291948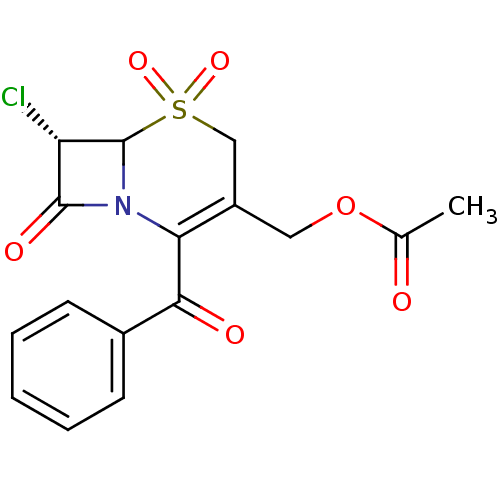 (Acetic acid (S)-2-benzoyl-7-chloro-5,5,8-trioxo-5l...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291927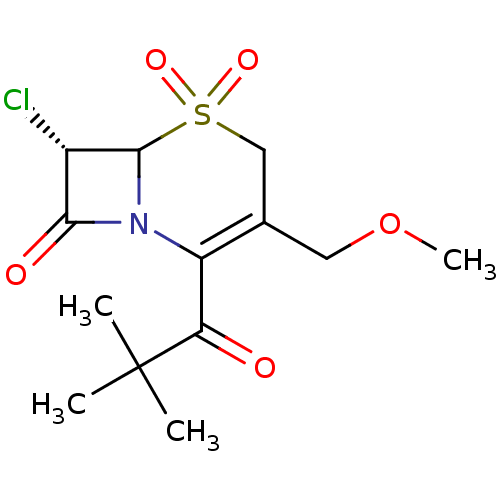 ((S)-7-Chloro-2-(2,2-dimethyl-propionyl)-3-methoxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291918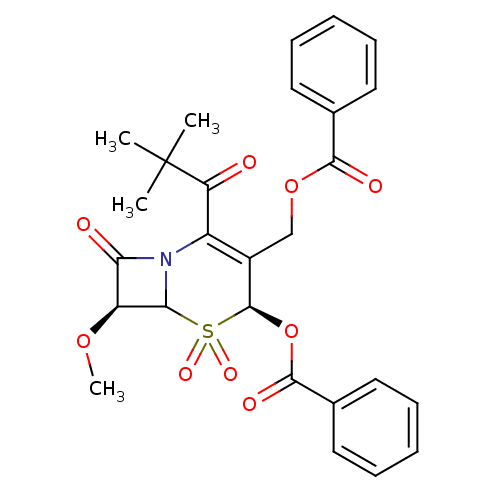 (2alpha-(benzoyloxy)3-[(benzoyloxy)methyl]-4-(tert-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280672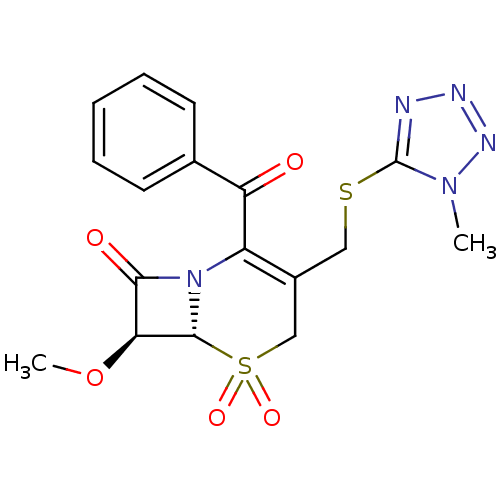 ((6R,7S)-2-Benzoyl-7-methoxy-3-(1-methyl-1H-tetrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.80 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280676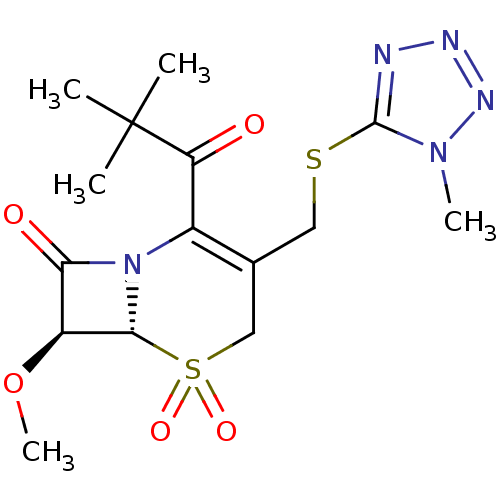 ((6R,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-3-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50015925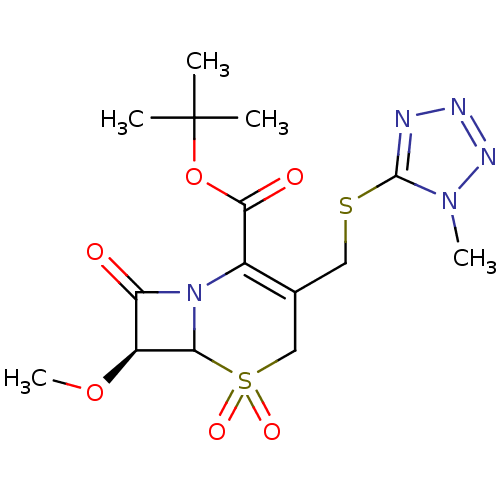 ((S)-7-Methoxy-3-(1-methyl-1H-tetrazol-5-ylsulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291942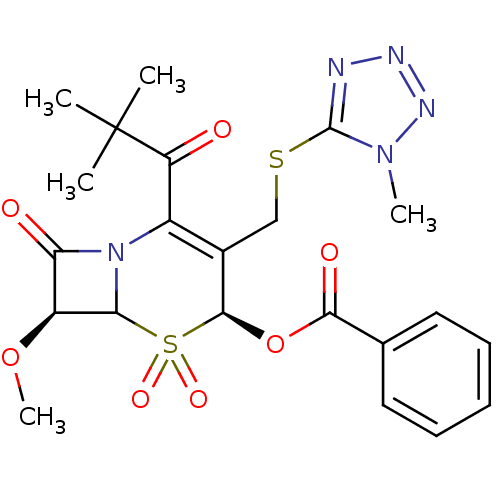 (Benzoic acid (4S,7S)-2-(2,2-dimethyl-propionyl)-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | >2 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291950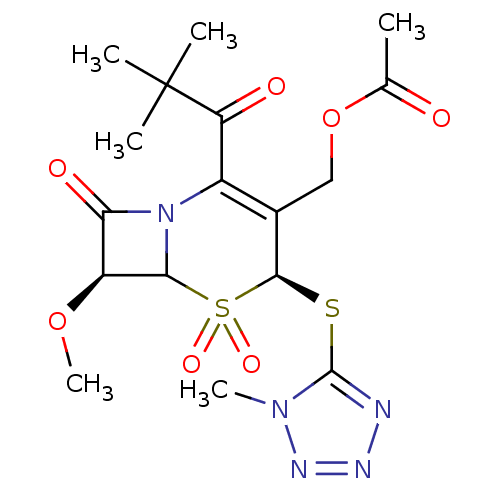 (Acetic acid (4S,7S)-2-(2,2-dimethyl-propionyl)-7-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291931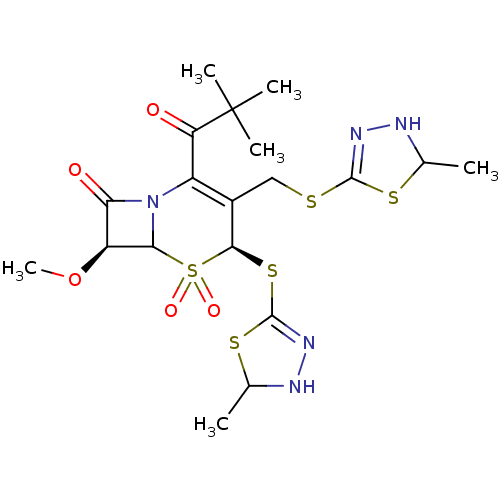 ((4S,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-4-(5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291920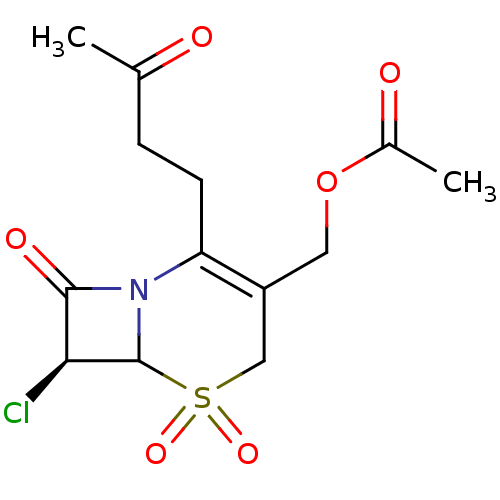 (Acetic acid (S)-7-chloro-5,5,8-trioxo-2-(3-oxo-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291914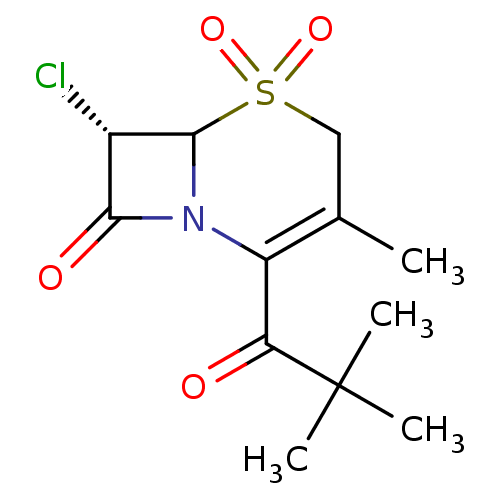 ((S)-7-Chloro-2-(2,2-dimethyl-propionyl)-3-methyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291949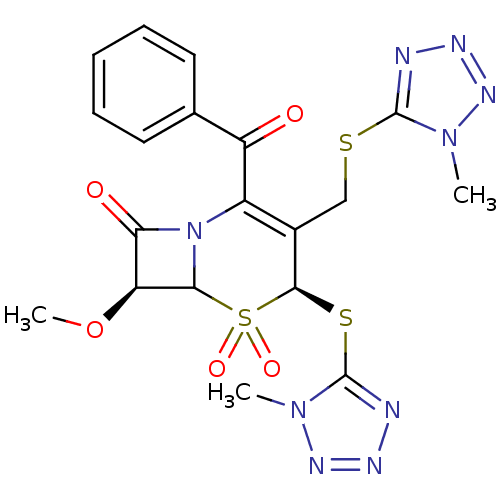 ((4S,7S)-2-Benzoyl-7-methoxy-4-(1-methyl-1H-tetrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291943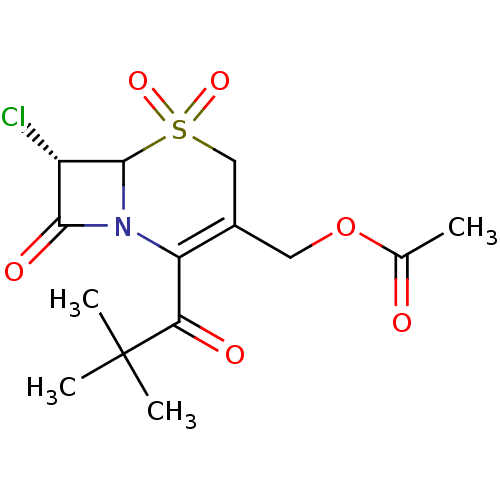 (Acetic acid (S)-7-chloro-2-(2,2-dimethyl-propionyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.30 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291919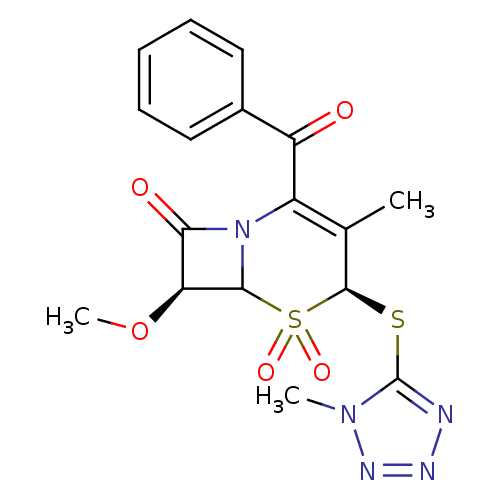 ((4S,7S)-2-Benzoyl-7-methoxy-3-methyl-4-(1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.30 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291923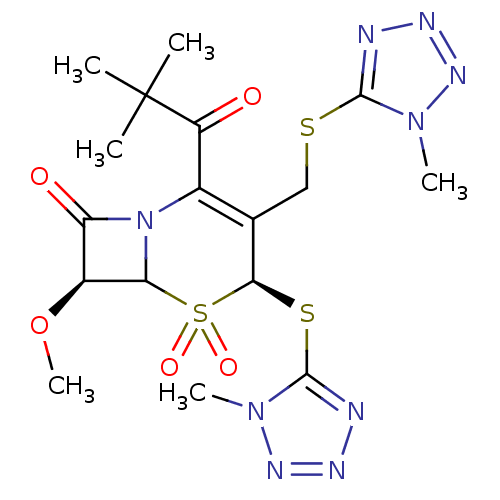 ((4S,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-4-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291929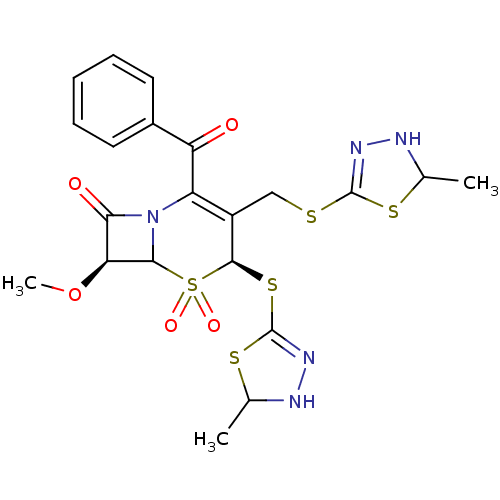 ((4S,7S)-2-Benzoyl-7-methoxy-4-(5-methyl-2,5-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 3.90 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291944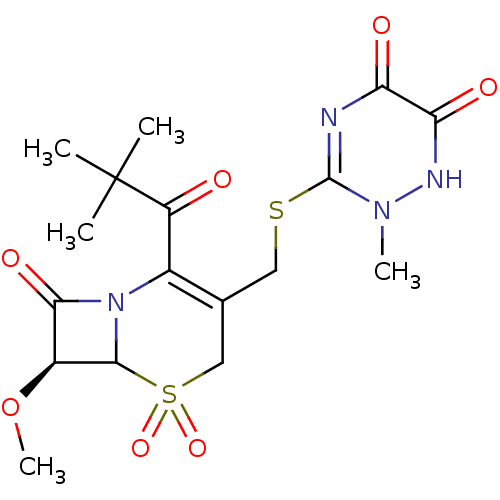 ((S)-2-(2,2-Dimethyl-propionyl)-3-(6-hydroxy-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291937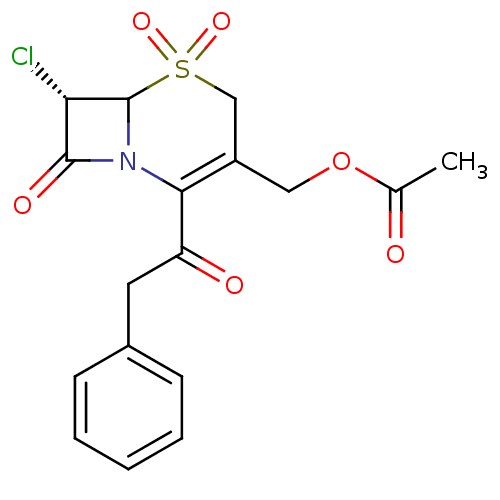 (Acetic acid (S)-7-chloro-5,5,8-trioxo-2-phenylacet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 4.90 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291936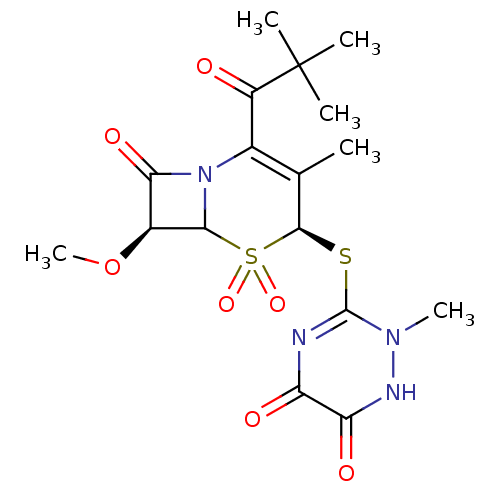 ((4S,7S)-2-(2,2-Dimethyl-propionyl)-4-(6-hydroxy-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 5.30 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50212647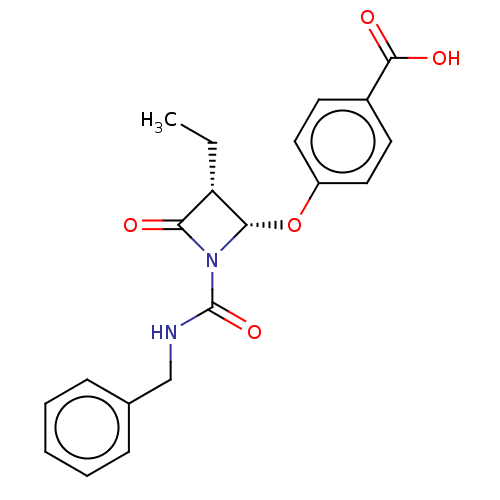 (CHEMBL75236) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | n/a | n/a | n/a | n/a | 5.60 | n/a | n/a | |
TBA | Assay Description Compound was evaluated for the inhibitory activity against Human leukocyte elastase; time-dependent inhibition was not observed | Citation and Details BindingDB Entry DOI: 10.7270/Q2V69MR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291946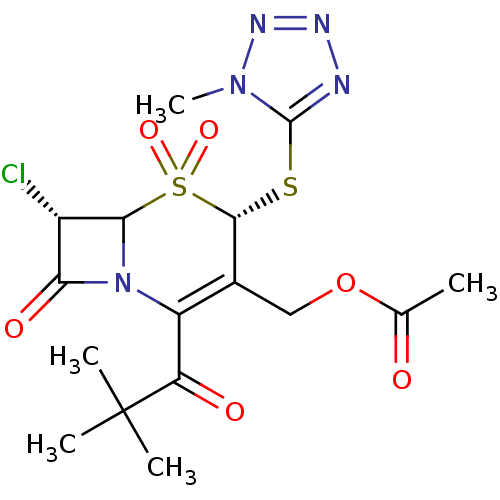 (Acetic acid (4S,7S)-7-chloro-2-(2,2-dimethyl-propi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 5.70 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291935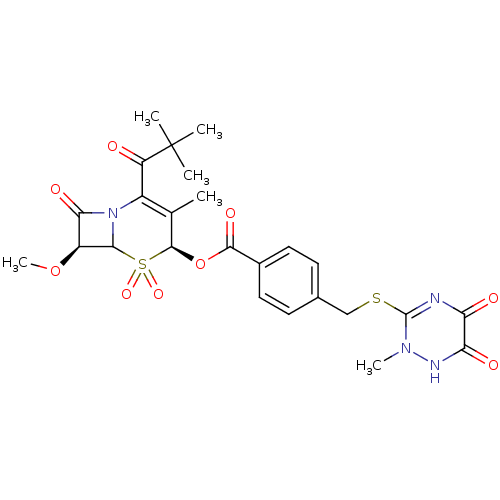 (4-(6-Hydroxy-2-methyl-5-oxo-2,5-dihydro-[1,2,4]tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 7 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291926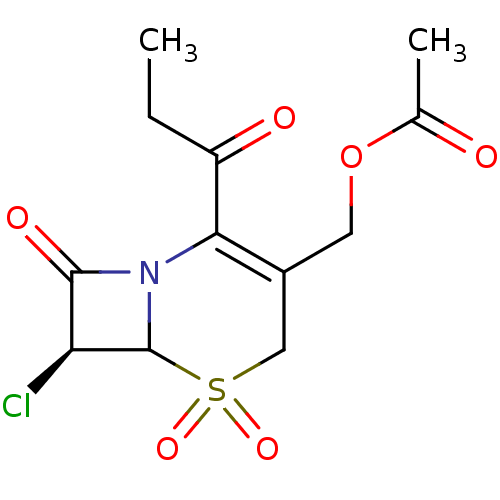 (Acetic acid (S)-7-chloro-5,5,8-trioxo-2-propionyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 7.30 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291945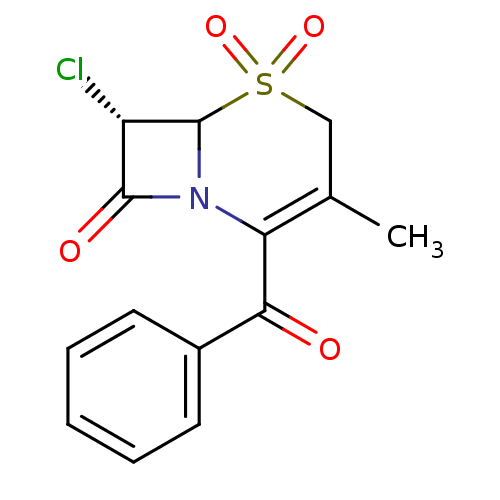 ((S)-2-Benzoyl-7-chloro-3-methyl-5,5-dioxo-5lambda*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 8 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50003159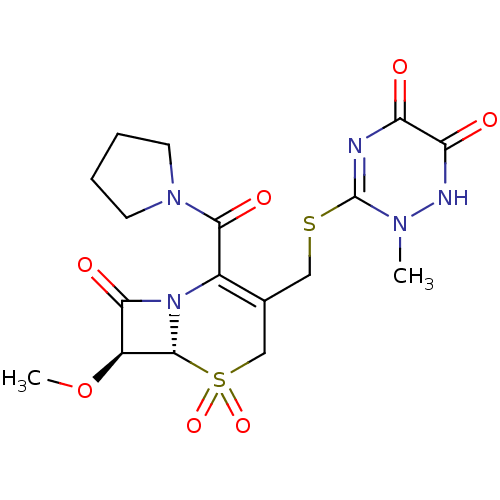 (3-(6-Hydroxy-2-methyl-5-oxo-2,5-dihydro-[1,2,4]tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 9.20 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50291754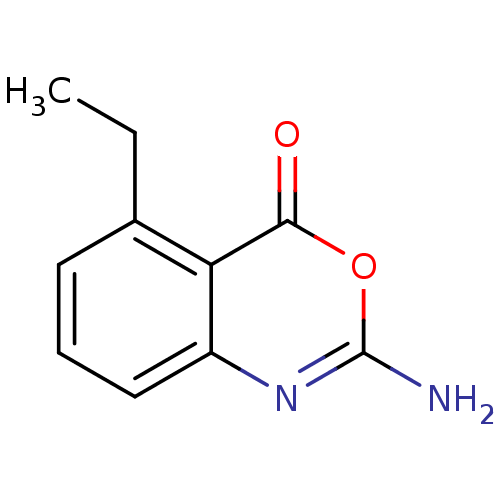 (2-Amino-5-ethyl-benzo[d][1,3]oxazin-4-one | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.01E+3 | n/a | n/a | n/a | n/a | 0.0000776 | 15.1 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406212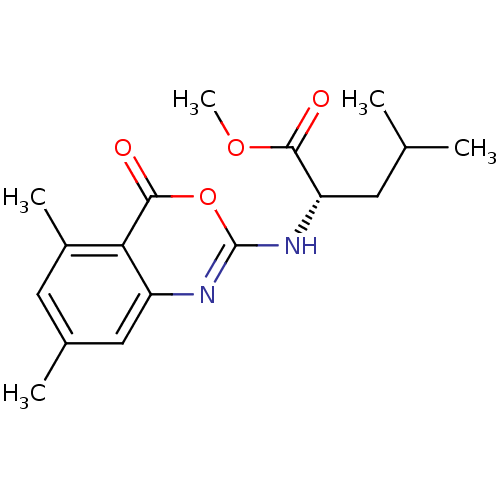 (CHEMBL357238) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | 0.0000200 | 15.8 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406303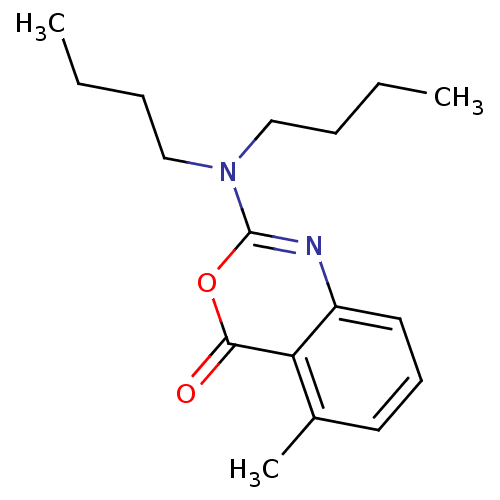 (CHEMBL145690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 398 | n/a | n/a | n/a | n/a | 0.0000240 | 66.1 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406244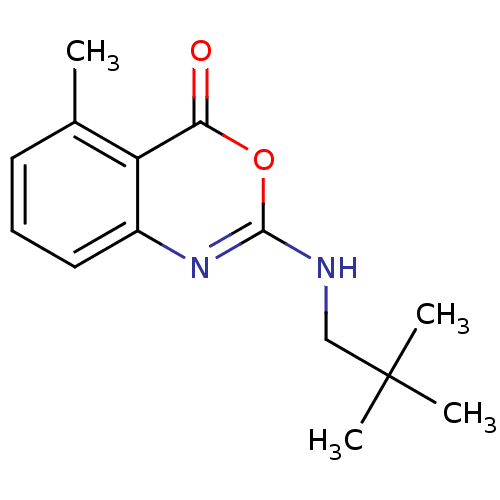 (CHEMBL444082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 251 | n/a | n/a | n/a | n/a | 0.0000182 | 75.9 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50286678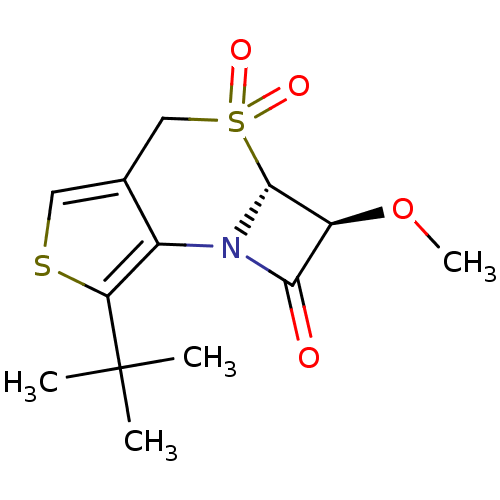 ((2S,2aR)-7-tert-Butyl-2-methoxy-3,3-dioxo-2,2a,3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 80 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) expressed as second order inhibition rate constant | Bioorg Med Chem Lett 5: 691-694 (1995) Article DOI: 10.1016/0960-894X(95)00095-B BindingDB Entry DOI: 10.7270/Q2Q2407G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280682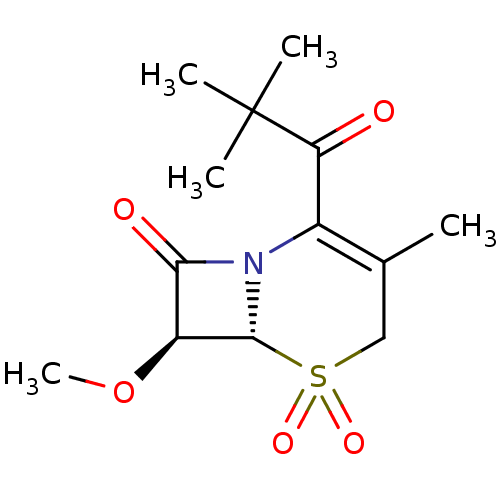 ((6R,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 90 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280682 ((6R,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 90 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase II (HLE-II) as second order rate constant | Bioorg Med Chem Lett 2: 1127-1132 (1992) Article DOI: 10.1016/S0960-894X(00)80632-0 BindingDB Entry DOI: 10.7270/Q2HT2P7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280682 ((6R,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 90 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) expressed as second order inhibition rate constant | Bioorg Med Chem Lett 5: 687-690 (1995) Article DOI: 10.1016/0960-894X(95)00094-A BindingDB Entry DOI: 10.7270/Q2TT4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280682 ((6R,7S)-2-(2,2-Dimethyl-propionyl)-7-methoxy-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 90 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) expressed as second order inhibition rate constant | Bioorg Med Chem Lett 5: 691-694 (1995) Article DOI: 10.1016/0960-894X(95)00095-B BindingDB Entry DOI: 10.7270/Q2Q2407G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406196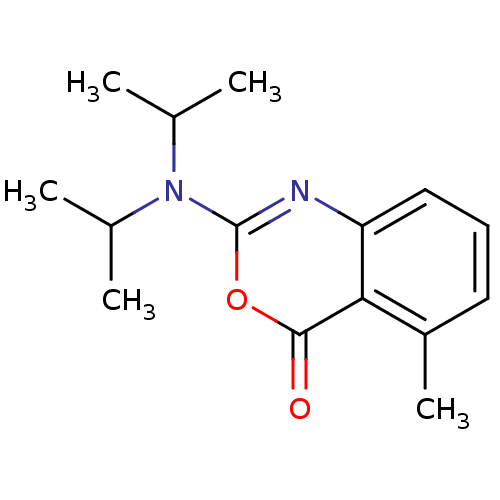 (CHEMBL335798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 398 | n/a | n/a | n/a | n/a | 0.0000389 | 97.7 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406254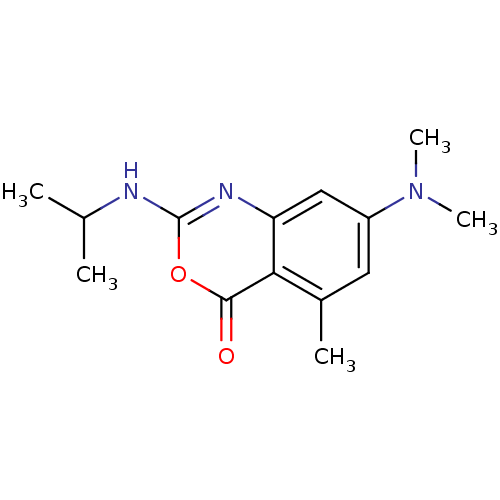 (CHEMBL344969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | 0.000129 | 102 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406210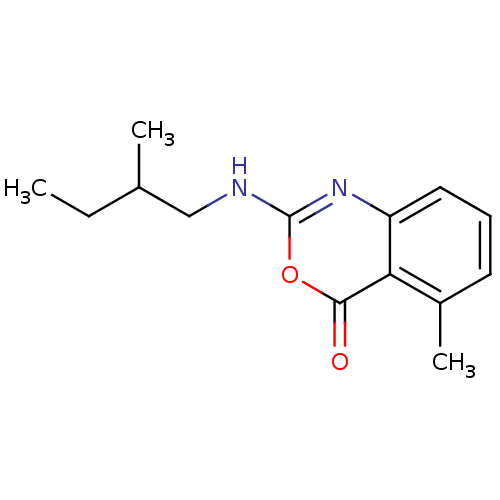 (CHEMBL443745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 631 | n/a | n/a | n/a | n/a | 0.0000741 | 112 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281004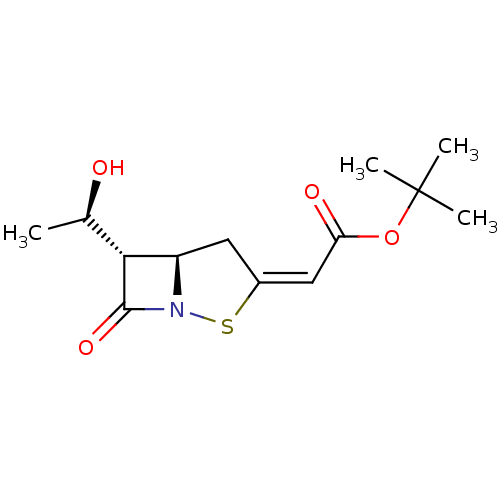 (CHEMBL419820 | [(5R,6S)-6-((R)-1-Hydroxy-ethyl)-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | 130 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Rate constant for the compound was determined (k2/ki) against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 3: 2271-2276 (1993) Article DOI: 10.1016/S0960-894X(01)80938-0 BindingDB Entry DOI: 10.7270/Q21N812T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281004 (CHEMBL419820 | [(5R,6S)-6-((R)-1-Hydroxy-ethyl)-7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | n/a | n/a | n/a | n/a | 130 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Rate constant for the compound was determined (k2/ki) against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 3: 2271-2276 (1993) Article DOI: 10.1016/S0960-894X(01)80938-0 BindingDB Entry DOI: 10.7270/Q21N812T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280675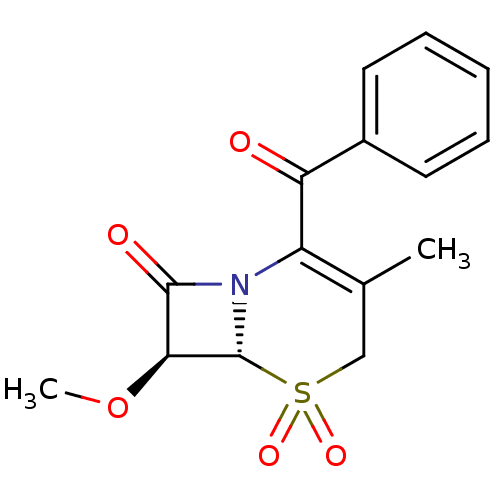 ((6R,7S)-2-Benzoyl-7-methoxy-3-methyl-5,5-dioxo-5la...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 130 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) expressed as second order inhibition rate constant | Bioorg Med Chem Lett 5: 687-690 (1995) Article DOI: 10.1016/0960-894X(95)00094-A BindingDB Entry DOI: 10.7270/Q2TT4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280675 ((6R,7S)-2-Benzoyl-7-methoxy-3-methyl-5,5-dioxo-5la...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 130 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) expressed as second order inhibition rate constant | Bioorg Med Chem Lett 5: 691-694 (1995) Article DOI: 10.1016/0960-894X(95)00095-B BindingDB Entry DOI: 10.7270/Q2Q2407G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280675 ((6R,7S)-2-Benzoyl-7-methoxy-3-methyl-5,5-dioxo-5la...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 130 | n/a | n/a |
Pharmacia Farmitalia Carlo Erba Curated by ChEMBL | Assay Description Tested for the rate constant against Human Leukocyte Elastase (HLE) inactivation | J Med Chem 37: 4003-19 (1994) BindingDB Entry DOI: 10.7270/Q2T43S5B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50280675 ((6R,7S)-2-Benzoyl-7-methoxy-3-methyl-5,5-dioxo-5la...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 130 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase II (HLE-II) as second order rate constant | Bioorg Med Chem Lett 2: 1127-1132 (1992) Article DOI: 10.1016/S0960-894X(00)80632-0 BindingDB Entry DOI: 10.7270/Q2HT2P7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50406306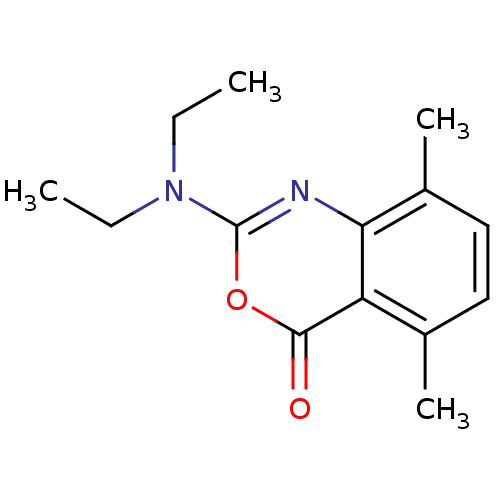 (CHEMBL145107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | 0.0000138 | 132 | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Component deacylation rate constant for interaction with human leukocyte elastase | J Med Chem 33: 464-79 (1990) Checked by Author BindingDB Entry DOI: 10.7270/Q2PR7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50281009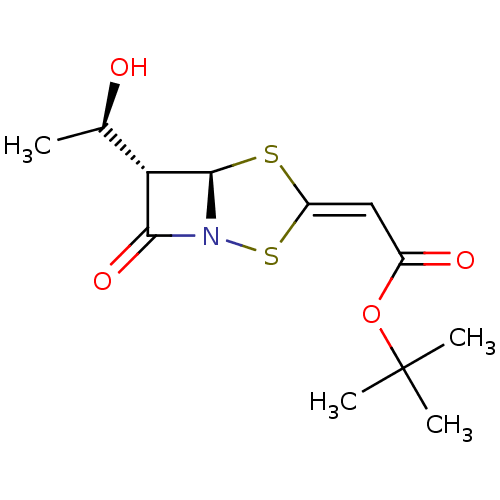 (CHEMBL74813 | [(5R,6S)-6-((R)-1-Hydroxy-ethyl)-7-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | n/a | n/a | 150 | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Rate constant for the compound was determined (k2/ki) against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 3: 2271-2276 (1993) Article DOI: 10.1016/S0960-894X(01)80938-0 BindingDB Entry DOI: 10.7270/Q21N812T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 318 total ) | Next | Last >> |