

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510776 (CHEMBL4438526) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) | Bioorg Med Chem Lett 29: 761-777 (2019) Article DOI: 10.1016/j.bmcl.2018.12.049 BindingDB Entry DOI: 10.7270/Q2S185SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353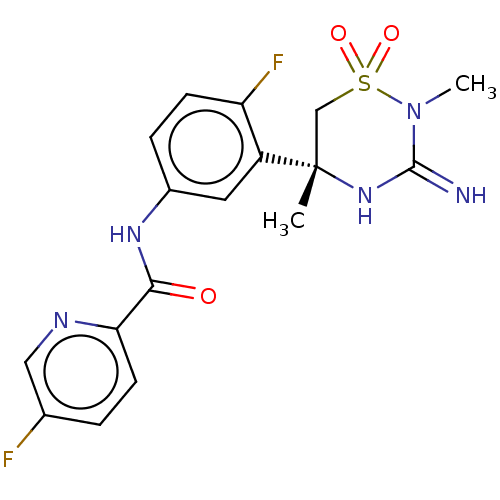 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM150688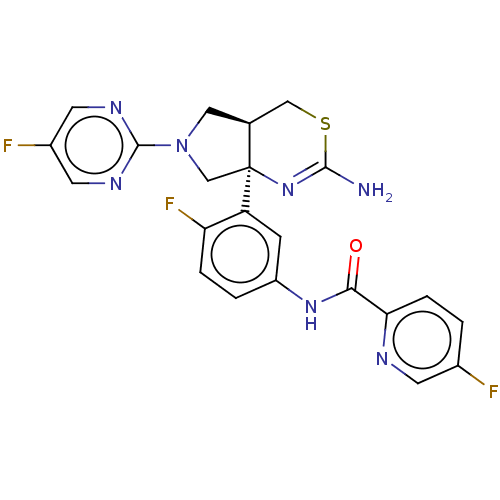 (US8987254, 3 | US9999624, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.388 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510816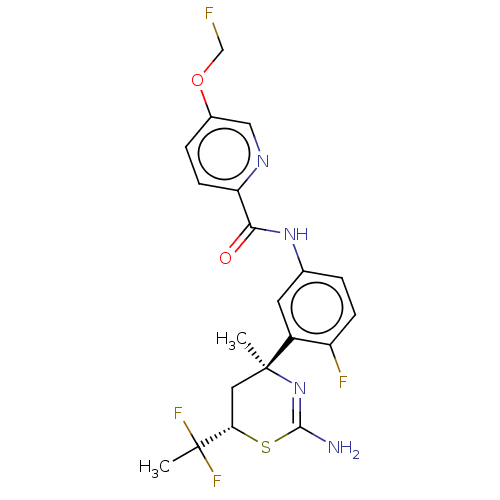 (CHEMBL4475802) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) | Bioorg Med Chem Lett 29: 761-777 (2019) Article DOI: 10.1016/j.bmcl.2018.12.049 BindingDB Entry DOI: 10.7270/Q2S185SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM150693 (US8987254, 8 | US9999624, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.555 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510987 (CHEMBL4472708) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510991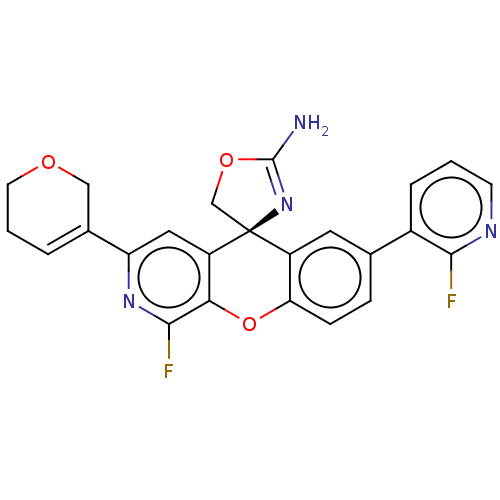 (CHEMBL4564735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM400979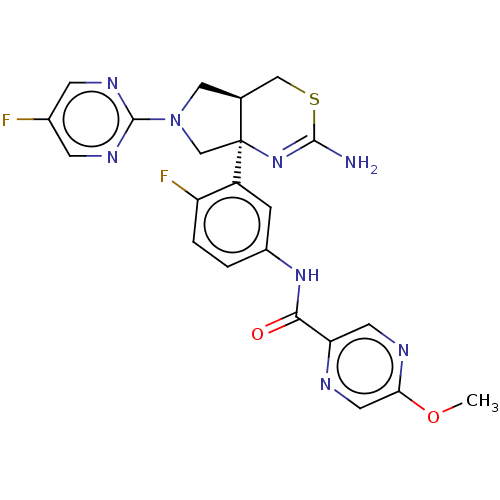 (US9999624, Compound 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00489 BindingDB Entry DOI: 10.7270/Q29W0KBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50452874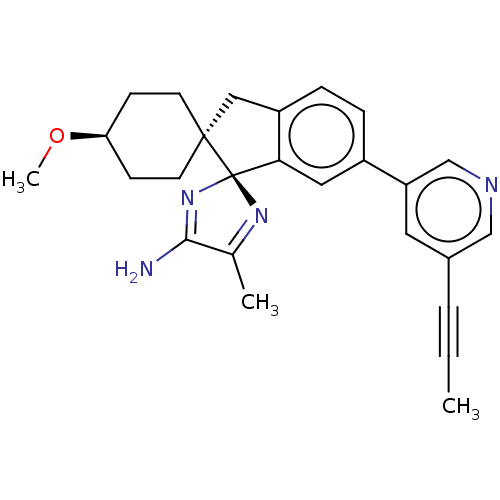 (CHEMBL4217620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50398264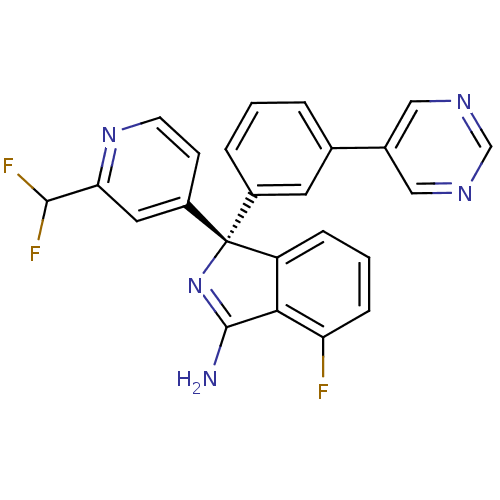 (CHEMBL2177913) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition measured after 6.... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM116254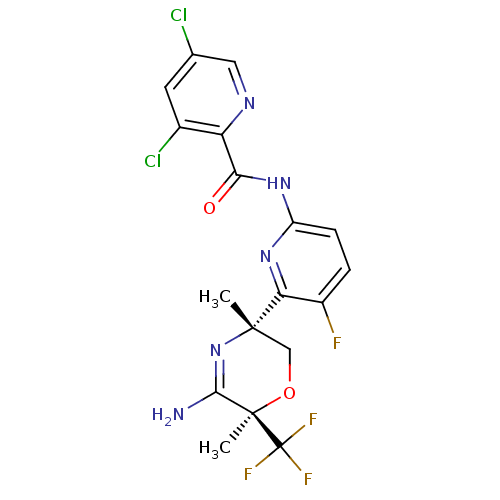 (US10035794, Example 31 | US10683287, Example 31 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate with BACE-cleavable sequence | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01300 BindingDB Entry DOI: 10.7270/Q27D302Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM124132 (US8754075, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM116254 (US10035794, Example 31 | US10683287, Example 31 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Novartis AG US Patent | Assay Description Recombinant BACE-2 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... | US Patent US8637508 (2014) BindingDB Entry DOI: 10.7270/Q2JD4VFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Rattus norvegicus) | BDBM50012639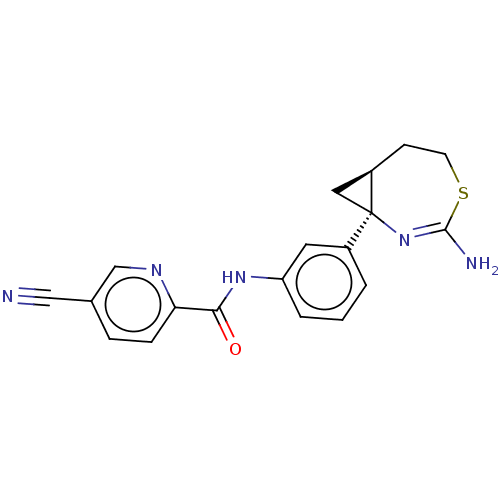 (CHEMBL3261053) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE2 in rat INS-1E cells assessed as TMEM27 cleavage preincubated for 2 hrs followed by doxycycline addition measured after 46 hrs by ... | Bioorg Med Chem Lett 24: 2033-45 (2014) Article DOI: 10.1016/j.bmcl.2014.03.025 BindingDB Entry DOI: 10.7270/Q2H41T0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM116254 (US10035794, Example 31 | US10683287, Example 31 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 4.5 | 25 |
Novartis AG US Patent | Assay Description Recombinant BACE-2 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... | US Patent US10035794 (2018) BindingDB Entry DOI: 10.7270/Q2RB76M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Rattus norvegicus) | BDBM50428341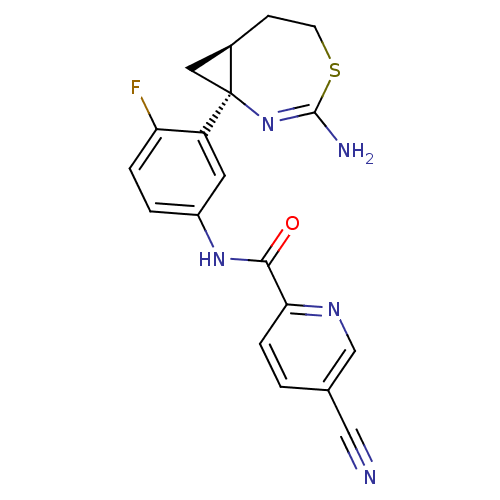 (CHEMBL2331713) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of BACE2-mediated TMEM27 cleavage in rat INS-1E cells expressing doxycycline-dependent human full-length TMEM27 by ELISA | ACS Med Chem Lett 4: 379-80 (2013) Article DOI: 10.1021/ml400060e BindingDB Entry DOI: 10.7270/Q2Z039GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510775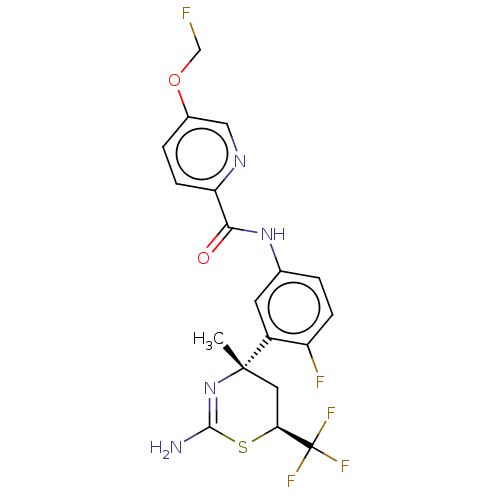 (CHEMBL4445405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) | Bioorg Med Chem Lett 29: 761-777 (2019) Article DOI: 10.1016/j.bmcl.2018.12.049 BindingDB Entry DOI: 10.7270/Q2S185SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM116254 (US10035794, Example 31 | US10683287, Example 31 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Recombinant BACE-2 (extracellular domain, expressed in baculovirus and purified using standard methods) at 0.1 to 10 nM concentrations is incubated w... | US Patent US10683287 (2020) BindingDB Entry DOI: 10.7270/Q2DZ0CCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM124141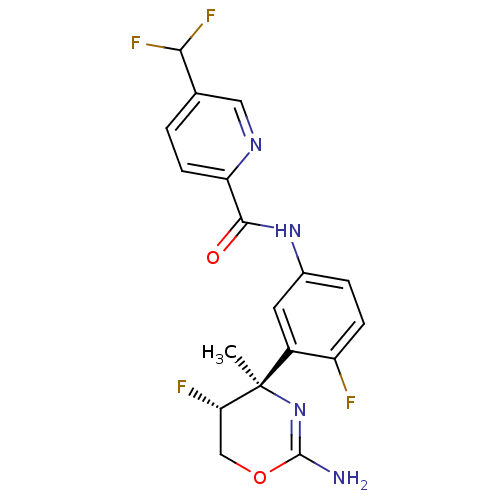 (US8754075, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay uses the principle of inhibition of human TMEM27 cleavage by endogenous cellular BACE2 in the Ins1e rat cell line and shedding from the cel... | US Patent US8754075 (2014) BindingDB Entry DOI: 10.7270/Q2FX784Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50559903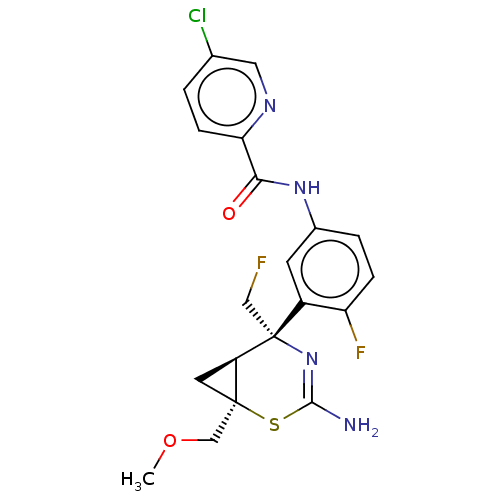 (CHEMBL4760131) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BACE2 using fluorescence substrate preincubated for 60 mins followed by substrate addition and measured after 60 mins... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127240 BindingDB Entry DOI: 10.7270/Q2Z323C8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM589168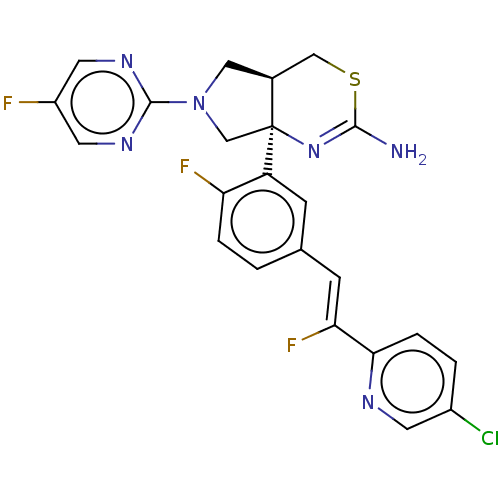 (US11548903, Example 101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The cDNAs for both human recombinant BACE1 and 2 with C-terminal 6-His Tags were cloned into transient protein expression vectors, which were subsequ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP2882 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510974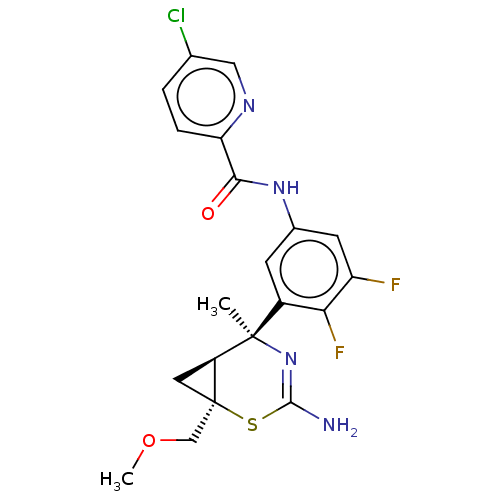 (CHEMBL4456863) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM372731 (6-((Z)-2-(3-((1S,5S,6S)-3-amino-1-((4aS,8aR)-decah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton | J Med Chem 49: 6652-5 (2006) BindingDB Entry DOI: 10.7270/Q23J3G8K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM589202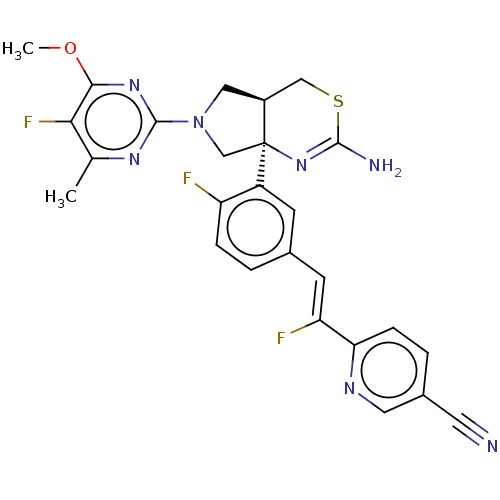 (US11548903, Example 130) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The cDNAs for both human recombinant BACE1 and 2 with C-terminal 6-His Tags were cloned into transient protein expression vectors, which were subsequ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP2882 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510973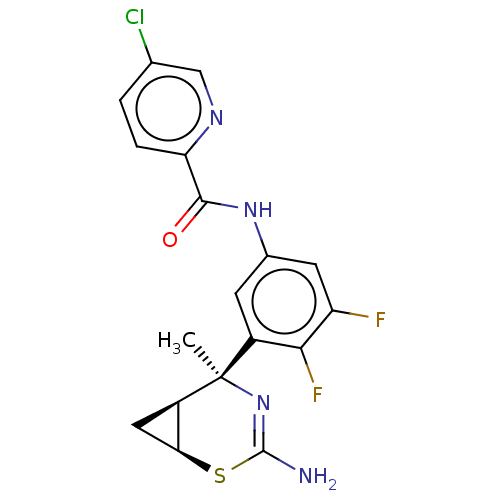 (CHEMBL4460278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510973 (CHEMBL4460278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged BACE2 expressed in mammalian expression system using FRET peptide substrate preincubated for 1... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM338840 (N-{3-[(6R)-4-Amino-6-methyl-6,7-dihydro[1,2,3]tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description This assay is a Fluorescence Resonance Energy Transfer Assay (FRET) based assay. The substrate for this assay contains the ┐Swedish┐ Lys-Met/Asn-Leu ... | US Patent US9751886 (2017) BindingDB Entry DOI: 10.7270/Q2Z321RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BACE2 (unknown origin) using APP harboring Swedish Lys/Met mutant-derived peptide as substrate incubated for 2 hrs by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01858 BindingDB Entry DOI: 10.7270/Q2GH9NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50587074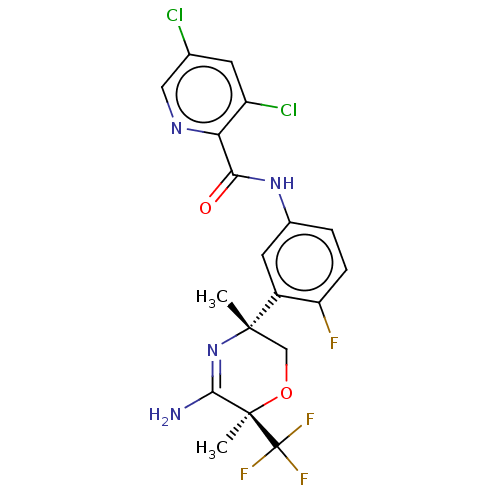 (CHEMBL5085959) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Rattus norvegicus) | BDBM50428343 (CHEMBL2331708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE2 in rat INS-1E cells assessed as TMEM27 cleavage preincubated for 2 hrs followed by doxycycline addition measured after 46 hrs by ... | Bioorg Med Chem Lett 24: 2033-45 (2014) Article DOI: 10.1016/j.bmcl.2014.03.025 BindingDB Entry DOI: 10.7270/Q2H41T0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM372698 ((1S,5S,6S)-3-amino-5-(5-((Z)-2-(5-chloropyridin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton | J Med Chem 49: 6652-5 (2006) BindingDB Entry DOI: 10.7270/Q23J3G8K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Rattus norvegicus) | BDBM50428343 (CHEMBL2331708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of BACE2-mediated TMEM27 cleavage in rat INS-1E cells expressing doxycycline-dependent human full-length TMEM27 by ELISA | ACS Med Chem Lett 4: 379-80 (2013) Article DOI: 10.1021/ml400060e BindingDB Entry DOI: 10.7270/Q2Z039GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Rattus norvegicus) | BDBM50428340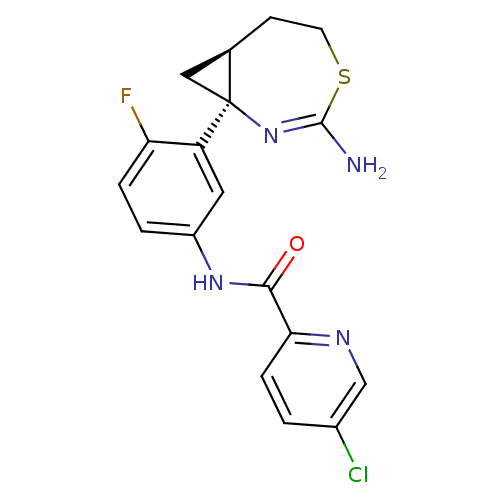 (CHEMBL2331712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE2 in rat INS-1E cells assessed as TMEM27 cleavage preincubated for 2 hrs followed by doxycycline addition measured after 46 hrs by ... | Bioorg Med Chem Lett 24: 2033-45 (2014) Article DOI: 10.1016/j.bmcl.2014.03.025 BindingDB Entry DOI: 10.7270/Q2H41T0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM501313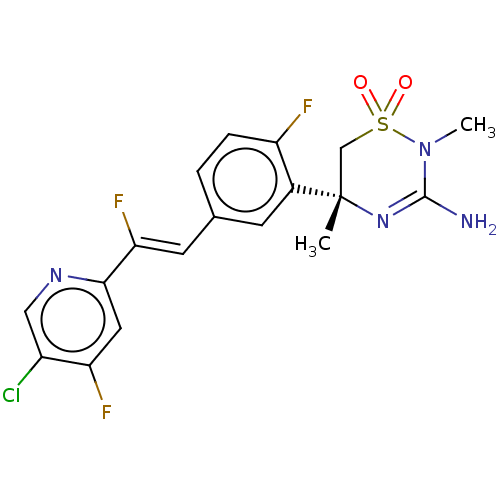 (US11021493, Example 232) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. US Patent | Assay Description The cDNAs for both human recombinant BACE1 and 2 with C-terminal 6-His Tags were cloned into transient protein expression vectors, which were subsequ... | US Patent US11021493 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM501185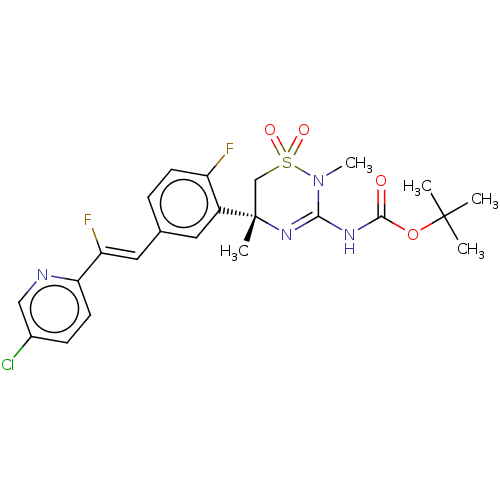 (US11021493, Example 104) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. US Patent | Assay Description The cDNAs for both human recombinant BACE1 and 2 with C-terminal 6-His Tags were cloned into transient protein expression vectors, which were subsequ... | US Patent US11021493 (2021) BindingDB Entry DOI: 10.7270/Q2CZ3B90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510978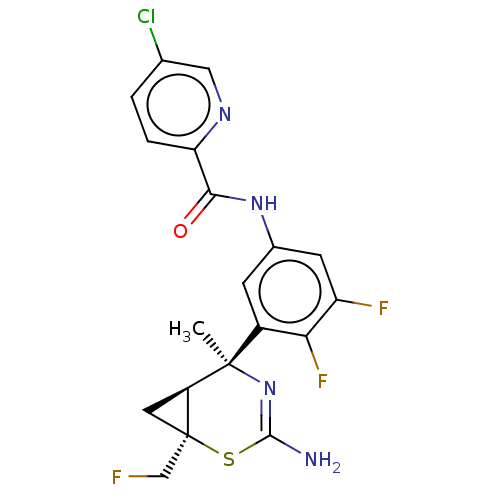 (CHEMBL4539909) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510980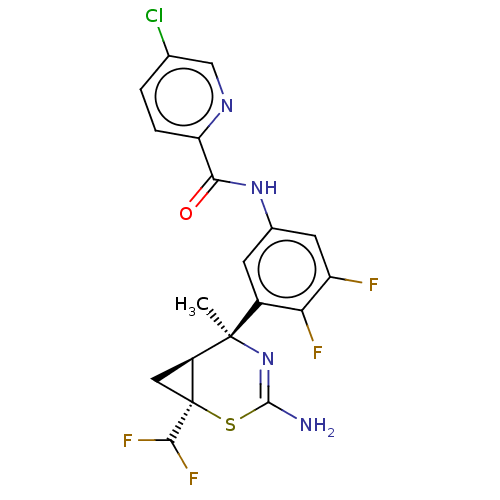 (CHEMBL4457316) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510981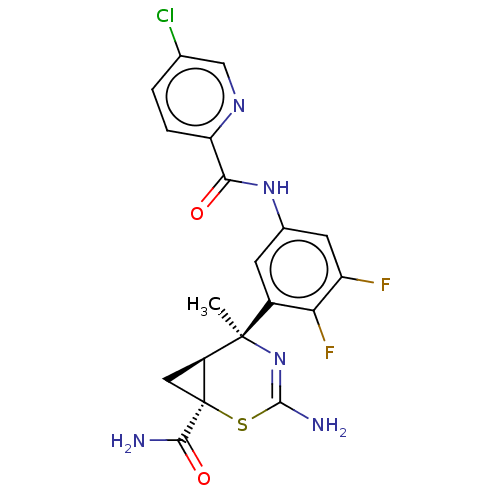 (CHEMBL4468455) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510982 (CHEMBL4473740) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510992 (CHEMBL4471141) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510983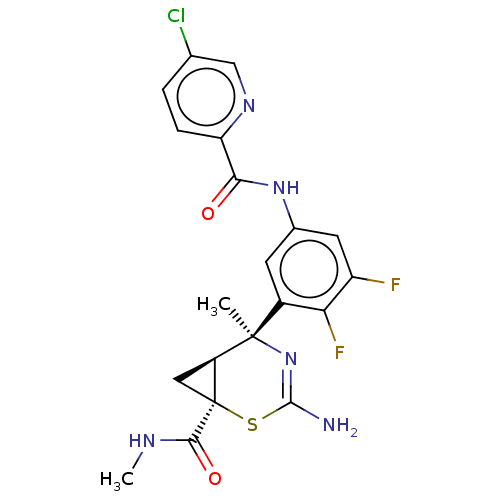 (CHEMBL4475533) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50510979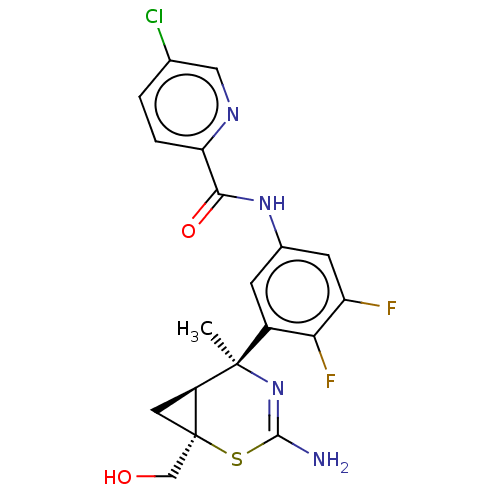 (CHEMBL4575752) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of BACE2 (unknown origin) using FRET peptide substrate preincubated for 1 hr followed by substrate addition and measured after 1 hr by FRE... | J Med Chem 63: 2263-2281 (2020) Article DOI: 10.1021/acs.jmedchem.9b01034 BindingDB Entry DOI: 10.7270/Q2ZK5M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM372804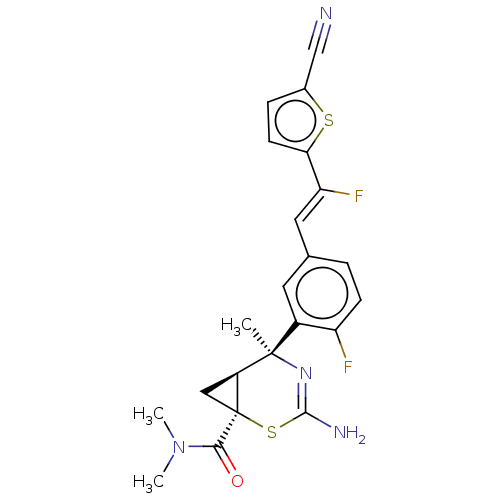 ((1S,5S,6S)-3-amino-5-(5-((Z)-2-(5-cyanothiophen-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton | J Med Chem 49: 6652-5 (2006) BindingDB Entry DOI: 10.7270/Q23J3G8K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Rattus norvegicus) | BDBM50428340 (CHEMBL2331712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of BACE2-mediated TMEM27 cleavage in rat INS-1E cells expressing doxycycline-dependent human full-length TMEM27 by ELISA | ACS Med Chem Lett 4: 379-80 (2013) Article DOI: 10.1021/ml400060e BindingDB Entry DOI: 10.7270/Q2Z039GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Rattus norvegicus) | BDBM50428342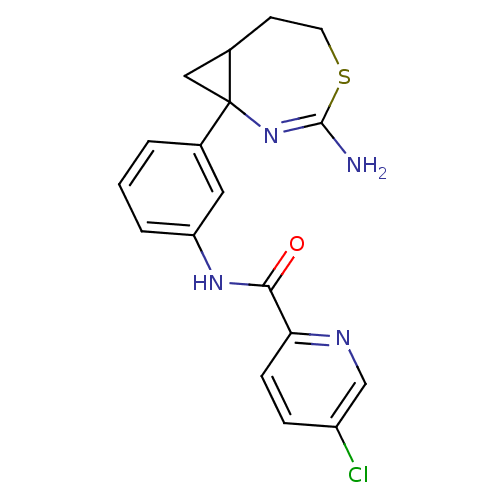 (CHEMBL2331710) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of BACE2-mediated TMEM27 cleavage in rat INS-1E cells expressing doxycycline-dependent human full-length TMEM27 by ELISA | ACS Med Chem Lett 4: 379-80 (2013) Article DOI: 10.1021/ml400060e BindingDB Entry DOI: 10.7270/Q2Z039GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM372735 (6-((Z)-2-(3-((1S,5S,6S)-3-amino-5-methyl-1-(4-(tri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton | J Med Chem 49: 6652-5 (2006) BindingDB Entry DOI: 10.7270/Q23J3G8K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM338839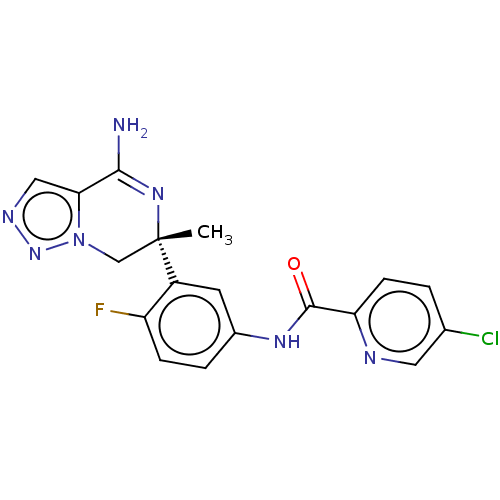 (US9751886, Compound 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description This assay is a Fluorescence Resonance Energy Transfer Assay (FRET) based assay. The substrate for this assay contains the ┐Swedish┐ Lys-Met/Asn-Leu ... | US Patent US9751886 (2017) BindingDB Entry DOI: 10.7270/Q2Z321RC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM589185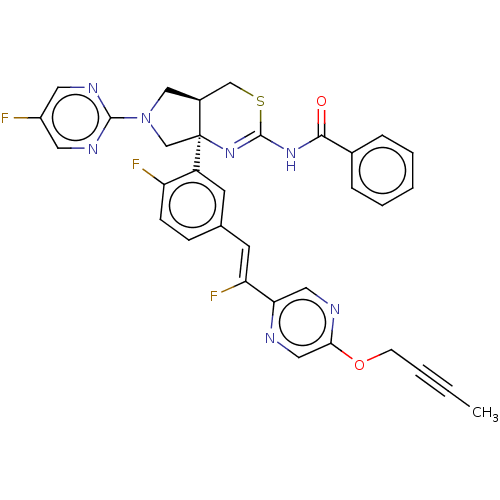 (US11548903, Example 117) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The cDNAs for both human recombinant BACE1 and 2 with C-terminal 6-His Tags were cloned into transient protein expression vectors, which were subsequ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NP2882 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM372704 ((1S,5S,6S)-3-amino-5-(5-((Z)-2-(5-bromopyridin-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.92 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton | J Med Chem 49: 6652-5 (2006) BindingDB Entry DOI: 10.7270/Q23J3G8K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM134356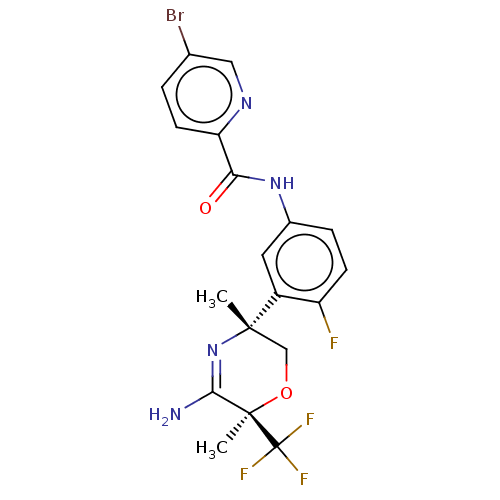 (US8846658, 68) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant BACE2 catalytic domain using FRET substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02143 BindingDB Entry DOI: 10.7270/Q20P13X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1705 total ) | Next | Last >> |