

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28428 ((2R,3R,4S,5R)-2-(6-amino-8-methyl-9H-purin-9-yl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28421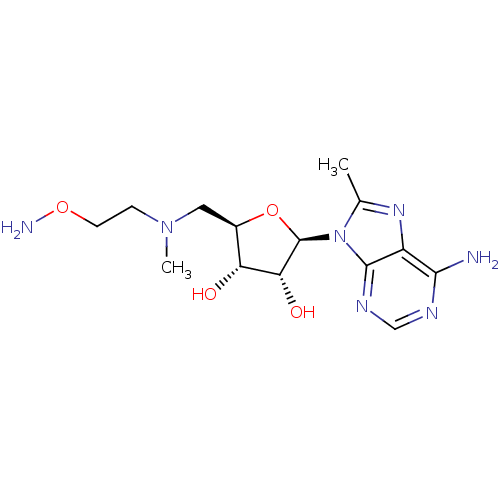 ((2R,3R,4S,5R)-2-(6-amino-8-methyl-9H-purin-9-yl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28427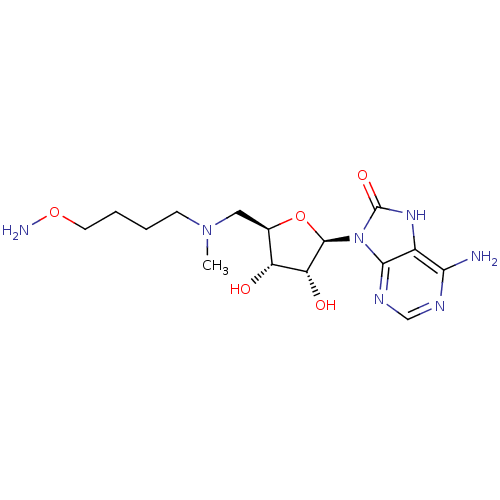 ((2R,3R,4S,5R)-2-(6-amino-8-hydroxy-9H-purin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28429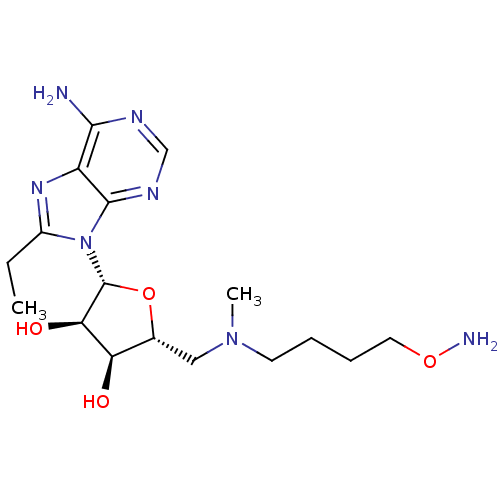 ((2R,3R,4S,5R)-2-(6-amino-8-ethyl-9H-purin-9-yl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28430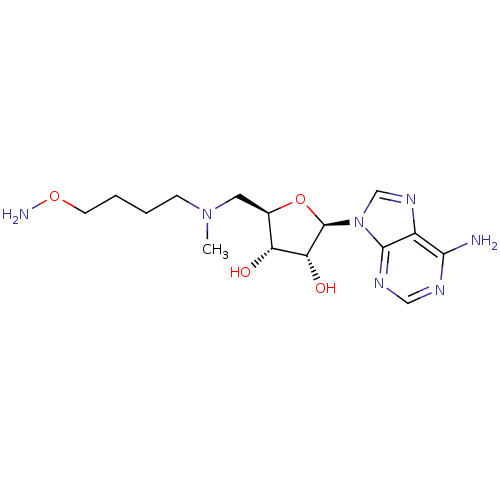 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-({[4-(am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28425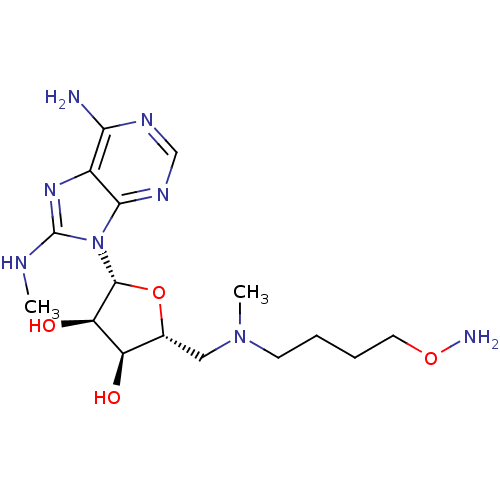 ((2R,3R,4S,5R)-2-[6-amino-8-(methylamino)-9H-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28431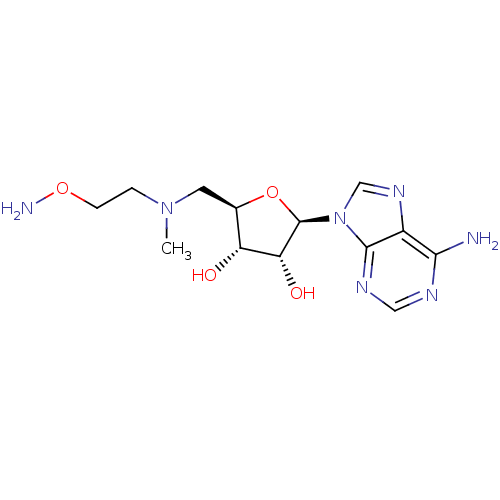 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-({[2-(am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28423 ((2R,3R,4S,5R)-2-[6-amino-8-(methylamino)-9H-purin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28436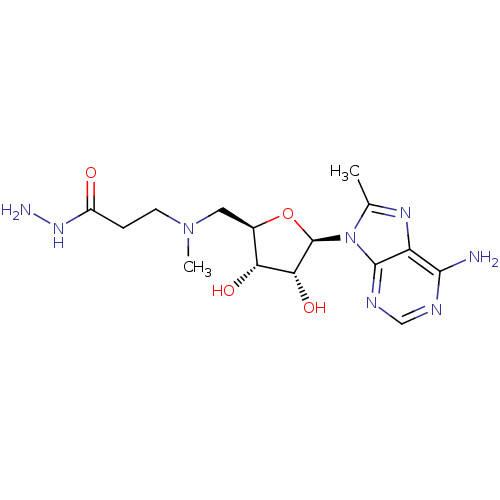 (3-({[(2R,3S,4R,5R)-5-(6-amino-8-methyl-9H-purin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28432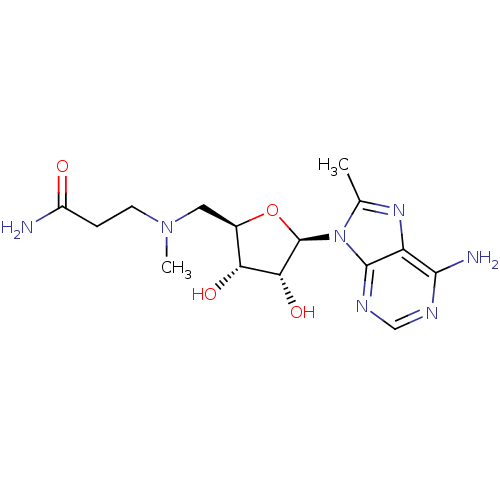 (3-({[(2R,3S,4R,5R)-5-(6-amino-8-methyl-9H-purin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28452 ((2R,3R,4S,5R)-2-(6-amino-8-methyl-9H-purin-9-yl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28437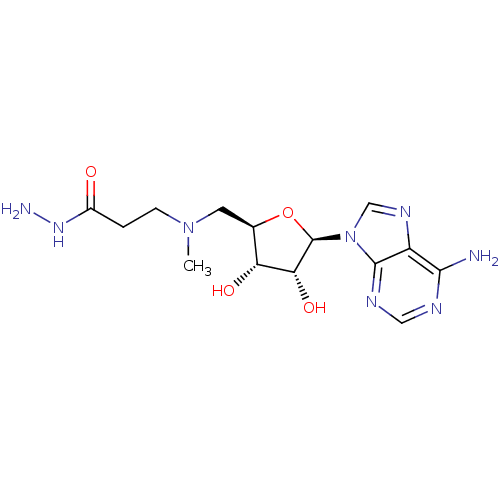 (3-({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28454 (AdoMet substrate analogue, 25b | {[(2S,3S,4R,5R)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28433 (3-({[(2R,3S,4R,5R)-5-(6-amino-8-ethyl-9H-purin-9-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28435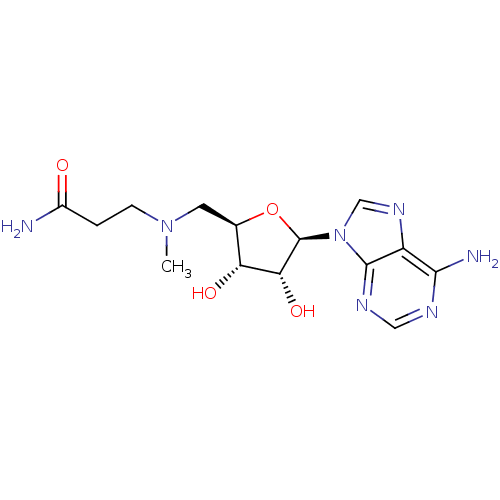 (3-({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254958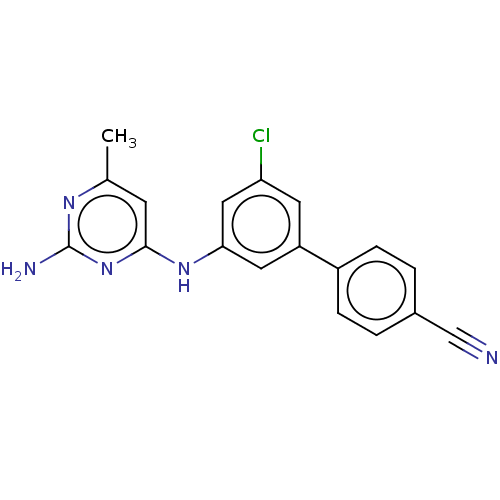 (CHEMBL4075752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50281292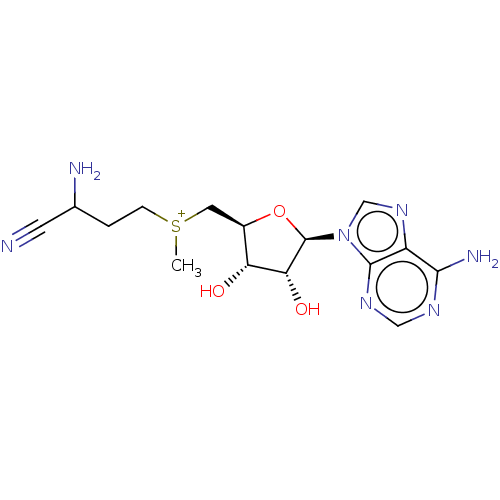 (2-amino-4-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against AdoMet-DC from Escherichia coli | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28453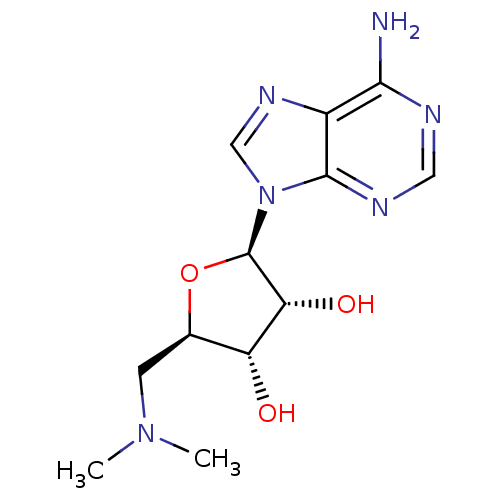 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-[(dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28455 (AdoMet substrate analogue, 25d | {[(2S,3S,4R,5R)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254815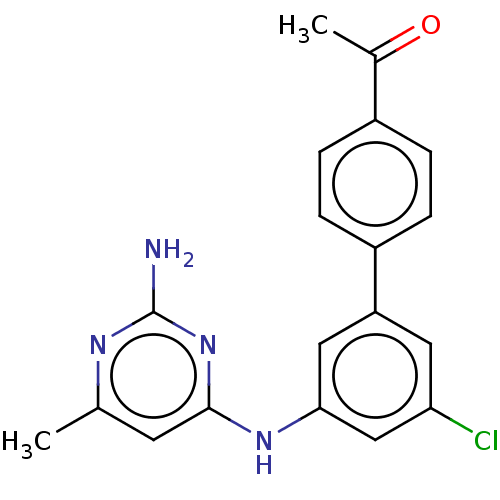 (CHEMBL4104060) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254905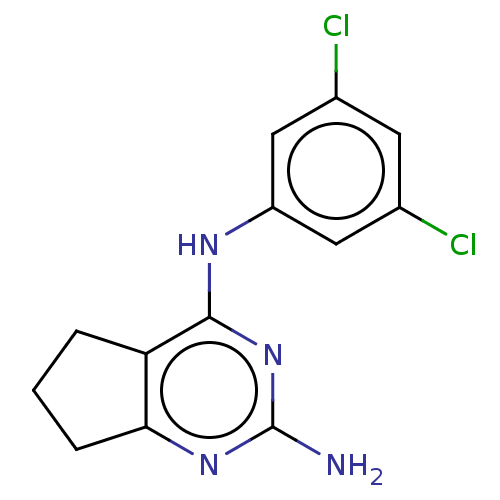 (CHEMBL4094809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50255043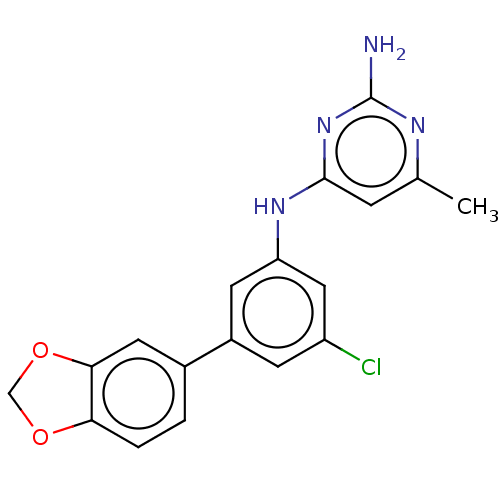 (CHEMBL4073190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50255044 (CHEMBL4077070) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254955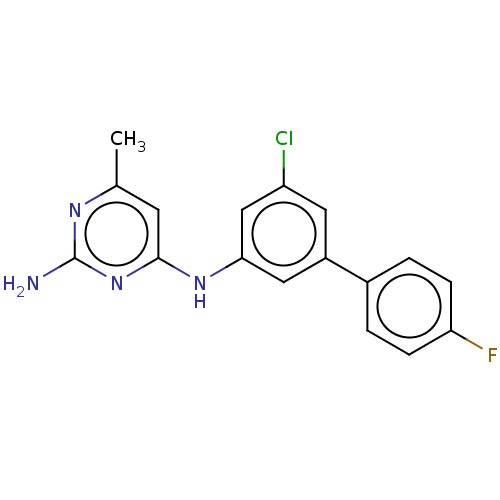 (CHEMBL4094762) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50255018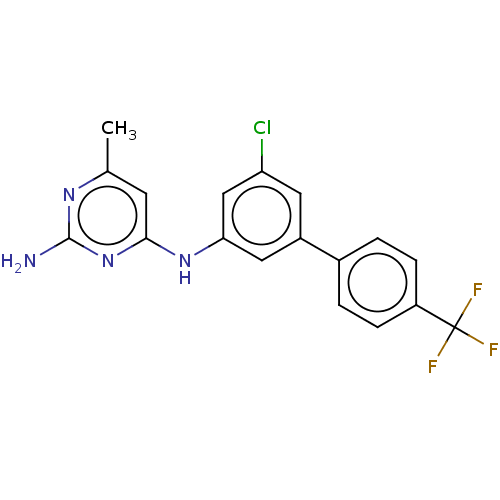 (CHEMBL4079196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254902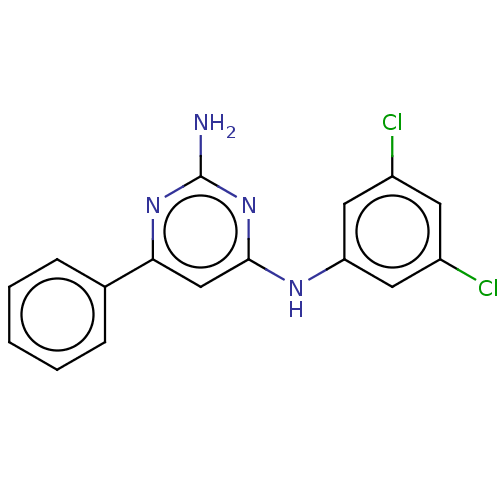 (CHEMBL4079242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28438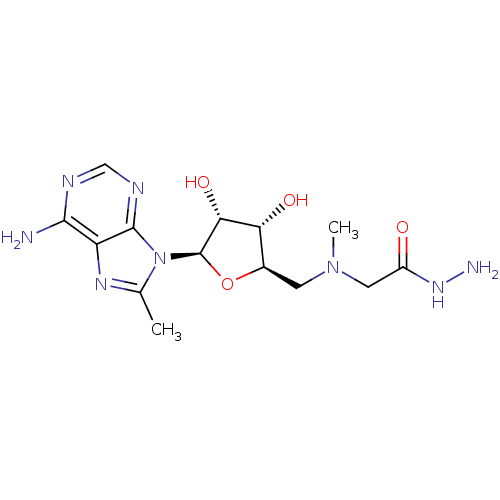 (2-({[(2R,3S,4R,5R)-5-(6-amino-8-methyl-9H-purin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254826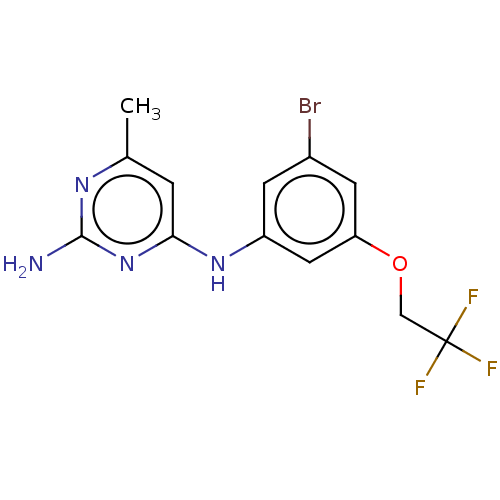 (CHEMBL4090615) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50281293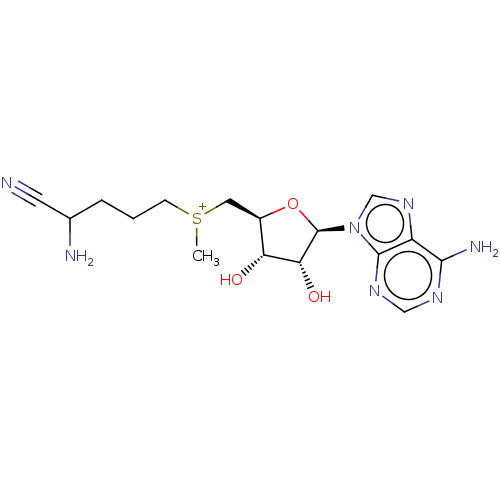 (2-amino-5-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated to inactivate the bacterial AdoMet-DC | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50255017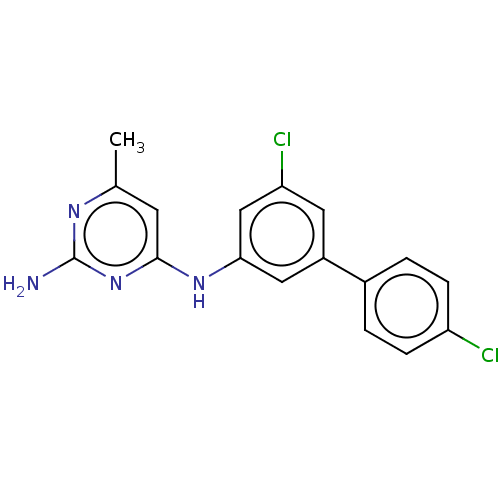 (CHEMBL4101315) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50255009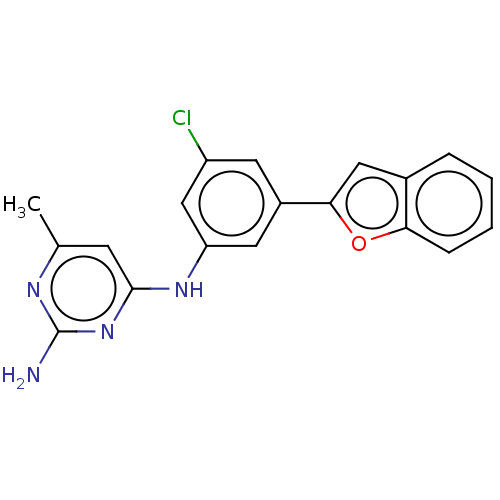 (CHEMBL4075494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254957 (CHEMBL4097210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254873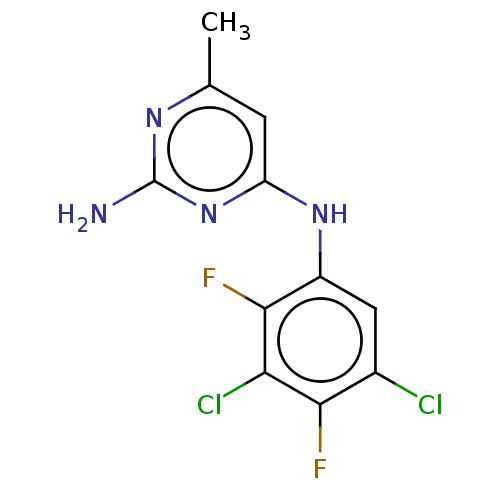 (CHEMBL4091216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254862 (CHEMBL4085397) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254860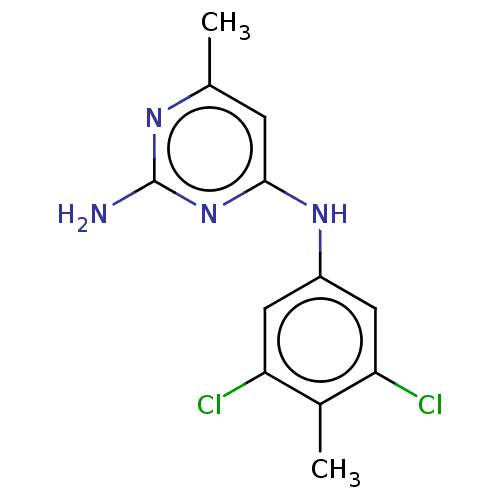 (CHEMBL4089063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50255078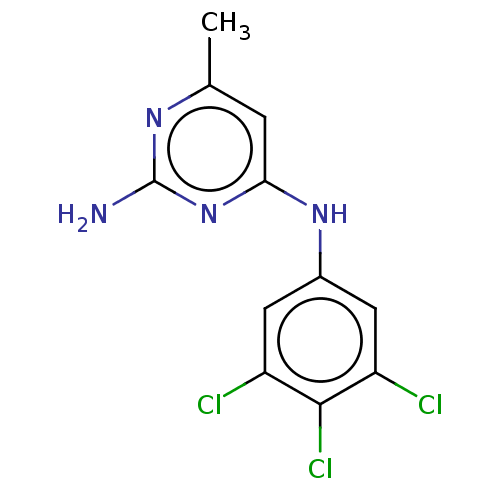 (CHEMBL4060869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50255077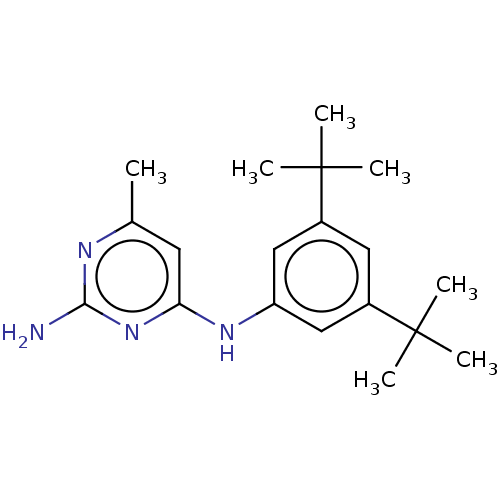 (CHEMBL4097036) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50255071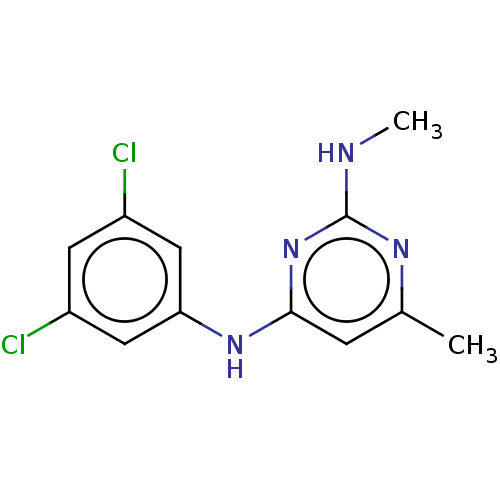 (CHEMBL4098044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28448 ((2R,3R,4S,5R)-2-(6-amino-8-methyl-9H-purin-9-yl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28443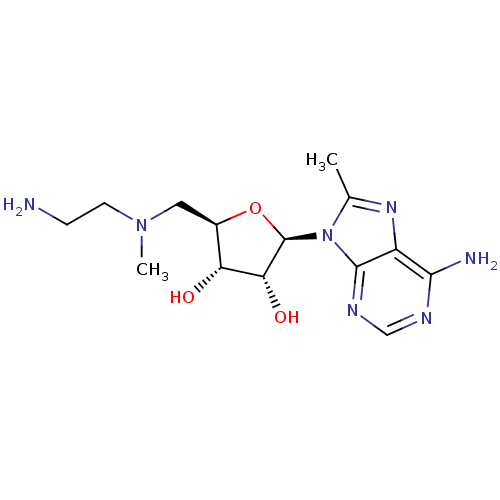 ((2R,3R,4S,5R)-2-(6-amino-8-methyl-9H-purin-9-yl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM28451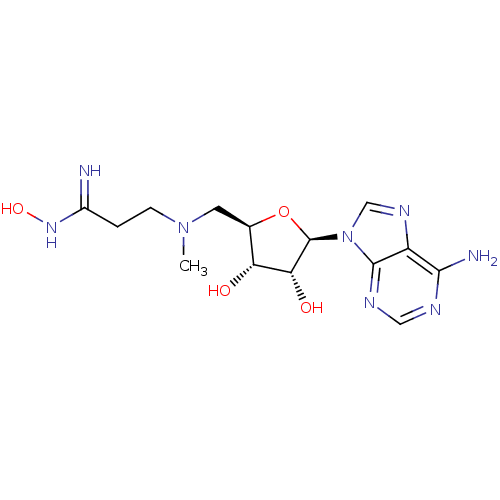 (3-({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University | Assay Description The C-terminal his-tagged AdoMetDC was assayed by measuring the release of 14CO2 from S-adenosyl-L-[carboxy-14C]methionine (Amersham Pharmacia Biotec... | J Med Chem 52: 1388-407 (2009) Article DOI: 10.1021/jm801126a BindingDB Entry DOI: 10.7270/Q2222S3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254859 (CHEMBL4069538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50366314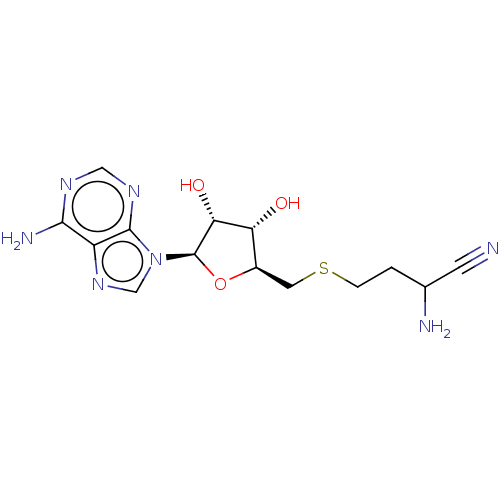 (CHEMBL3392211 | CHEMBL607699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against AdoMet-DC from Escherichia coli | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254816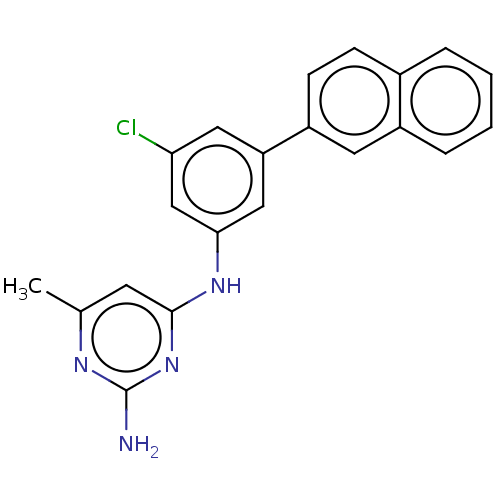 (CHEMBL4066551) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254817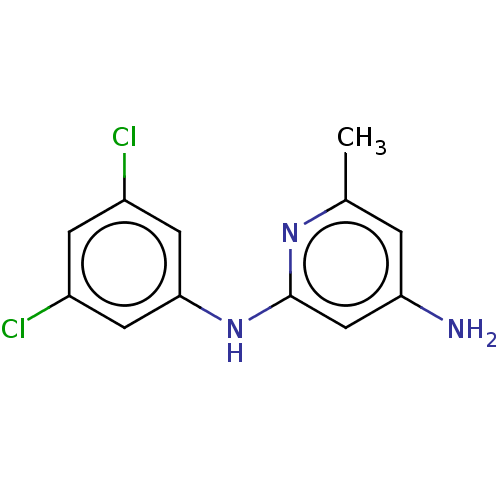 (CHEMBL4103347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254818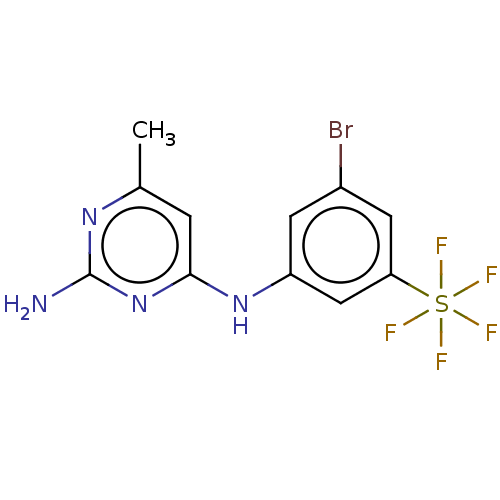 (CHEMBL4070203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254819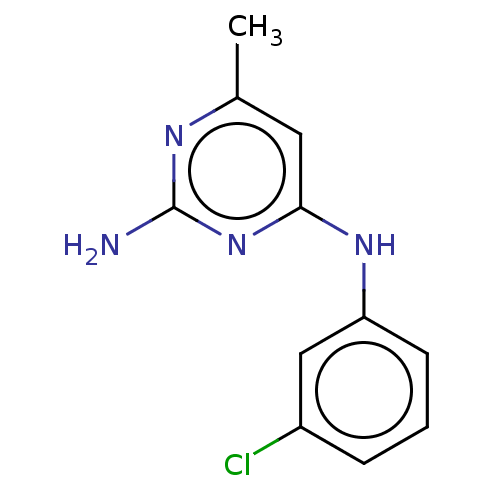 (CHEMBL4104760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254820 (CHEMBL4099923) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254821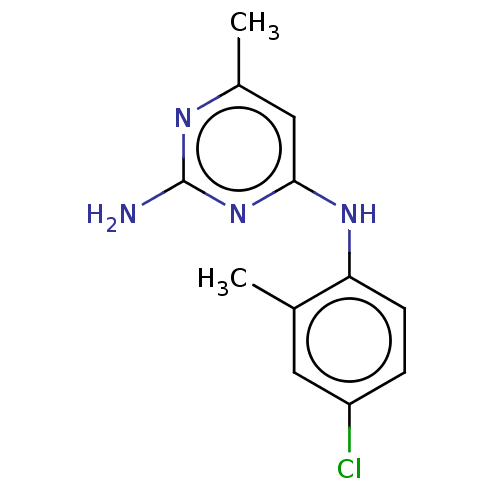 (CHEMBL4070829) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50254822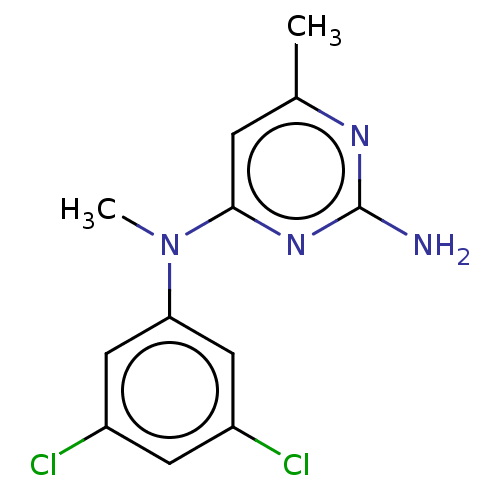 (CHEMBL4062599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Scynexis, Inc. (now Avista Pharma Solutions) , 3501 Tricenter Boulevard, Suite C, Durham, North Carolina 27713, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged AdoMetDC expressed in Escherichia coli BL21/DE3 cells at pH 7.2 by RapidFire-MS-based enzyme a... | J Med Chem 61: 1182-1203 (2018) Article DOI: 10.1021/acs.jmedchem.7b01654 BindingDB Entry DOI: 10.7270/Q22N54R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 123 total ) | Next | Last >> |