

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054973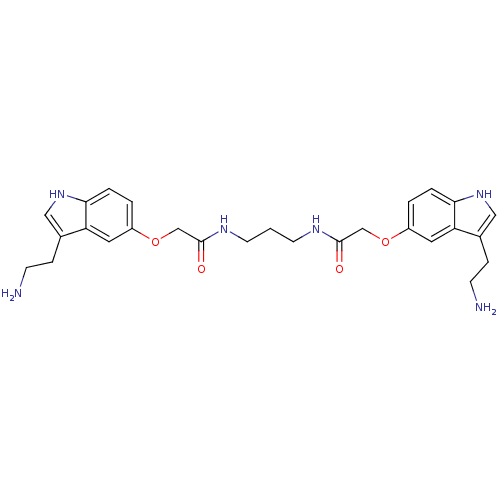 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(3-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054985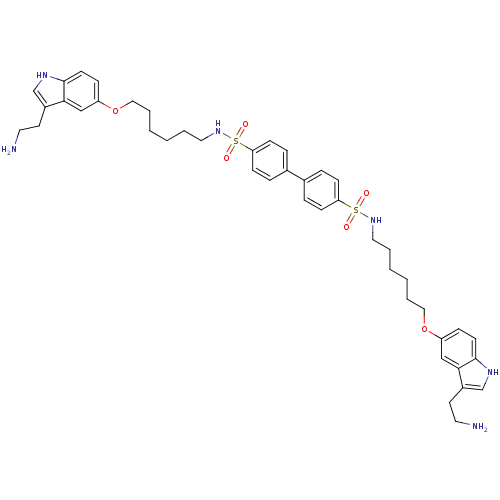 (Biphenyl-4,4'-disulfonic acid bis-({6-[3-(2-amino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054979 (2-{5-[2-(4-{2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054987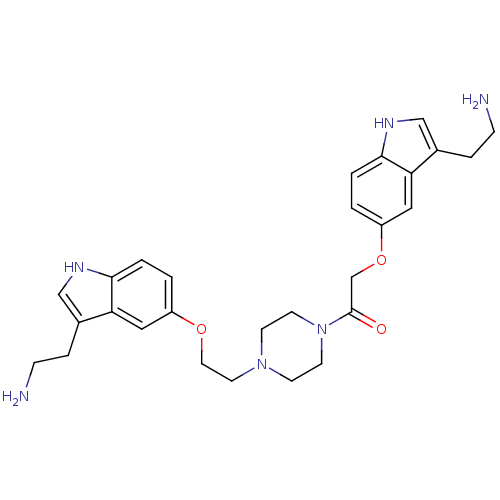 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(4-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054980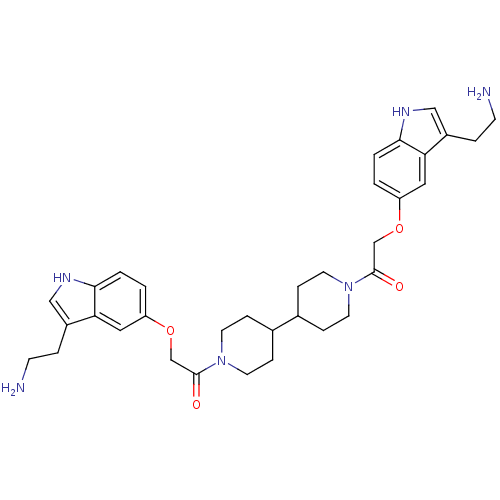 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(1'-{2-[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054986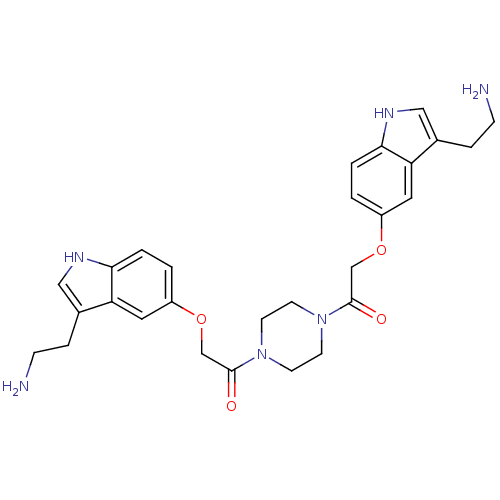 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(4-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054981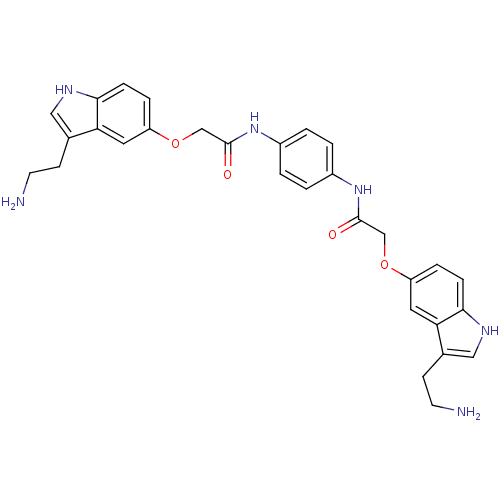 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(4-{2-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054988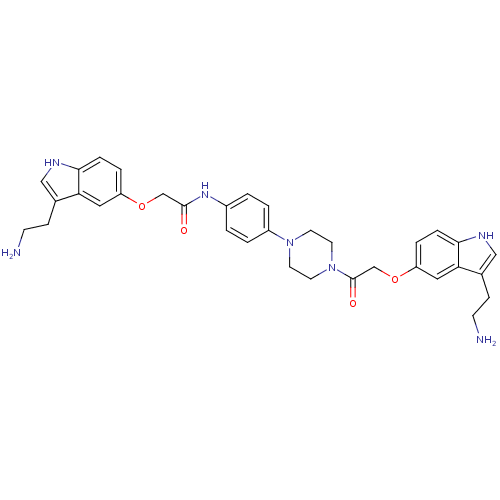 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-[4-(4-{2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054976 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-{2-[3-(2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50069315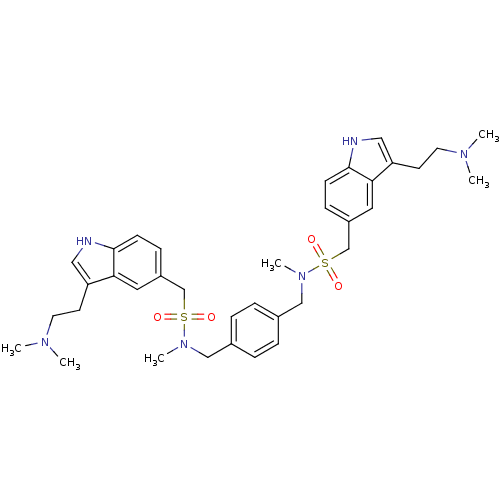 (C-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Effective concentration of the compound determined by measuring inhibition of forskolin-stimulated c-AMP formation at 5-hydroxytryptamine 1B receptor... | Bioorg Med Chem Lett 8: 675-80 (1999) BindingDB Entry DOI: 10.7270/Q24B30F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054977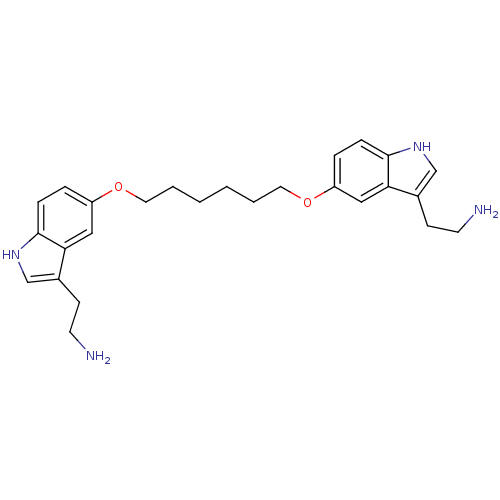 (2-(5-{6-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-hexyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054978 (2-(5-{3-[3-(2-Amino-ethyl)-1H-indol-5-yloxymethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054983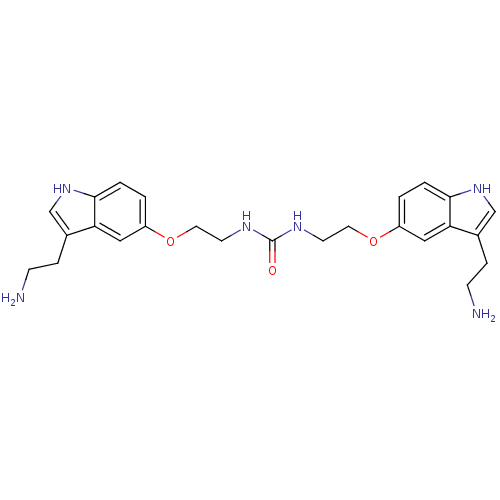 (1,3-Bis-{2-[3-(2-amino-ethyl)-1H-indol-5-yloxy]-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50290912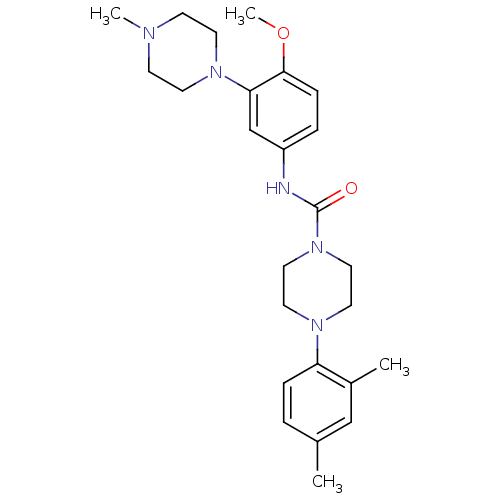 (4-(2,4-Dimethyl-phenyl)-piperazine-1-carboxylic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for intrinsic activity against human cloned 5-hydroxytryptamine 1B receptor; full agonist | Bioorg Med Chem Lett 7: 3183-3188 (1997) Article DOI: 10.1016/S0960-894X(97)10164-0 BindingDB Entry DOI: 10.7270/Q23R0SW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054984 (2-(5-{2-[3-(2-Amino-ethyl)-1H-indol-5-yloxymethyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.07 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50033447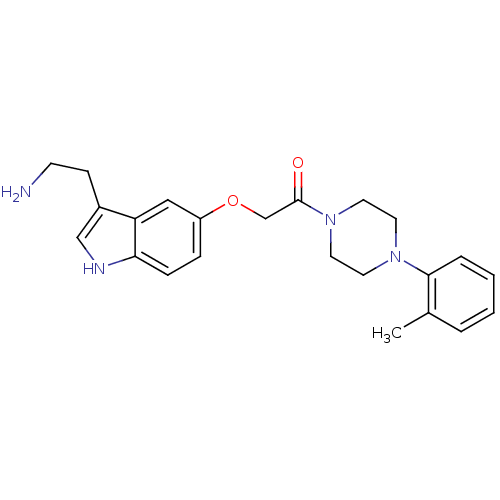 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(4-o-toly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for intrinsic activity against human cloned 5-hydroxytryptamine 1B receptor | Bioorg Med Chem Lett 7: 3183-3188 (1997) Article DOI: 10.1016/S0960-894X(97)10164-0 BindingDB Entry DOI: 10.7270/Q23R0SW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054974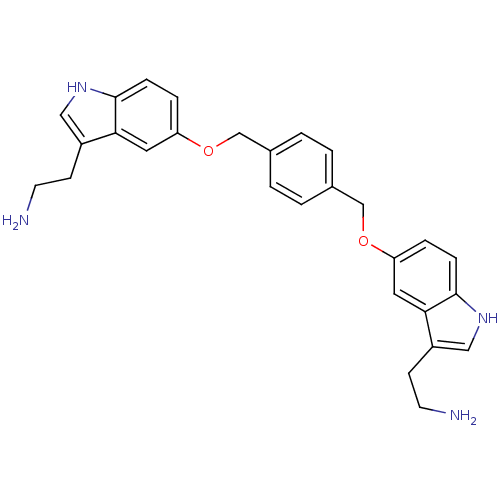 (2-(5-((4-((3-(2-aminoethyl)-1H-indol-5-yloxy)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054982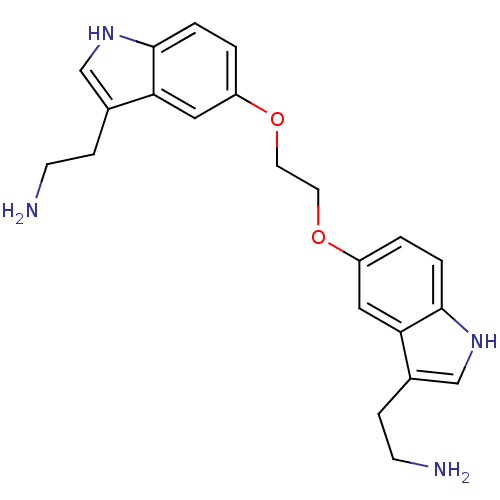 (2-(5-{2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description The intrinsic activity of the compound was evaluated for its ability to inhibit forskolin-stimulated c-AMP formation mediated by cloned 5-hydroxytryp... | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.82 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50413552 (CHEMBL472289) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5HT1B assessed as GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 19: 2338-42 (2009) Article DOI: 10.1016/j.bmcl.2009.02.056 BindingDB Entry DOI: 10.7270/Q2J967MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50290915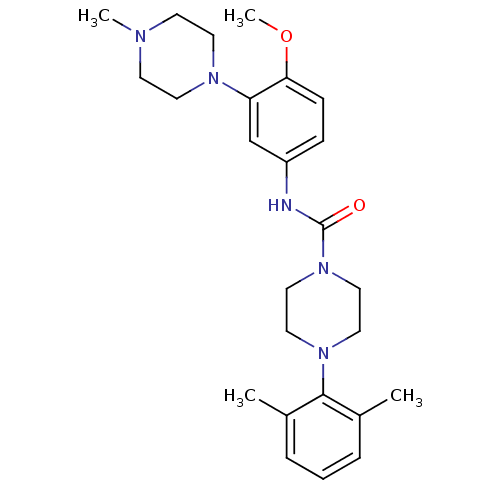 (4-(2,6-Dimethyl-phenyl)-piperazine-1-carboxylic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for intrinsic activity against human cloned 5-hydroxytryptamine 1B receptor | Bioorg Med Chem Lett 7: 3183-3188 (1997) Article DOI: 10.1016/S0960-894X(97)10164-0 BindingDB Entry DOI: 10.7270/Q23R0SW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50103594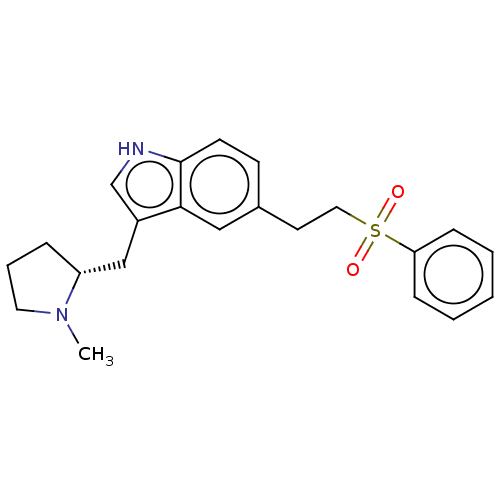 (CHEBI:50922 | Eletriptan | UK-116044 | UK-116044-0...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at 5-HT1B receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114045 BindingDB Entry DOI: 10.7270/Q29K4G4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50413566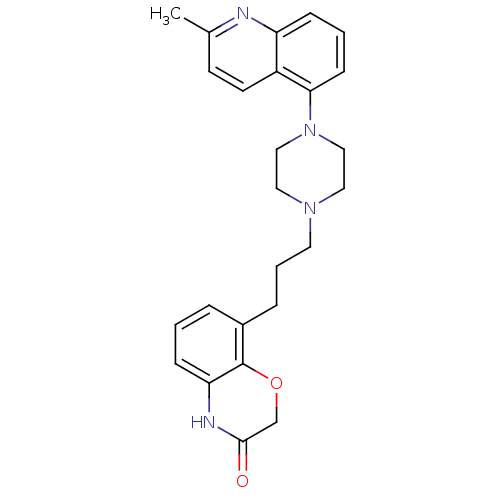 (CHEMBL470849) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5HT1B assessed as GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 19: 2338-42 (2009) Article DOI: 10.1016/j.bmcl.2009.02.056 BindingDB Entry DOI: 10.7270/Q2J967MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50033383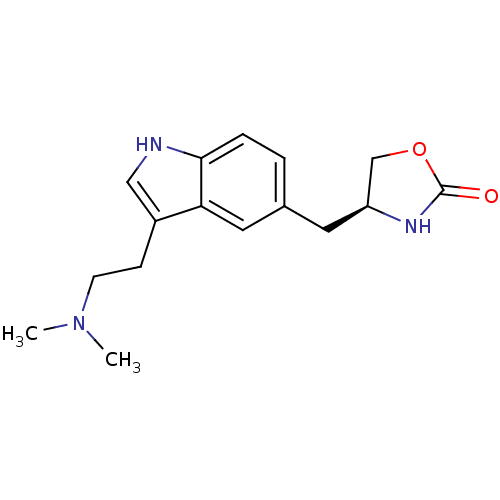 ((S)-4-((3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Effective concentration of the compound determined by measuring inhibition of forskolin-stimulated c-AMP formation at 5-hydroxytryptamine 1B receptor... | Bioorg Med Chem Lett 8: 675-80 (1999) BindingDB Entry DOI: 10.7270/Q24B30F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50061297 (1-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-piperazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Agonist activity at 5-hydroxytryptamine 1B receptor by measuring the inhibition of forskolin-stimulated cAMP formation | J Med Chem 40: 3974-8 (1998) Article DOI: 10.1021/jm9703552 BindingDB Entry DOI: 10.7270/Q2FT8K57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50061304 (2-[8-(4-Methyl-piperazin-1-yl)-naphthalen-2-yloxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Agonist activity at 5-hydroxytryptamine 1B receptor by measuring the inhibition of forskolin-stimulated cAMP formation | J Med Chem 40: 3974-8 (1998) Article DOI: 10.1021/jm9703552 BindingDB Entry DOI: 10.7270/Q2FT8K57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50413563 (CHEMBL469376) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5HT1B assessed as GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 19: 2338-42 (2009) Article DOI: 10.1016/j.bmcl.2009.02.056 BindingDB Entry DOI: 10.7270/Q2J967MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50454685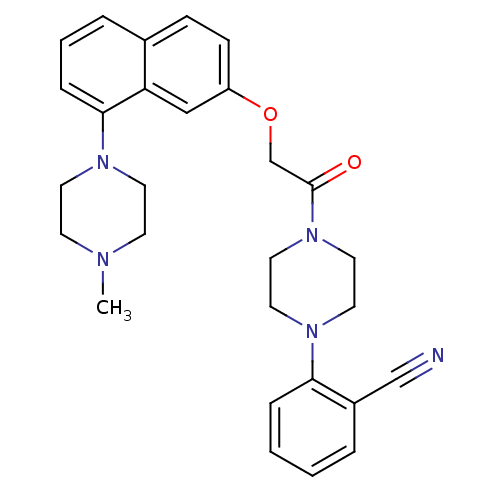 (CHEMBL2112660) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Agonist activity at 5-hydroxytryptamine 1B receptor by measuring the inhibition of forskolin-stimulated cAMP formation | J Med Chem 40: 3974-8 (1998) Article DOI: 10.1021/jm9703552 BindingDB Entry DOI: 10.7270/Q2FT8K57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50456728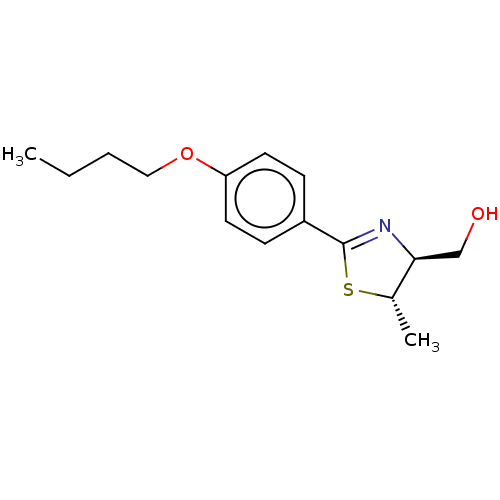 (CHEMBL4212246) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
University of the Philippines Curated by ChEMBL | Assay Description Partial agonist activity at 5-HT1B receptor (unknown origin) by Tango assay | J Nat Prod 80: 2360-2370 (2017) Article DOI: 10.1021/acs.jnatprod.7b00317 BindingDB Entry DOI: 10.7270/Q24F1TB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50061296 (1-[4-(2,3-Dimethoxy-phenyl)-piperazin-1-yl]-2-[8-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Agonist activity at 5-hydroxytryptamine 1B receptor by measuring the inhibition of forskolin-stimulated cAMP formation | J Med Chem 40: 3974-8 (1998) Article DOI: 10.1021/jm9703552 BindingDB Entry DOI: 10.7270/Q2FT8K57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of Forskolin-stimulated adenylate cyclase activity against 5-hydroxytryptamine 1A receptor of guinea pig hippocampus | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank PubMed | n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Effective concentration of the compound determined by measuring inhibition of forskolin-stimulated c-AMP formation at 5-hydroxytryptamine 1B receptor... | Bioorg Med Chem Lett 8: 675-80 (1999) BindingDB Entry DOI: 10.7270/Q24B30F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070407 (2N,2N-dipropyl-8-[6-(3-dipropylamino-1,2,3,4-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description The intrinsic activity of the compound was evaluated for its ability to inhibit forskolin-stimulated c-AMP formation mediated by cloned 5-hydroxytryp... | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50413562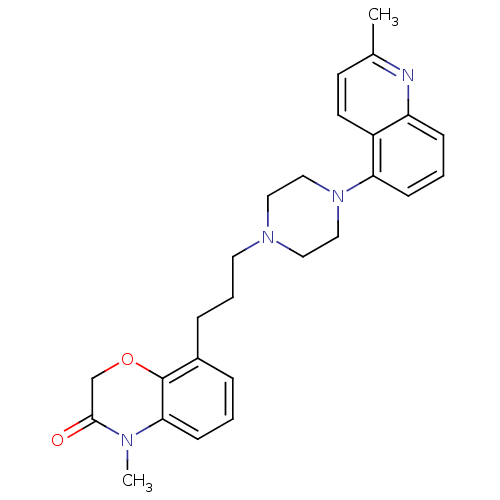 (CHEMBL469375) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5HT1B assessed as GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 19: 2338-42 (2009) Article DOI: 10.1016/j.bmcl.2009.02.056 BindingDB Entry DOI: 10.7270/Q2J967MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50417422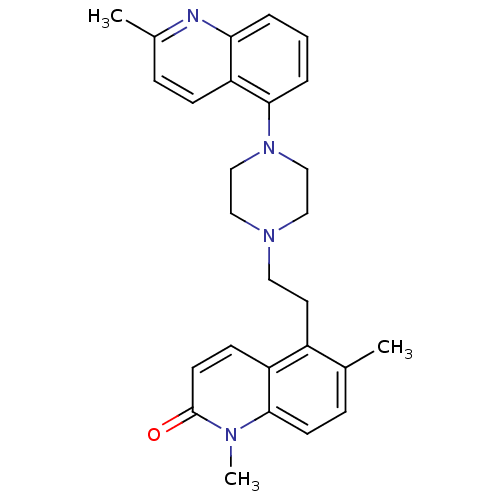 (CHEMBL1289048) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1B receptor expressed in CHO cells assessed as stimulation of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50065417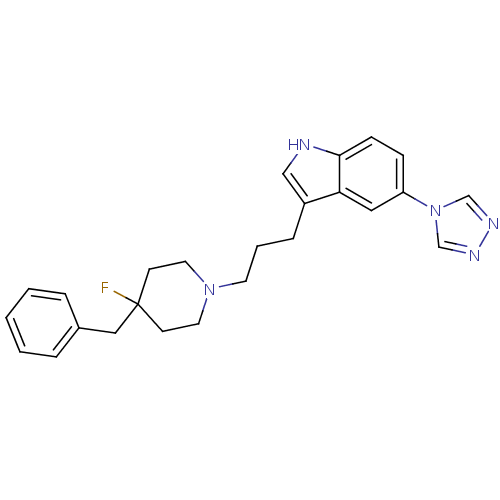 (3-[3-(4-Benzyl-4-fluoro-piperidin-1-yl)-propyl]-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with human 5-hydroxytryptamine 1B receptor | J Med Chem 42: 2087-104 (1999) Article DOI: 10.1021/jm981133m BindingDB Entry DOI: 10.7270/Q2765DHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 77 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre Fabre Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated c-AMP formation by human 5-hydroxytryptamine 1B receptor expressed in CHO-K1 cells | J Med Chem 39: 4920-7 (1997) Article DOI: 10.1021/jm960552l BindingDB Entry DOI: 10.7270/Q23B5Z7M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50065419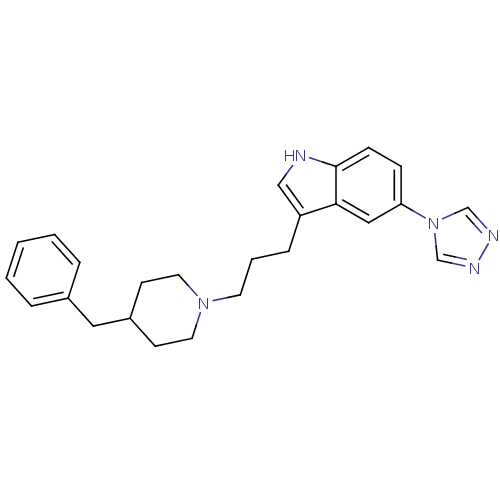 (3-[3-(4-Benzyl-piperidin-1-yl)-propyl]-5-[1,2,4]tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with human 5-hydroxytryptamine 1B receptor | J Med Chem 42: 2087-104 (1999) Article DOI: 10.1021/jm981133m BindingDB Entry DOI: 10.7270/Q2765DHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50400902 (1-(2-(2,4-dimethylphenylsulfanyl)phenyl)piperazine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Partial agonist activity at human 5HT1B receptor expressed in CHO cells assessed as [35S]GTPgammaS binding | J Med Chem 54: 3206-21 (2011) Article DOI: 10.1021/jm101459g BindingDB Entry DOI: 10.7270/Q2W37XFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50456732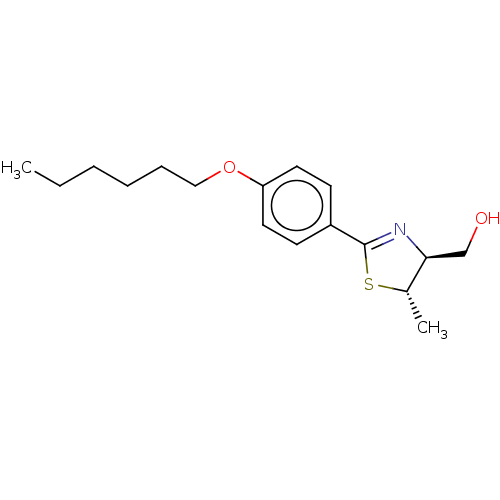 (CHEMBL4207355) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
University of the Philippines Curated by ChEMBL | Assay Description Partial agonist activity at 5-HT1B receptor (unknown origin) by Tango assay | J Nat Prod 80: 2360-2370 (2017) Article DOI: 10.1021/acs.jnatprod.7b00317 BindingDB Entry DOI: 10.7270/Q24F1TB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50417410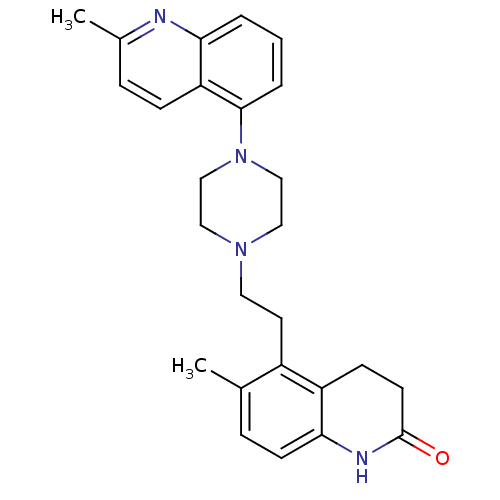 (CHEMBL1290596) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1B receptor expressed in CHO cells assessed as stimulation of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description The intrinsic activity of the compound was evaluated for its ability to inhibit forskolin-stimulated c-AMP formation mediated by cloned 5-hydroxytryp... | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50077814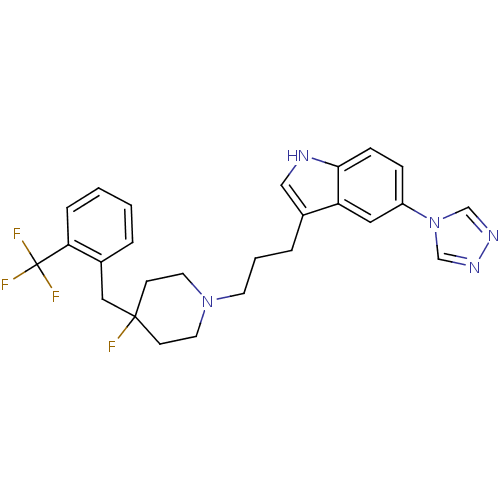 (3-{3-[4-Fluoro-4-(2-trifluoromethyl-benzyl)-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with human 5-hydroxytryptamine 1B receptor | J Med Chem 42: 2087-104 (1999) Article DOI: 10.1021/jm981133m BindingDB Entry DOI: 10.7270/Q2765DHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50417423 (CHEMBL1289164) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1B receptor expressed in CHO cells assessed as stimulation of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50417421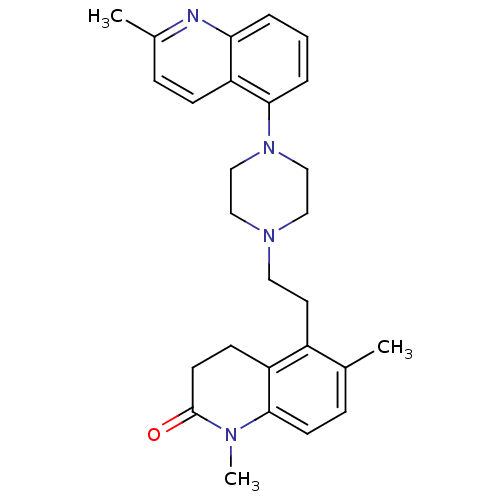 (CHEMBL1290597) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 398 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1B receptor expressed in CHO cells assessed as stimulation of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50060437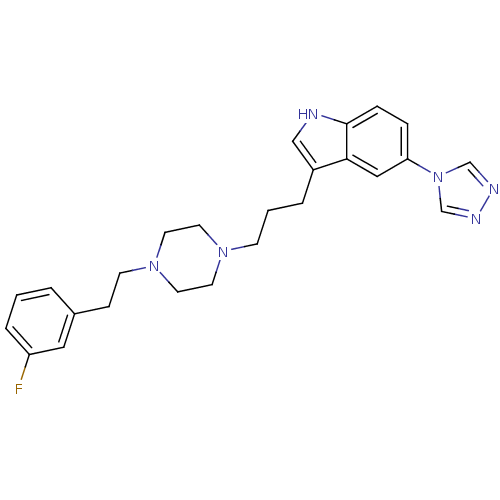 (3-(3-{4-[2-(3-Fluoro-phenyl)-ethyl]-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with human 5-hydroxytryptamine 1B receptor | J Med Chem 42: 2087-104 (1999) Article DOI: 10.1021/jm981133m BindingDB Entry DOI: 10.7270/Q2765DHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50065421 (3-(3-{4-Fluoro-4-[2-(3-fluoro-phenyl)-ethyl]-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with human 5-hydroxytryptamine 1B receptor | J Med Chem 42: 2087-104 (1999) Article DOI: 10.1021/jm981133m BindingDB Entry DOI: 10.7270/Q2765DHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50065418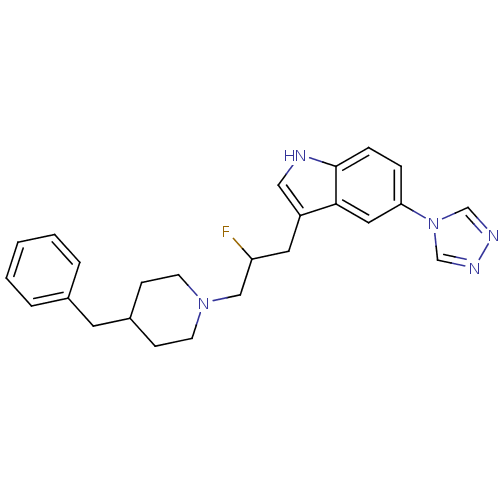 (3-[3-(4-Benzyl-piperidin-1-yl)-2-fluoro-propyl]-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with human 5-hydroxytryptamine 1B receptor | J Med Chem 42: 2087-104 (1999) Article DOI: 10.1021/jm981133m BindingDB Entry DOI: 10.7270/Q2765DHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50417412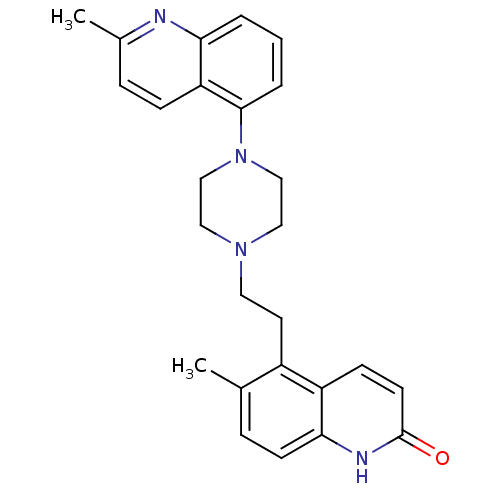 (CHEMBL1289047) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1B receptor expressed in CHO cells assessed as stimulation of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |