

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060440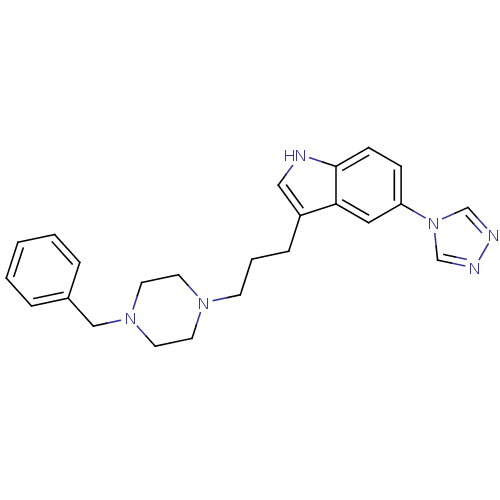 (3-[3-(4-Benzyl-piperazin-1-yl)-propyl]-5-[1,2,4]tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist induced [35S]-GTP-gammaS, binding in CHO cells expressing 5-HT 1d receptor | J Med Chem 41: 2667-70 (1998) Article DOI: 10.1021/jm980204e BindingDB Entry DOI: 10.7270/Q22Z14PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060440 (3-[3-(4-Benzyl-piperazin-1-yl)-propyl]-5-[1,2,4]tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing 5-hydroxytryptamine 1D receptor | J Med Chem 40: 3501-3 (1997) Article DOI: 10.1021/jm9704560 BindingDB Entry DOI: 10.7270/Q2DZ07D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074188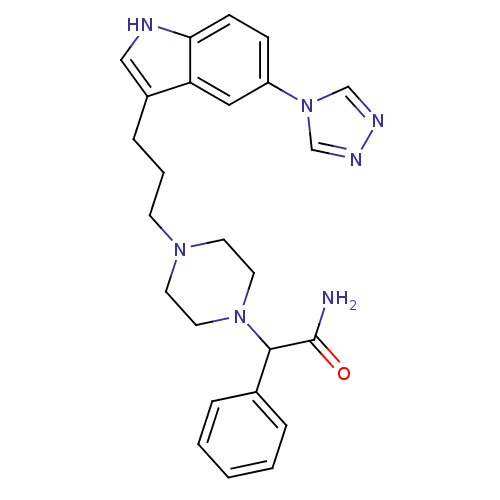 (2-Phenyl-2-{4-[3-(5-[1,2,4]triazol-4-yl-1H-indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060440 (3-[3-(4-Benzyl-piperazin-1-yl)-propyl]-5-[1,2,4]tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50077814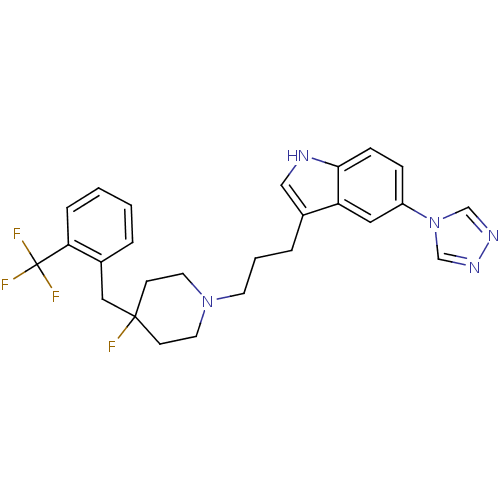 (3-{3-[4-Fluoro-4-(2-trifluoromethyl-benzyl)-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 42: 2087-104 (1999) Article DOI: 10.1021/jm981133m BindingDB Entry DOI: 10.7270/Q2765DHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074205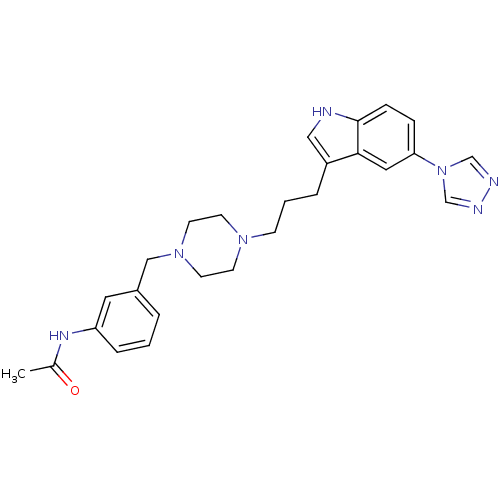 (CHEMBL161176 | N-(3-{4-[3-(5-[1,2,4]Triazol-4-yl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50073688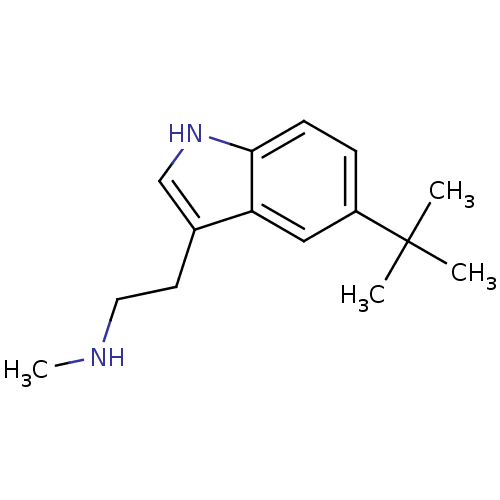 (CHEMBL357034 | [2-(5-tert-Butyl-1H-indol-3-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to inhibit forskolin-stimulated adenylate cyclase in a cell line expressing human 5-hydroxytryptamine 1D receptor | J Med Chem 42: 526-31 (1999) Article DOI: 10.1021/jm9805945 BindingDB Entry DOI: 10.7270/Q2668CBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50286671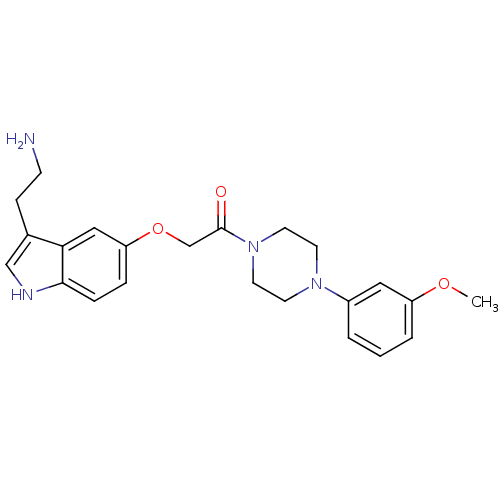 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-[4-(3-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability inhibit forskolin-stimulated activity of adenylate cyclase coupled to human 5-hydroxytryptamine 1D receptor beta ... | Bioorg Med Chem Lett 5: 663-666 (1995) Article DOI: 10.1016/0960-894X(95)00091-7 BindingDB Entry DOI: 10.7270/Q2ZK5GMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074194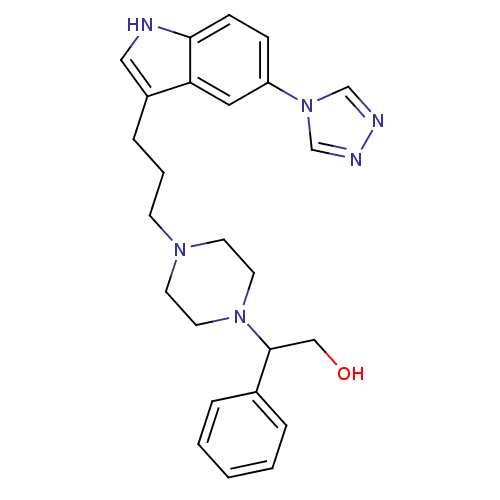 (2-Phenyl-2-{4-[3-(5-[1,2,4]triazol-4-yl-1H-indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074206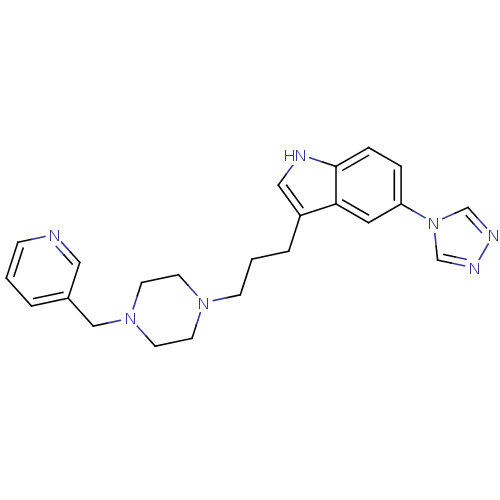 (3-[3-(4-Pyridin-3-ylmethyl-piperazin-1-yl)-propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074202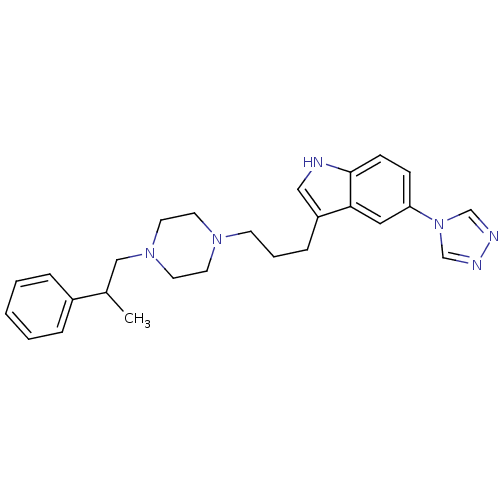 (3-{3-[4-(2-Phenyl-propyl)-piperazin-1-yl]-propyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074186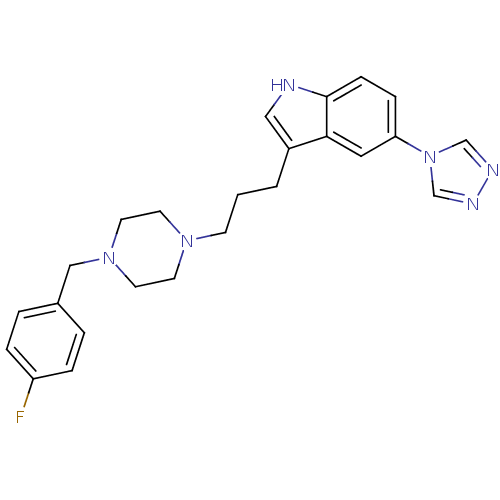 (3-{3-[4-(4-Fluoro-benzyl)-piperazin-1-yl]-propyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074198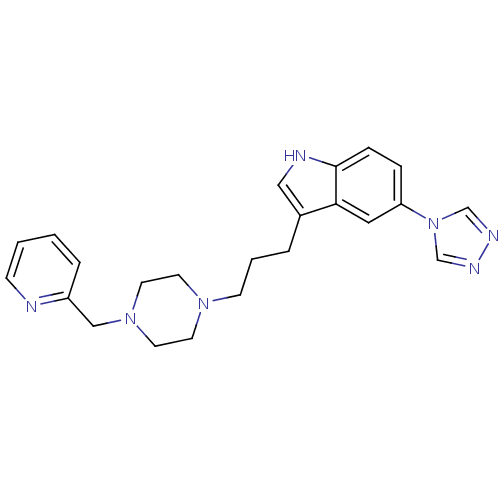 (3-[3-(4-Pyridin-2-ylmethyl-piperazin-1-yl)-propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50136469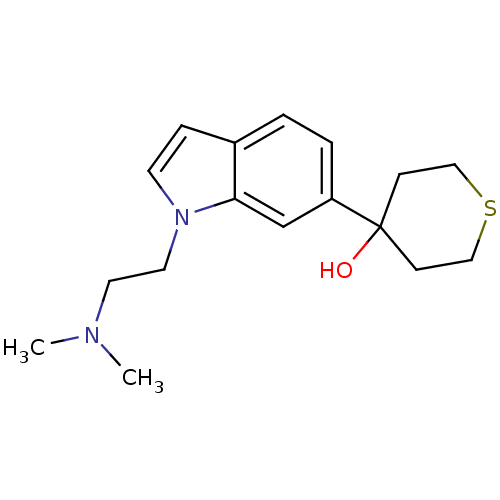 (4-[1-(2-Dimethylamino-ethyl)-1H-indol-6-yl]-tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Ability to inhibit foskolin-stimulated adenylate cyclase activity in Chinese hamster ovary (CHO) stable cell lines expressing human 5-hydroxytryptami... | Bioorg Med Chem Lett 13: 4409-13 (2003) BindingDB Entry DOI: 10.7270/Q2HT2NR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074178 ((1-Methyl-1-phenyl-ethyl)-{(S)-1-[2-(5-[1,2,4]tria...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the 5-hydroxytryptamine 1D receptor | J Med Chem 42: 677-90 (1999) Article DOI: 10.1021/jm9805687 BindingDB Entry DOI: 10.7270/Q23777W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074203 (3-[3-(4-Furan-3-ylmethyl-piperazin-1-yl)-propyl]-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060426 (CHEMBL296161 | Dimethyl-[2-(5-[1,2,4]triazol-4-yl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the 5-hydroxytryptamine 1D receptor | J Med Chem 42: 677-90 (1999) Article DOI: 10.1021/jm9805687 BindingDB Entry DOI: 10.7270/Q23777W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50065417 (3-[3-(4-Benzyl-4-fluoro-piperidin-1-yl)-propyl]-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 42: 2087-104 (1999) Article DOI: 10.1021/jm981133m BindingDB Entry DOI: 10.7270/Q2765DHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060426 (CHEMBL296161 | Dimethyl-[2-(5-[1,2,4]triazol-4-yl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the 5-hydroxytryptamine 1D receptor | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060426 (CHEMBL296161 | Dimethyl-[2-(5-[1,2,4]triazol-4-yl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Measurement of agonist induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor. | J Med Chem 40: 3497-500 (1997) Article DOI: 10.1021/jm9704558 BindingDB Entry DOI: 10.7270/Q2JQ103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50033439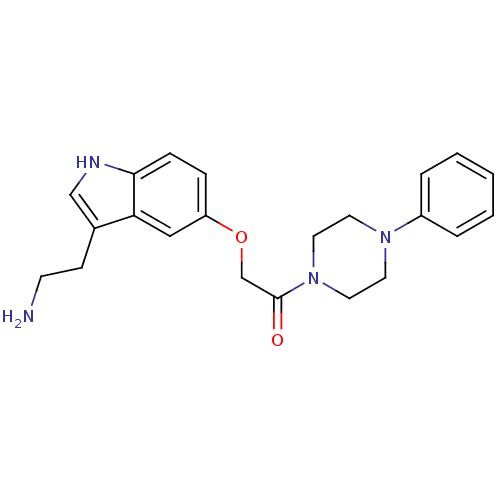 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(4-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability inhibit forskolin-stimulated activity of adenylate cyclase coupled to human 5-HT 1Dbeta receptor in CHO-K1 cells | Bioorg Med Chem Lett 5: 663-666 (1995) Article DOI: 10.1016/0960-894X(95)00091-7 BindingDB Entry DOI: 10.7270/Q2ZK5GMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50080890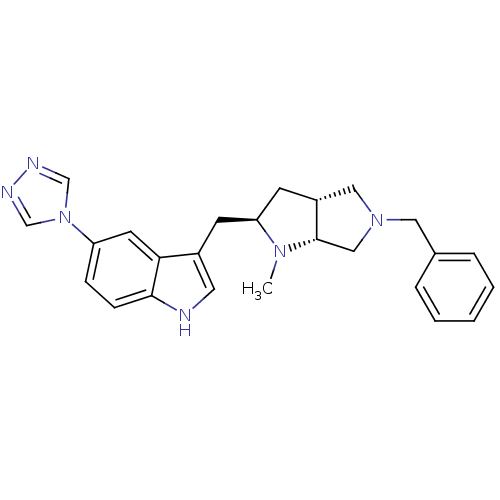 (3-((2R,3aR,6aR)-5-Benzyl-1-methyl-octahydro-pyrrol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Measurement of agonist induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor | Bioorg Med Chem Lett 9: 2491-6 (1999) BindingDB Entry DOI: 10.7270/Q27M074P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074201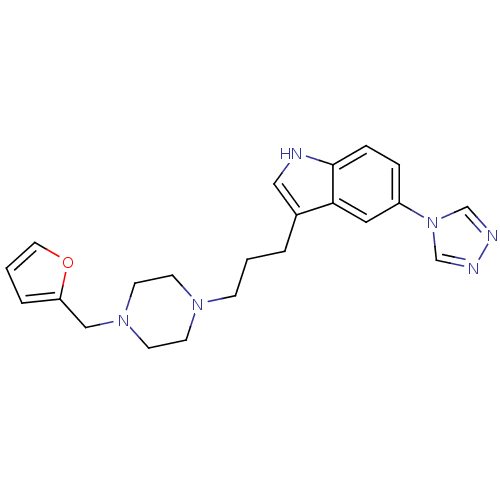 (3-[3-(4-Furan-2-ylmethyl-piperazin-1-yl)-propyl]-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50065417 (3-[3-(4-Benzyl-4-fluoro-piperidin-1-yl)-propyl]-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist induced [35S]-GTP-gammaS, binding in CHO cells expressing 5-HT 1d receptor | J Med Chem 41: 2667-70 (1998) Article DOI: 10.1021/jm980204e BindingDB Entry DOI: 10.7270/Q22Z14PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50083144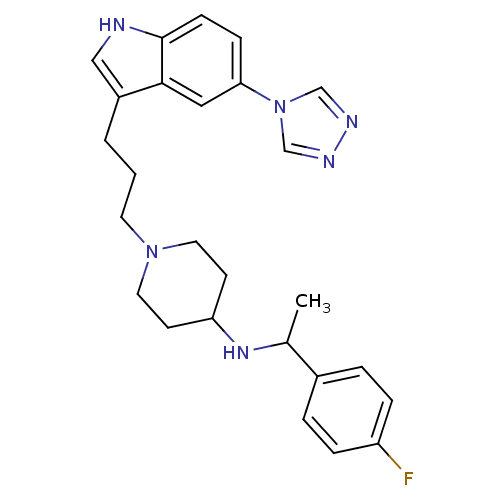 (CHEMBL146285 | [1-(4-Fluoro-phenyl)-ethyl]-{1-[3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]- GTPgammaS binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor | J Med Chem 42: 4981-5001 (2000) BindingDB Entry DOI: 10.7270/Q20P0Z77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074159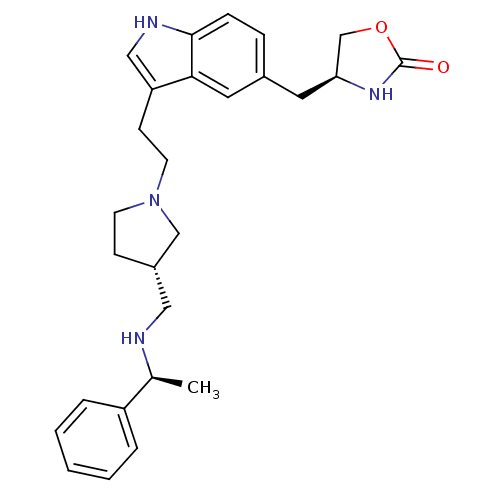 ((S)-4-[3-(2-{(S)-3-[((S)-1-Phenyl-ethylamino)-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the 5-hydroxytryptamine 1D receptor | J Med Chem 42: 677-90 (1999) Article DOI: 10.1021/jm9805687 BindingDB Entry DOI: 10.7270/Q23777W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50412441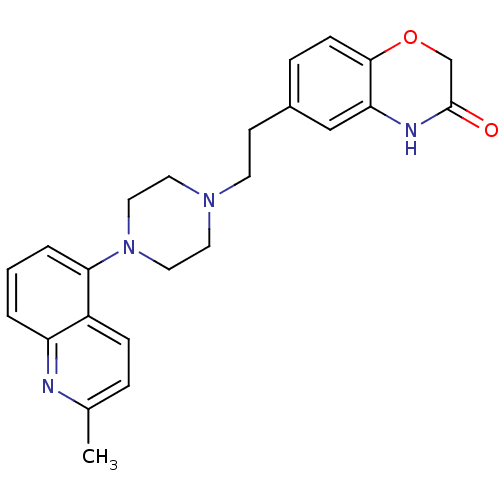 (CHEMBL490417 | SB-744185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 19: 2338-42 (2009) Article DOI: 10.1016/j.bmcl.2009.02.056 BindingDB Entry DOI: 10.7270/Q2J967MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060439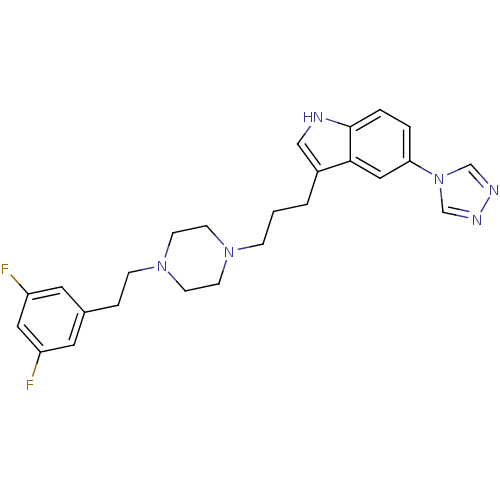 (3-(3-{4-[2-(3,5-Difluoro-phenyl)-ethyl]-piperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing 5-hydroxytryptamine 1D receptor | J Med Chem 40: 3501-3 (1997) Article DOI: 10.1021/jm9704560 BindingDB Entry DOI: 10.7270/Q2DZ07D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074200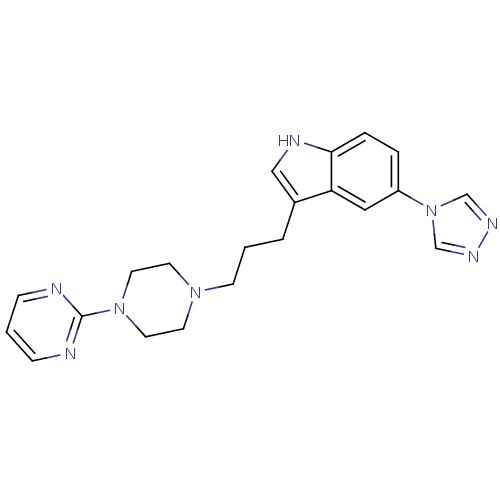 (3-[3-(4-Pyrimidin-2-yl-piperazin-1-yl)-propyl]-5-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50033447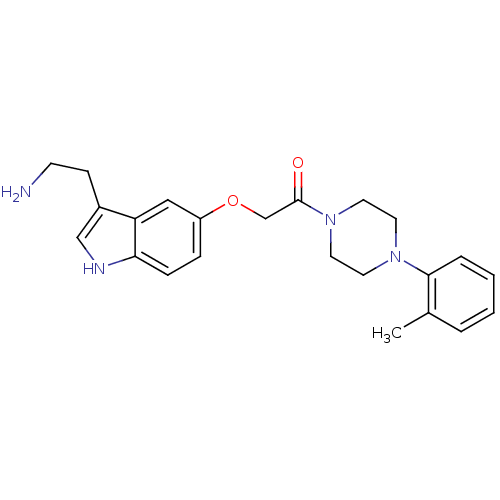 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-1-(4-o-toly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability inhibit forskolin-stimulated activity of adenylate cyclase coupled to human 5-hydroxytryptamine 1D receptor beta ... | Bioorg Med Chem Lett 5: 663-666 (1995) Article DOI: 10.1016/0960-894X(95)00091-7 BindingDB Entry DOI: 10.7270/Q2ZK5GMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50065419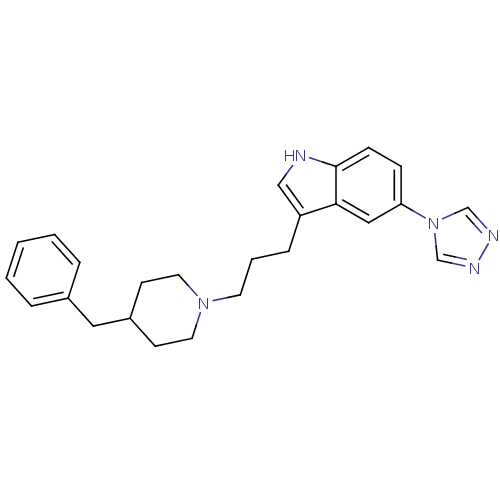 (3-[3-(4-Benzyl-piperidin-1-yl)-propyl]-5-[1,2,4]tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist induced [35S]-GTP-gammaS, binding in CHO cells expressing 5-HT 1d receptor | J Med Chem 41: 2667-70 (1998) Article DOI: 10.1021/jm980204e BindingDB Entry DOI: 10.7270/Q22Z14PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50065419 (3-[3-(4-Benzyl-piperidin-1-yl)-propyl]-5-[1,2,4]tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 42: 2087-104 (1999) Article DOI: 10.1021/jm981133m BindingDB Entry DOI: 10.7270/Q2765DHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074164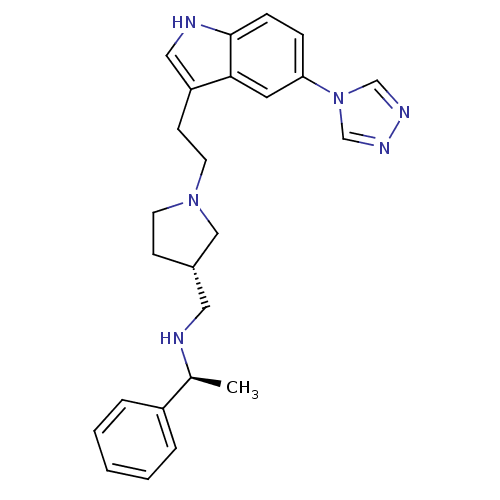 (((S)-1-Phenyl-ethyl)-{(S)-1-[2-(5-[1,2,4]triazol-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the 5-hydroxytryptamine 1D receptor | J Med Chem 42: 677-90 (1999) Article DOI: 10.1021/jm9805687 BindingDB Entry DOI: 10.7270/Q23777W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060434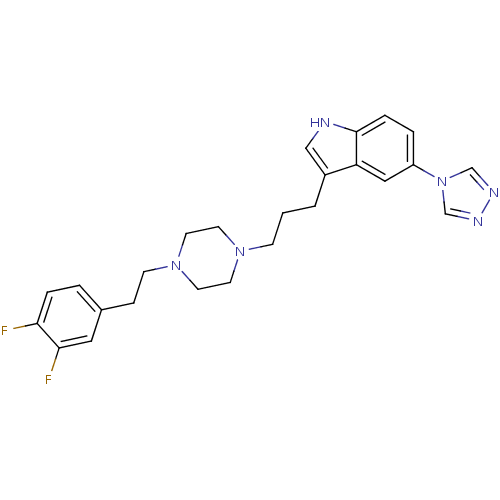 (3-(3-{4-[2-(3,4-Difluoro-phenyl)-ethyl]-piperazin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060425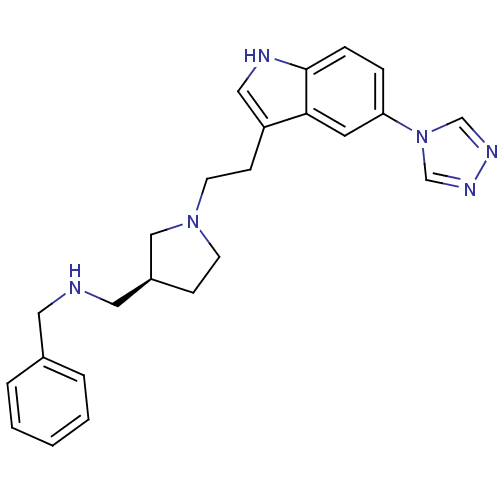 (Benzyl-{(S)-1-[2-(5-[1,2,4]triazol-4-yl-1H-indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Measurement of agonist induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor | Bioorg Med Chem Lett 9: 2491-6 (1999) BindingDB Entry DOI: 10.7270/Q27M074P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074180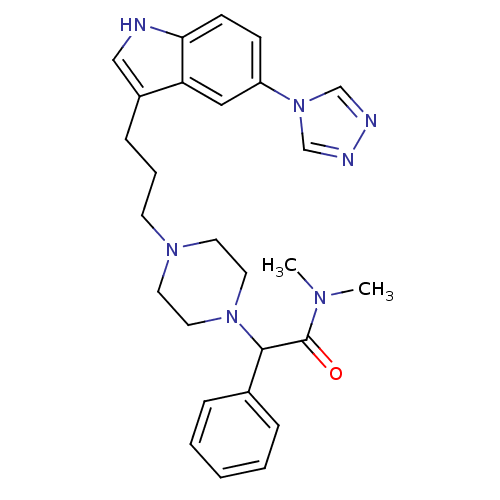 (CHEMBL164035 | N,N-Dimethyl-2-phenyl-2-{4-[3-(5-[1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060425 (Benzyl-{(S)-1-[2-(5-[1,2,4]triazol-4-yl-1H-indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the 5-hydroxytryptamine 1D receptor | J Med Chem 42: 677-90 (1999) Article DOI: 10.1021/jm9805687 BindingDB Entry DOI: 10.7270/Q23777W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060425 (Benzyl-{(S)-1-[2-(5-[1,2,4]triazol-4-yl-1H-indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the 5-hydroxytryptamine 1D receptor | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50083137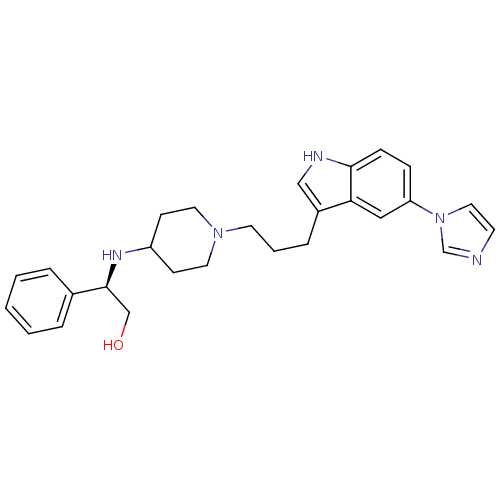 ((R)-2-{1-[3-(5-Imidazol-1-yl-1H-indol-3-yl)-propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]- GTPgammaS binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor | J Med Chem 42: 4981-5001 (2000) BindingDB Entry DOI: 10.7270/Q20P0Z77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060429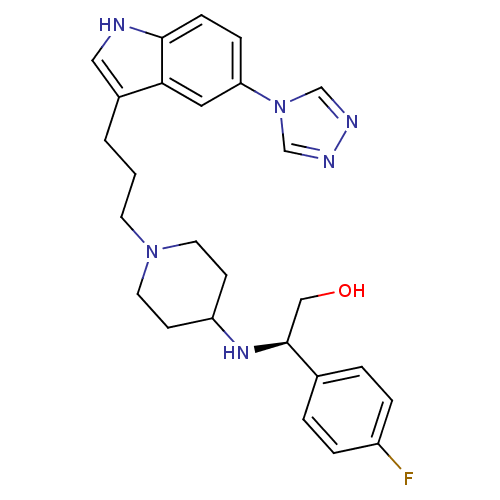 ((R)-2-(4-Fluoro-phenyl)-2-{1-[3-(5-[1,2,4]triazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]- GTPgammaS binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor | J Med Chem 42: 4981-5001 (2000) BindingDB Entry DOI: 10.7270/Q20P0Z77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060422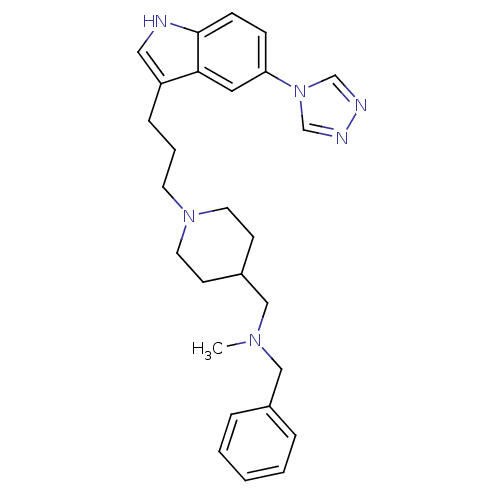 (Benzyl-methyl-{1-[3-(5-[1,2,4]triazol-4-yl-1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]- GTPgammaS binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor | J Med Chem 42: 4981-5001 (2000) BindingDB Entry DOI: 10.7270/Q20P0Z77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50083083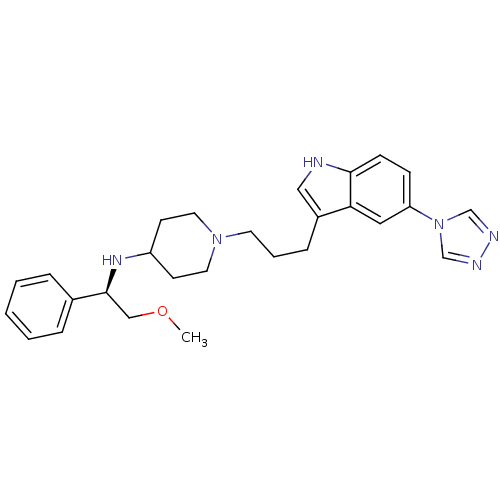 (((R)-2-Methoxy-1-phenyl-ethyl)-{1-[3-(5-[1,2,4]tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]- GTPgammaS binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor | J Med Chem 42: 4981-5001 (2000) BindingDB Entry DOI: 10.7270/Q20P0Z77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060422 (Benzyl-methyl-{1-[3-(5-[1,2,4]triazol-4-yl-1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Measurement of agonist induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor. | J Med Chem 40: 3497-500 (1997) Article DOI: 10.1021/jm9704558 BindingDB Entry DOI: 10.7270/Q2JQ103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50065418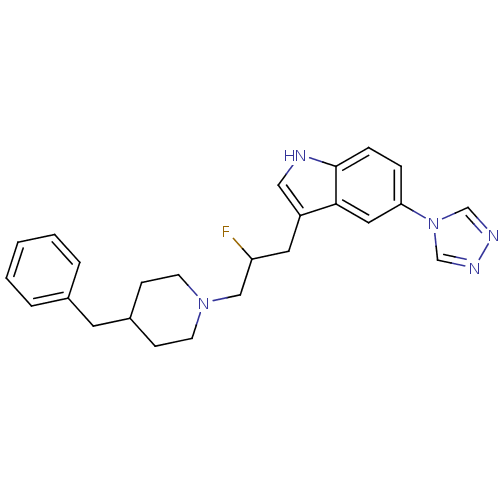 (3-[3-(4-Benzyl-piperidin-1-yl)-2-fluoro-propyl]-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 42: 2087-104 (1999) Article DOI: 10.1021/jm981133m BindingDB Entry DOI: 10.7270/Q2765DHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060444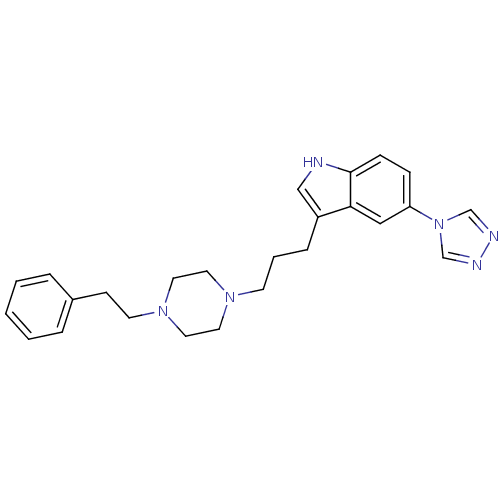 (3-[3-(4-Phenethyl-piperazin-1-yl)-propyl]-5-[1,2,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing 5-hydroxytryptamine 1D receptor | J Med Chem 40: 3501-3 (1997) Article DOI: 10.1021/jm9704560 BindingDB Entry DOI: 10.7270/Q2DZ07D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060425 (Benzyl-{(S)-1-[2-(5-[1,2,4]triazol-4-yl-1H-indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Measurement of agonist induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with 5-hydroxytryptamine 1D receptor. | J Med Chem 40: 3497-500 (1997) Article DOI: 10.1021/jm9704558 BindingDB Entry DOI: 10.7270/Q2JQ103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060437 (3-(3-{4-[2-(3-Fluoro-phenyl)-ethyl]-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50060437 (3-(3-{4-[2-(3-Fluoro-phenyl)-ethyl]-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Agonist-induced [35S]-GTP-gammaS, binding in CHO cells stably transfected with human 5-hydroxytryptamine 1D receptor | J Med Chem 42: 2087-104 (1999) Article DOI: 10.1021/jm981133m BindingDB Entry DOI: 10.7270/Q2765DHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50417427 (CHEMBL1290716) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human 5-HT1D receptor expressed in CHO cells assessed as stimulation of GTPgammaS binding by scintillation proximity assay | Bioorg Med Chem Lett 20: 7092-6 (2010) Article DOI: 10.1016/j.bmcl.2010.09.085 BindingDB Entry DOI: 10.7270/Q2MC919V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50074189 (3-[3-(4-Pyridin-4-ylmethyl-piperazin-1-yl)-propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Stimulation of [35S]-GTP-gammaS, binding in CHO cells expressing the human 5-hydroxytryptamine 1D receptor. | J Med Chem 42: 691-705 (1999) Article DOI: 10.1021/jm980569z BindingDB Entry DOI: 10.7270/Q2ZG6RDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 193 total ) | Next | Last >> |