 Found 998 hits of ic50 data for polymerid = 3609
Found 998 hits of ic50 data for polymerid = 3609 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205961
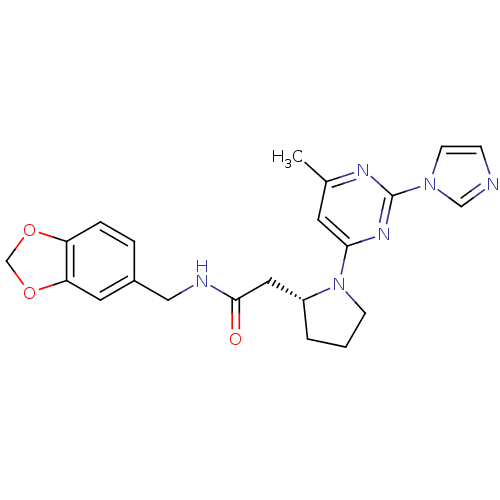
(2-((R)-1-(2-(1H-imidazol-1-yl)-6-methylpyrimidin-4...)Show SMILES Cc1cc(nc(n1)-n1ccnc1)N1CCC[C@@H]1CC(=O)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C22H24N6O3/c1-15-9-20(26-22(25-15)27-8-6-23-13-27)28-7-2-3-17(28)11-21(29)24-12-16-4-5-18-19(10-16)31-14-30-18/h4-6,8-10,13,17H,2-3,7,11-12,14H2,1H3,(H,24,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205952
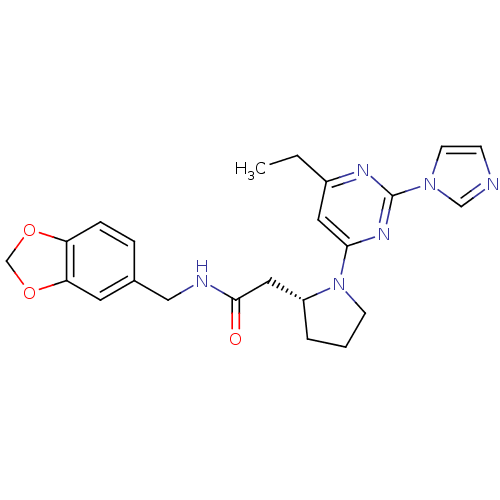
(2-((R)-1-(2-(1H-imidazol-1-yl)-6-methylpyrimidin-4...)Show SMILES CCc1cc(nc(n1)-n1ccnc1)N1CCC[C@@H]1CC(=O)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C23H26N6O3/c1-2-17-11-21(27-23(26-17)28-9-7-24-14-28)29-8-3-4-18(29)12-22(30)25-13-16-5-6-19-20(10-16)32-15-31-19/h5-7,9-11,14,18H,2-4,8,12-13,15H2,1H3,(H,25,30)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205910
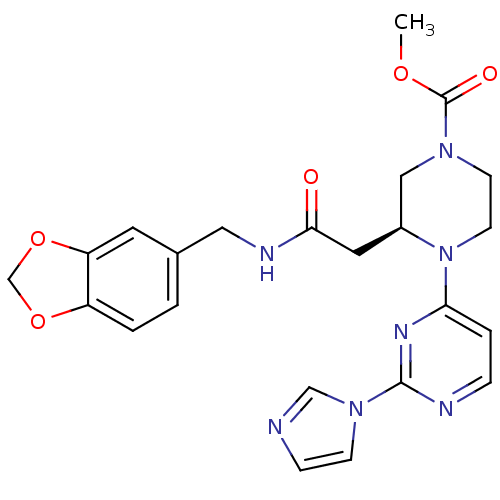
((3S)-methyl 4-(2-(1H-imidazol-1-yl)pyrimidin-4-yl)...)Show SMILES COC(=O)N1CCN([C@@H](CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C23H25N7O5/c1-33-23(32)28-8-9-30(20-4-5-25-22(27-20)29-7-6-24-14-29)17(13-28)11-21(31)26-12-16-2-3-18-19(10-16)35-15-34-18/h2-7,10,14,17H,8-9,11-13,15H2,1H3,(H,26,31)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205937
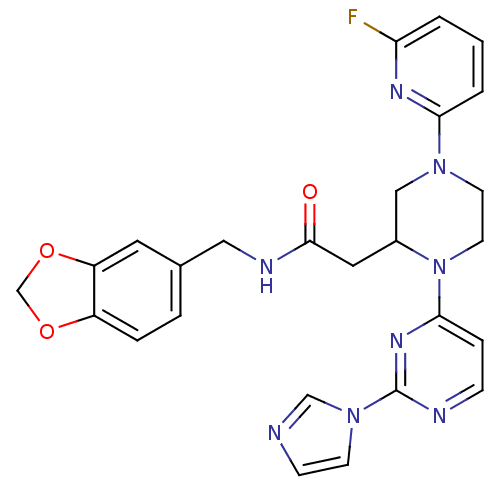
(CHEMBL385325 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES Fc1cccc(n1)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C26H25FN8O3/c27-22-2-1-3-23(31-22)33-10-11-35(24-6-7-29-26(32-24)34-9-8-28-16-34)19(15-33)13-25(36)30-14-18-4-5-20-21(12-18)38-17-37-20/h1-9,12,16,19H,10-11,13-15,17H2,(H,30,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205950
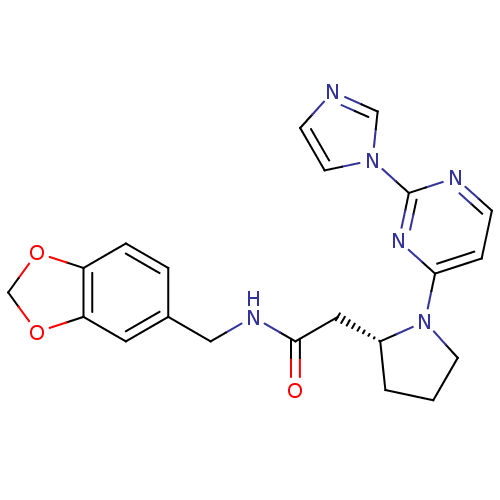
(2-((R)-1-(2-(1H-imidazol-1-yl)pyrimidin-4-yl)pyrro...)Show SMILES O=C(C[C@H]1CCCN1c1ccnc(n1)-n1ccnc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C21H22N6O3/c28-20(24-12-15-3-4-17-18(10-15)30-14-29-17)11-16-2-1-8-27(16)19-5-6-23-21(25-19)26-9-7-22-13-26/h3-7,9-10,13,16H,1-2,8,11-12,14H2,(H,24,28)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205947

((2R)-1-(2-(1H-imidazol-1-yl)-6-methylpyrimidin-4-y...)Show SMILES Cc1cc(nc(n1)-n1ccnc1)N1CCC[C@@H]1C(=O)NCCc1ccc2OCOc2c1 Show InChI InChI=1S/C22H24N6O3/c1-15-11-20(26-22(25-15)27-10-8-23-13-27)28-9-2-3-17(28)21(29)24-7-6-16-4-5-18-19(12-16)31-14-30-18/h4-5,8,10-13,17H,2-3,6-7,9,14H2,1H3,(H,24,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50111438
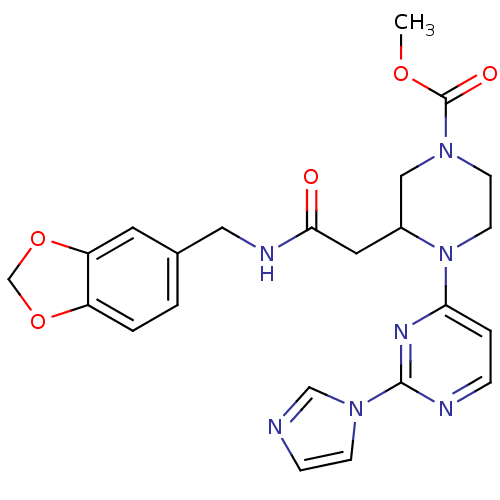
(3-{[(Benzo[1,3]dioxol-5-ylmethyl)-carbamoyl]-methy...)Show SMILES COC(=O)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C23H25N7O5/c1-33-23(32)28-8-9-30(20-4-5-25-22(27-20)29-7-6-24-14-29)17(13-28)11-21(31)26-12-16-2-3-18-19(10-16)35-15-34-18/h2-7,10,14,17H,8-9,11-13,15H2,1H3,(H,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205911
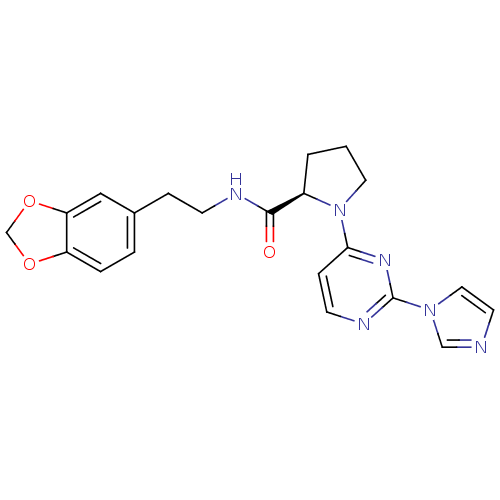
(2-((R)-1-(2-(1H-imidazol-1-yl)-6-methylpyrimidin-4...)Show SMILES O=C(NCCc1ccc2OCOc2c1)[C@H]1CCCN1c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C21H22N6O3/c28-20(23-7-5-15-3-4-17-18(12-15)30-14-29-17)16-2-1-10-27(16)19-6-8-24-21(25-19)26-11-9-22-13-26/h3-4,6,8-9,11-13,16H,1-2,5,7,10,14H2,(H,23,28)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205932

(CHEMBL373623 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES CN1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C22H25N7O3/c1-27-8-9-29(20-4-5-24-22(26-20)28-7-6-23-14-28)17(13-27)11-21(30)25-12-16-2-3-18-19(10-16)32-15-31-18/h2-7,10,14,17H,8-9,11-13,15H2,1H3,(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205956
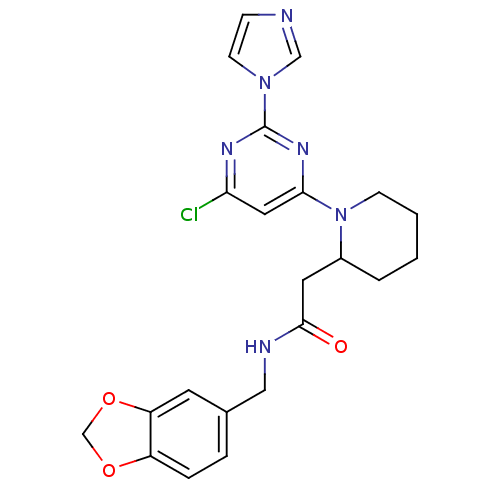
(CHEMBL223788 | N-(1,3-benzodioxol-5-ylmethyl)-1-[6...)Show SMILES Clc1cc(nc(n1)-n1ccnc1)N1CCCCC1CC(=O)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C22H23ClN6O3/c23-19-11-20(27-22(26-19)28-8-6-24-13-28)29-7-2-1-3-16(29)10-21(30)25-12-15-4-5-17-18(9-15)32-14-31-17/h4-6,8-9,11,13,16H,1-3,7,10,12,14H2,(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205957
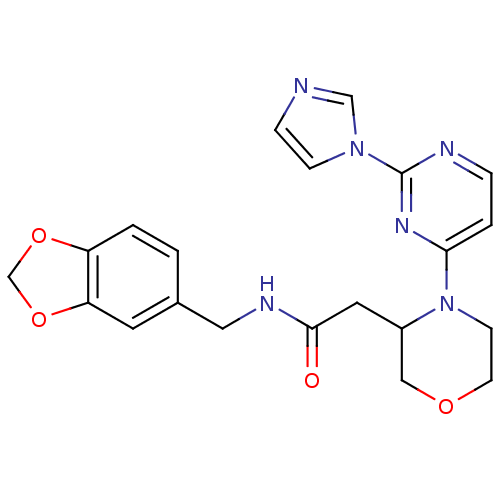
(CHEMBL220932 | N-[(1,3-benzodioxol-5-yl)methyl]-4-...)Show SMILES O=C(CC1COCCN1c1ccnc(n1)-n1ccnc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C21H22N6O4/c28-20(24-11-15-1-2-17-18(9-15)31-14-30-17)10-16-12-29-8-7-27(16)19-3-4-23-21(25-19)26-6-5-22-13-26/h1-6,9,13,16H,7-8,10-12,14H2,(H,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205926
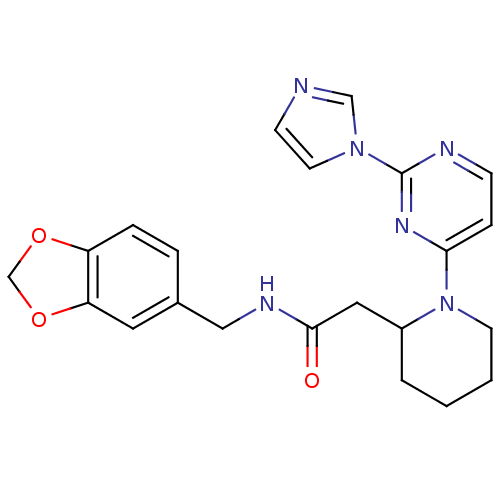
(CHEMBL442041 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES O=C(CC1CCCCN1c1ccnc(n1)-n1ccnc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C22H24N6O3/c29-21(25-13-16-4-5-18-19(11-16)31-15-30-18)12-17-3-1-2-9-28(17)20-6-7-24-22(26-20)27-10-8-23-14-27/h4-8,10-11,14,17H,1-3,9,12-13,15H2,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205954

(CHEMBL385334 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES O=C(CC1CN(CCN1c1ccnc(n1)-n1ccnc1)C(=O)c1ccccc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C28H27N7O4/c36-26(31-16-20-6-7-23-24(14-20)39-19-38-23)15-22-17-33(27(37)21-4-2-1-3-5-21)12-13-35(22)25-8-9-30-28(32-25)34-11-10-29-18-34/h1-11,14,18,22H,12-13,15-17,19H2,(H,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205905
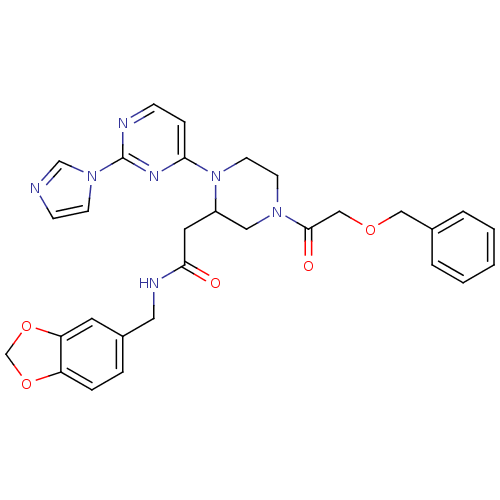
(CHEMBL376713 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES O=C(CC1CN(CCN1c1ccnc(n1)-n1ccnc1)C(=O)COCc1ccccc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C30H31N7O5/c38-28(33-16-23-6-7-25-26(14-23)42-21-41-25)15-24-17-35(29(39)19-40-18-22-4-2-1-3-5-22)12-13-37(24)27-8-9-32-30(34-27)36-11-10-31-20-36/h1-11,14,20,24H,12-13,15-19,21H2,(H,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205907

((2R)-N-(2-(benzo[d][1,3]dioxol-5-yl)ethyl)-1-(6-et...)Show SMILES CCc1cc(nc(n1)-n1ccnc1)N1CCC[C@@H]1C(=O)NCCc1ccc2OCOc2c1 Show InChI InChI=1S/C23H26N6O3/c1-2-17-13-21(27-23(26-17)28-11-9-24-14-28)29-10-3-4-18(29)22(30)25-8-7-16-5-6-19-20(12-16)32-15-31-19/h5-6,9,11-14,18H,2-4,7-8,10,15H2,1H3,(H,25,30)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205973
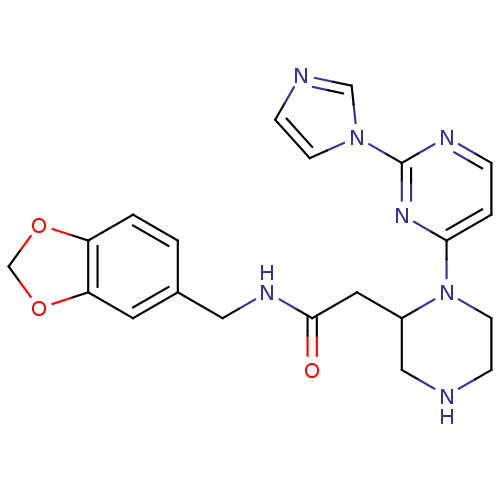
(CHEMBL411343 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES O=C(CC1CNCCN1c1ccnc(n1)-n1ccnc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C21H23N7O3/c29-20(25-11-15-1-2-17-18(9-15)31-14-30-17)10-16-12-22-6-8-28(16)19-3-4-24-21(26-19)27-7-5-23-13-27/h1-5,7,9,13,16,22H,6,8,10-12,14H2,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205938

(CHEMBL424928 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES O=C(CC1CN(Cc2ccco2)CCN1c1ccnc(n1)-n1ccnc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C26H27N7O4/c34-25(29-14-19-3-4-22-23(12-19)37-18-36-22)13-20-15-31(16-21-2-1-11-35-21)9-10-33(20)24-5-6-28-26(30-24)32-8-7-27-17-32/h1-8,11-12,17,20H,9-10,13-16,18H2,(H,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50111438

(3-{[(Benzo[1,3]dioxol-5-ylmethyl)-carbamoyl]-methy...)Show SMILES COC(=O)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C23H25N7O5/c1-33-23(32)28-8-9-30(20-4-5-25-22(27-20)29-7-6-24-14-29)17(13-28)11-21(31)26-12-16-2-3-18-19(10-16)35-15-34-18/h2-7,10,14,17H,8-9,11-13,15H2,1H3,(H,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against the partially purified human Inducible nitric oxide synthase |
J Med Chem 45: 1543-58 (2002)
BindingDB Entry DOI: 10.7270/Q2CN74ND |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205909

(CHEMBL223782 | N-(1,3-benzodioxol-5-ylmethyl)-4-(2...)Show SMILES O=C(CC1CN(CCN1c1ccnc(n1)-n1ccnc1)C(=O)c1ccco1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C26H25N7O5/c34-24(29-14-18-3-4-20-22(12-18)38-17-37-20)13-19-15-31(25(35)21-2-1-11-36-21)9-10-33(19)23-5-6-28-26(30-23)32-8-7-27-16-32/h1-8,11-12,16,19H,9-10,13-15,17H2,(H,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205968

(CHEMBL373881 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES CS(=O)(=O)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C22H25N7O5S/c1-35(31,32)28-8-9-29(20-4-5-24-22(26-20)27-7-6-23-14-27)17(13-28)11-21(30)25-12-16-2-3-18-19(10-16)34-15-33-18/h2-7,10,14,17H,8-9,11-13,15H2,1H3,(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205930
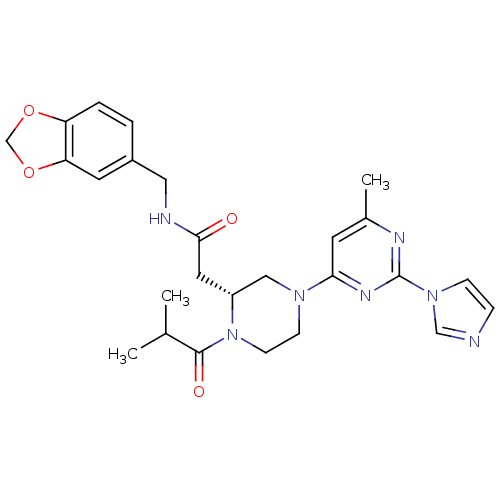
(2-((R)-4-(2-(1H-imidazol-1-yl)-6-methylpyrimidin-4...)Show SMILES CC(C)C(=O)N1CCN(C[C@H]1CC(=O)NCc1ccc2OCOc2c1)c1cc(C)nc(n1)-n1ccnc1 Show InChI InChI=1S/C26H31N7O4/c1-17(2)25(35)33-9-8-31(23-10-18(3)29-26(30-23)32-7-6-27-15-32)14-20(33)12-24(34)28-13-19-4-5-21-22(11-19)37-16-36-21/h4-7,10-11,15,17,20H,8-9,12-14,16H2,1-3H3,(H,28,34)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205971
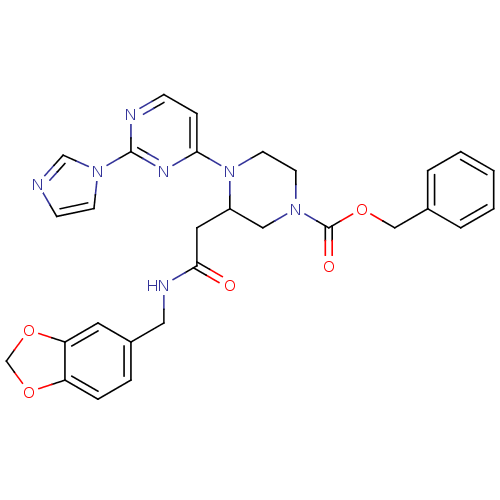
(CHEMBL223792 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES O=C(CC1CN(CCN1c1ccnc(n1)-n1ccnc1)C(=O)OCc1ccccc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C29H29N7O5/c37-27(32-16-22-6-7-24-25(14-22)41-20-40-24)15-23-17-34(29(38)39-18-21-4-2-1-3-5-21)12-13-36(23)26-8-9-31-28(33-26)35-11-10-30-19-35/h1-11,14,19,23H,12-13,15-18,20H2,(H,32,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205904
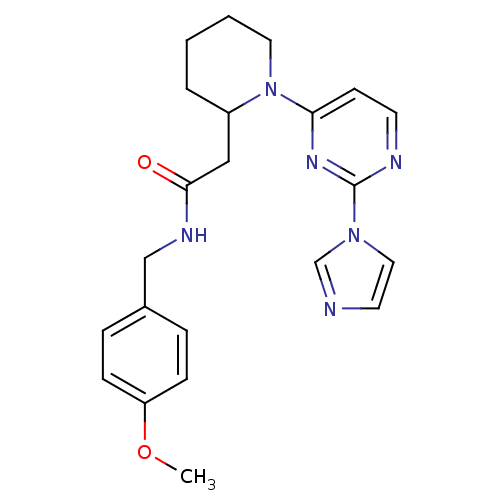
(1-[2-(1H-imidazol-1-yl)-4-pyrimidinyl]-N-[(4-metho...)Show SMILES COc1ccc(CNC(=O)CC2CCCCN2c2ccnc(n2)-n2ccnc2)cc1 Show InChI InChI=1S/C22H26N6O2/c1-30-19-7-5-17(6-8-19)15-25-21(29)14-18-4-2-3-12-28(18)20-9-10-24-22(26-20)27-13-11-23-16-27/h5-11,13,16,18H,2-4,12,14-15H2,1H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205964
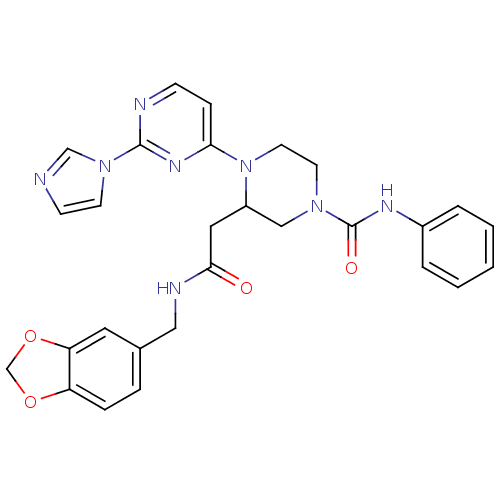
(CHEMBL374319 | N-(1,3-benzodioxol-5-ylmethyl)-1-[2...)Show SMILES O=C(CC1CN(CCN1c1ccnc(n1)-n1ccnc1)C(=O)Nc1ccccc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C28H28N8O4/c37-26(31-16-20-6-7-23-24(14-20)40-19-39-23)15-22-17-34(28(38)32-21-4-2-1-3-5-21)12-13-36(22)25-8-9-30-27(33-25)35-11-10-29-18-35/h1-11,14,18,22H,12-13,15-17,19H2,(H,31,37)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205965

(CHEMBL222364 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES CCOC(=O)CN1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C25H29N7O5/c1-2-35-24(34)15-30-9-10-32(22-5-6-27-25(29-22)31-8-7-26-16-31)19(14-30)12-23(33)28-13-18-3-4-20-21(11-18)37-17-36-20/h3-8,11,16,19H,2,9-10,12-15,17H2,1H3,(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205931
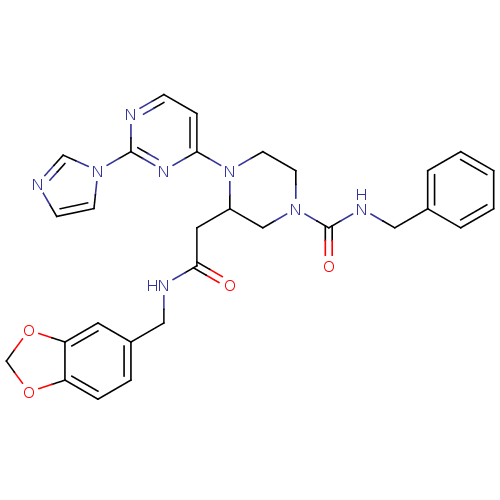
(CHEMBL223791 | N-(1,3-benzodioxol-5-ylmethyl)-1-[2...)Show SMILES O=C(CC1CN(CCN1c1ccnc(n1)-n1ccnc1)C(=O)NCc1ccccc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C29H30N8O4/c38-27(32-17-22-6-7-24-25(14-22)41-20-40-24)15-23-18-35(29(39)33-16-21-4-2-1-3-5-21)12-13-37(23)26-8-9-31-28(34-26)36-11-10-30-19-36/h1-11,14,19,23H,12-13,15-18,20H2,(H,32,38)(H,33,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205974
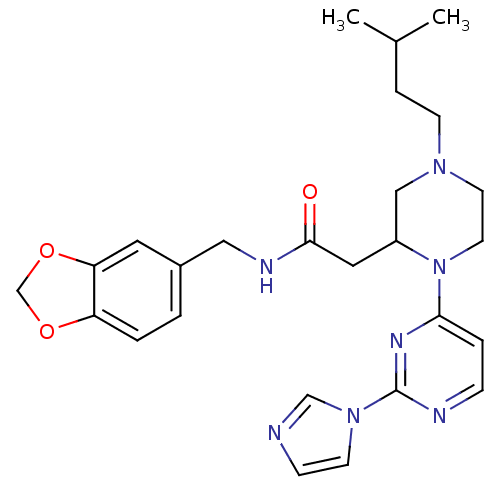
(CHEMBL375404 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES CC(C)CCN1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C26H33N7O3/c1-19(2)6-9-31-11-12-33(24-5-7-28-26(30-24)32-10-8-27-17-32)21(16-31)14-25(34)29-15-20-3-4-22-23(13-20)36-18-35-22/h3-5,7-8,10,13,17,19,21H,6,9,11-12,14-16,18H2,1-2H3,(H,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205924
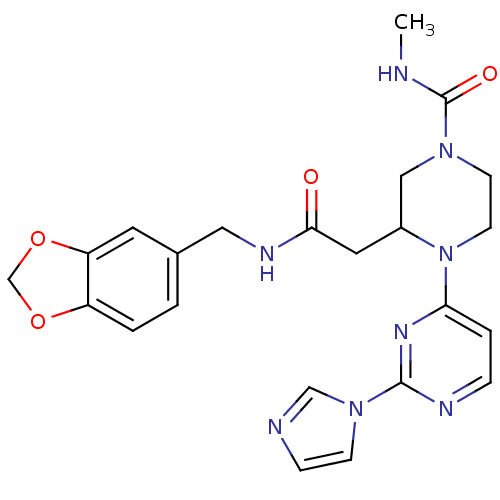
(CHEMBL223384 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES CNC(=O)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C23H26N8O4/c1-24-23(33)29-8-9-31(20-4-5-26-22(28-20)30-7-6-25-14-30)17(13-29)11-21(32)27-12-16-2-3-18-19(10-16)35-15-34-18/h2-7,10,14,17H,8-9,11-13,15H2,1H3,(H,24,33)(H,27,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205921
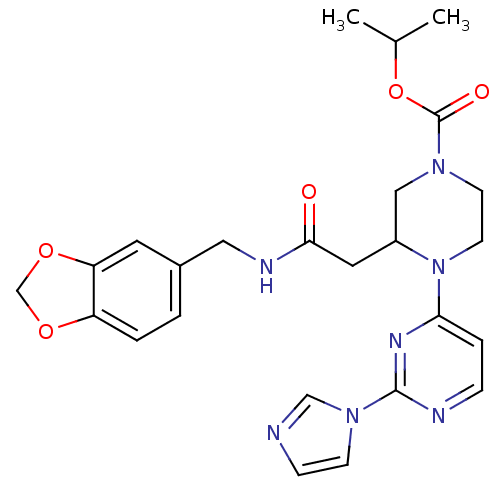
(3-[2-[(1,3-benzodioxol-5-ylmethyl)amino]-2-oxoethy...)Show SMILES CC(C)OC(=O)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C25H29N7O5/c1-17(2)37-25(34)30-9-10-32(22-5-6-27-24(29-22)31-8-7-26-15-31)19(14-30)12-23(33)28-13-18-3-4-20-21(11-18)36-16-35-20/h3-8,11,15,17,19H,9-10,12-14,16H2,1-2H3,(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205920

(CHEMBL223634 | N-[(1,3-benzodioxol-5-yl)methyl]-1-...)Show SMILES O=C(CC1CN(Cc2ccccc2)CCN1c1ccnc(n1)-n1ccnc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C28H29N7O3/c36-27(31-16-22-6-7-24-25(14-22)38-20-37-24)15-23-18-33(17-21-4-2-1-3-5-21)12-13-35(23)26-8-9-30-28(32-26)34-11-10-29-19-34/h1-11,14,19,23H,12-13,15-18,20H2,(H,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205929
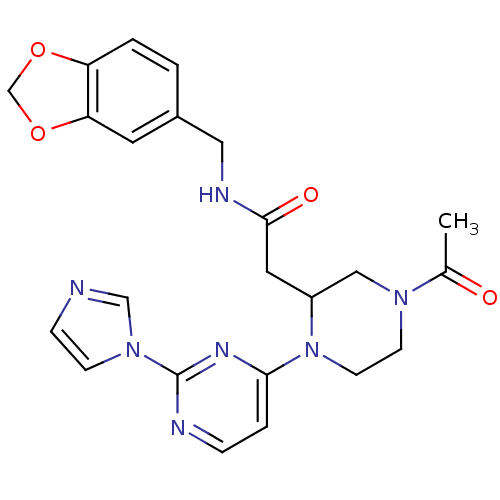
(4-(acetyl)-N-[(1,3-benzodioxol-5-yl)methyl]-1-[2-(...)Show SMILES CC(=O)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C23H25N7O4/c1-16(31)28-8-9-30(21-4-5-25-23(27-21)29-7-6-24-14-29)18(13-28)11-22(32)26-12-17-2-3-19-20(10-17)34-15-33-19/h2-7,10,14,18H,8-9,11-13,15H2,1H3,(H,26,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205976

(CHEMBL223838 | N-[(1,3-benzodioxol-5-yl)methyl]-4-...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C33H34N8O5S/c1-38(2)27-7-3-6-26-25(27)5-4-8-30(26)47(43,44)40-15-16-41(31-11-12-35-33(37-31)39-14-13-34-21-39)24(20-40)18-32(42)36-19-23-9-10-28-29(17-23)46-22-45-28/h3-14,17,21,24H,15-16,18-20,22H2,1-2H3,(H,36,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50111448

(CHEMBL440473 | N-Benzo[1,3]dioxol-5-ylmethyl-2-[1-...)Show SMILES Clc1cc(nc(n1)-n1ccnc1)N1CCNCC1CC(=O)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C21H22ClN7O3/c22-18-9-19(27-21(26-18)28-5-3-24-12-28)29-6-4-23-11-15(29)8-20(30)25-10-14-1-2-16-17(7-14)32-13-31-16/h1-3,5,7,9,12,15,23H,4,6,8,10-11,13H2,(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against the partially purified human Inducible nitric oxide synthase |
J Med Chem 45: 1543-58 (2002)
BindingDB Entry DOI: 10.7270/Q2CN74ND |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205953

(3-[2-[(1,3-benzodioxol-5-ylmethyl)amino]-2-oxoethy...)Show SMILES O=C(CC1CN(CCN1c1ccnc(n1)-n1ccnc1)C(=O)Oc1ccccc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C28H27N7O5/c36-26(31-16-20-6-7-23-24(14-20)39-19-38-23)15-21-17-33(28(37)40-22-4-2-1-3-5-22)12-13-35(21)25-8-9-30-27(32-25)34-11-10-29-18-34/h1-11,14,18,21H,12-13,15-17,19H2,(H,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205903
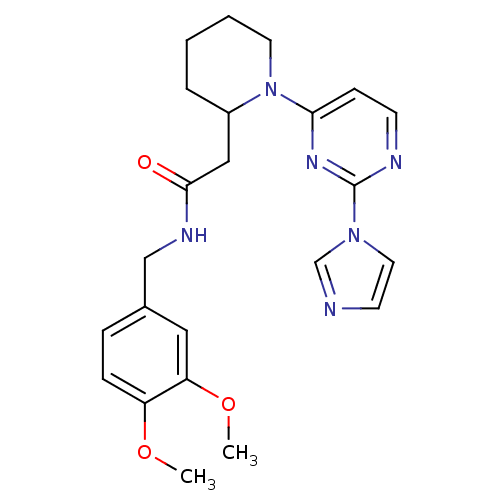
(1-[2-(1H-imidazol-1-yl)pyrimidin-4-yl]-N-[(3,4-dim...)Show SMILES COc1ccc(CNC(=O)CC2CCCCN2c2ccnc(n2)-n2ccnc2)cc1OC Show InChI InChI=1S/C23H28N6O3/c1-31-19-7-6-17(13-20(19)32-2)15-26-22(30)14-18-5-3-4-11-29(18)21-8-9-25-23(27-21)28-12-10-24-16-28/h6-10,12-13,16,18H,3-5,11,14-15H2,1-2H3,(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50230993

((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...)Show InChI InChI=1S/C7H16N4O2/c1-10-7(9)11-4-2-3-5(8)6(12)13/h5H,2-4,8H2,1H3,(H,12,13)(H3,9,10,11)/t5-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins measured after 45 mins by ... |
ACS Med Chem Lett 3: 227-231 (2012)
Article DOI: 10.1021/ml200268w
BindingDB Entry DOI: 10.7270/Q2TX3GF4 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205955
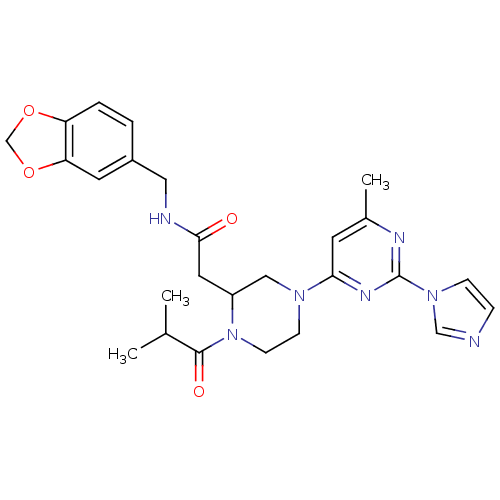
(CHEMBL220771 | N-[(1,3-benzodioxol-5-yl)methyl]-4-...)Show SMILES CC(C)C(=O)N1CCN(CC1CC(=O)NCc1ccc2OCOc2c1)c1cc(C)nc(n1)-n1ccnc1 Show InChI InChI=1S/C26H31N7O4/c1-17(2)25(35)33-9-8-31(23-10-18(3)29-26(30-23)32-7-6-27-15-32)14-20(33)12-24(34)28-13-19-4-5-21-22(11-19)37-16-36-21/h4-7,10-11,15,17,20H,8-9,12-14,16H2,1-3H3,(H,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM331831

(US10189791, CDDO-Im 1)Show SMILES CC1(C)CCC2(CCC3(C)C(C2C1)C(=O)C=C1C2(C)C=C(C#N)C(=O)C(C)(C)C2CCC31C)C(=O)n1ccnc1 |t:17,21| Show InChI InChI=1S/C34H43N3O3/c1-29(2)10-12-34(28(40)37-15-14-36-20-37)13-11-33(7)26(22(34)18-29)23(38)16-25-31(5)17-21(19-35)27(39)30(3,4)24(31)8-9-32(25,33)6/h14-17,20,22,24,26H,8-13,18H2,1-7H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College
US Patent
| Assay Description
Because oxidative and inflammatory stress contribute to carcinogenesis (Albini & Sporn (2007) Nature Rev. Cancer 7:139-147), it was determined whethe... |
US Patent US10189791 (2019)
BindingDB Entry DOI: 10.7270/Q2571F3P |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124519

(5,8-Difluoro-2-furan-2-yl-1,2-dihydro-quinazolin-4...)Show InChI InChI=1S/C12H9F2N3O/c13-6-3-4-7(14)10-9(6)11(15)17-12(16-10)8-2-1-5-18-8/h1-5,12,16H,(H2,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R& D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human inducible nitiric oxide synthase |
J Med Chem 46: 913-6 (2003)
Article DOI: 10.1021/jm0255926
BindingDB Entry DOI: 10.7270/Q2S75H3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205980
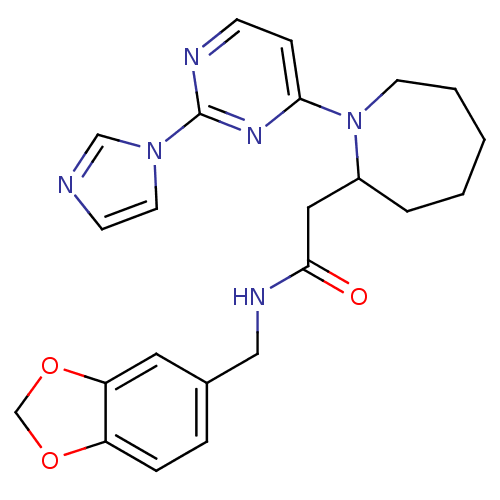
(CHEMBL223442 | N-[(1,3-benzodioxol-5-yl)methyl]hex...)Show SMILES O=C(CC1CCCCCN1c1ccnc(n1)-n1ccnc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C23H26N6O3/c30-22(26-14-17-5-6-19-20(12-17)32-16-31-19)13-18-4-2-1-3-10-29(18)21-7-8-25-23(27-21)28-11-9-24-15-28/h5-9,11-12,15,18H,1-4,10,13-14,16H2,(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205914
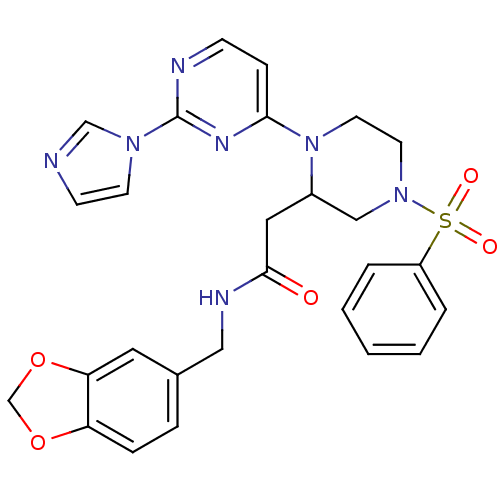
(CHEMBL220156 | N-(1,3-benzodioxol-5-ylmethyl)-1-[2...)Show SMILES O=C(CC1CN(CCN1c1ccnc(n1)-n1ccnc1)S(=O)(=O)c1ccccc1)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C27H27N7O5S/c35-26(30-16-20-6-7-23-24(14-20)39-19-38-23)15-21-17-33(40(36,37)22-4-2-1-3-5-22)12-13-34(21)25-8-9-29-27(31-25)32-11-10-28-18-32/h1-11,14,18,21H,12-13,15-17,19H2,(H,30,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205944
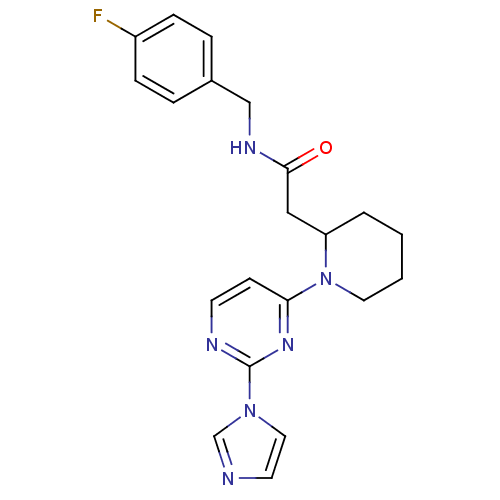
(CHEMBL436535 | N-[(4-fluorophenyl)methyl]-1-[2-(1H...)Show SMILES Fc1ccc(CNC(=O)CC2CCCCN2c2ccnc(n2)-n2ccnc2)cc1 Show InChI InChI=1S/C21H23FN6O/c22-17-6-4-16(5-7-17)14-25-20(29)13-18-3-1-2-11-28(18)19-8-9-24-21(26-19)27-12-10-23-15-27/h4-10,12,15,18H,1-3,11,13-14H2,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205940

(1-[2-(1H-imidazol-1-yl)-4-pyrimidinyl]-N-[2-(4-met...)Show SMILES COc1ccc(CCNC(=O)CC2CCCCN2c2ccnc(n2)-n2ccnc2)cc1 Show InChI InChI=1S/C23H28N6O2/c1-31-20-7-5-18(6-8-20)9-11-25-22(30)16-19-4-2-3-14-29(19)21-10-12-26-23(27-21)28-15-13-24-17-28/h5-8,10,12-13,15,17,19H,2-4,9,11,14,16H2,1H3,(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205970
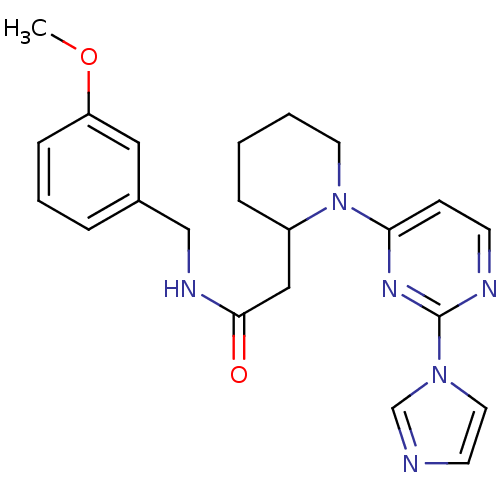
(1-[2-(1H-imidazol-1-yl)pyrimidin-4-yl]-N-[(3-metho...)Show SMILES COc1cccc(CNC(=O)CC2CCCCN2c2ccnc(n2)-n2ccnc2)c1 Show InChI InChI=1S/C22H26N6O2/c1-30-19-7-4-5-17(13-19)15-25-21(29)14-18-6-2-3-11-28(18)20-8-9-24-22(26-20)27-12-10-23-16-27/h4-5,7-10,12-13,16,18H,2-3,6,11,14-15H2,1H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50340003

((R)-2-(3-amino-1-phenylpropylthio)-6-methylnicotin...)Show InChI InChI=1S/C16H17N3S/c1-12-7-8-14(11-18)16(19-12)20-15(9-10-17)13-5-3-2-4-6-13/h2-8,15H,9-10,17H2,1H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339998
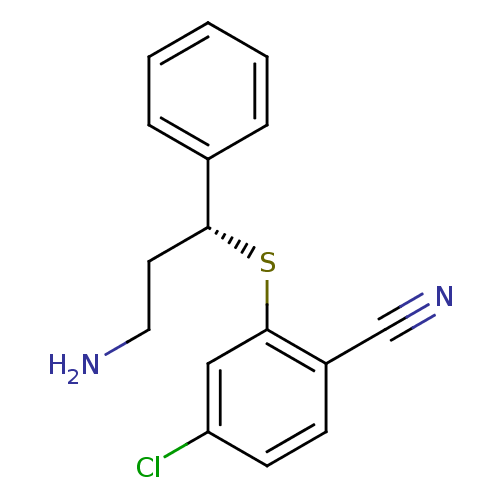
((R)-2-(3-amino-1-phenylpropylthio)-4-chlorobenzoni...)Show InChI InChI=1S/C16H15ClN2S/c17-14-7-6-13(11-19)16(10-14)20-15(8-9-18)12-4-2-1-3-5-12/h1-7,10,15H,8-9,18H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50339997
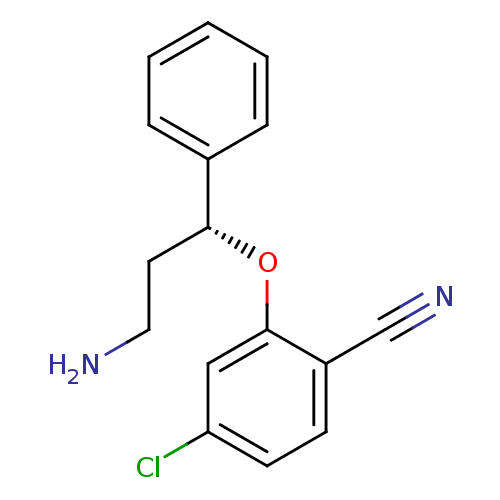
((R)-2-(3-amino-1-phenylpropoxy)-4-chlorobenzonitri...)Show InChI InChI=1S/C16H15ClN2O/c17-14-7-6-13(11-19)16(10-14)20-15(8-9-18)12-4-2-1-3-5-12/h1-7,10,15H,8-9,18H2/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant iNOS |
Bioorg Med Chem Lett 21: 2468-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.061
BindingDB Entry DOI: 10.7270/Q29G5N4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50205939

(CHEMBL221264 | N-(1,3-benzodioxol-5-ylmethyl)-1-[2...)Show SMILES Cc1cc(nc(n1)-n1ccnc1)N1CCCCC1CC(=O)NCc1ccc2OCOc2c1 Show InChI InChI=1S/C23H26N6O3/c1-16-10-21(27-23(26-16)28-9-7-24-14-28)29-8-3-2-4-18(29)12-22(30)25-13-17-5-6-19-20(11-17)32-15-31-19/h5-7,9-11,14,18H,2-4,8,12-13,15H2,1H3,(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of cytokine-induced iNOS expressed in human A172 cells assessed as inhibition of NO formation |
J Med Chem 50: 1146-57 (2007)
Article DOI: 10.1021/jm061319i
BindingDB Entry DOI: 10.7270/Q2SX6CWJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM331833
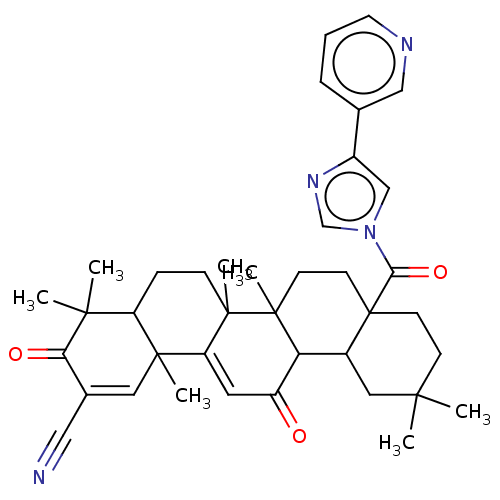
(US10189791, CDDO-3P-Im 3)Show SMILES CC1(C)CCC2(CCC3(C)C(C2C1)C(=O)C=C1C2(C)C=C(C#N)C(=O)C(C)(C)C2CCC31C)C(=O)n1cnc(c1)-c1cccnc1 |t:17,21| Show InChI InChI=1S/C39H46N4O3/c1-34(2)12-14-39(33(46)43-22-27(42-23-43)24-9-8-16-41-21-24)15-13-38(7)31(26(39)19-34)28(44)17-30-36(5)18-25(20-40)32(45)35(3,4)29(36)10-11-37(30,38)6/h8-9,16-18,21-23,26,29,31H,10-15,19H2,1-7H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College
US Patent
| Assay Description
Because oxidative and inflammatory stress contribute to carcinogenesis (Albini & Sporn (2007) Nature Rev. Cancer 7:139-147), it was determined whethe... |
US Patent US10189791 (2019)
BindingDB Entry DOI: 10.7270/Q2571F3P |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50363262

(CHEMBL1944717)Show SMILES Fc1ccc2c(CN(c3cccc(Cl)c3)c3cnccn3)cc(=O)[nH]c2c1F Show InChI InChI=1S/C20H13ClF2N4O/c21-13-2-1-3-14(9-13)27(17-10-24-6-7-25-17)11-12-8-18(28)26-20-15(12)4-5-16(22)19(20)23/h1-10H,11H2,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Afraxis
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS expressed in HEK293 cells assessed as NO production by 2,3-diaminonapthalene-based fluorescence assay |
Bioorg Med Chem Lett 22: 1237-41 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.073
BindingDB Entry DOI: 10.7270/Q26W9BJ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data