 Found 7640 hits of ic50 data for polymerid = 3619
Found 7640 hits of ic50 data for polymerid = 3619 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9017
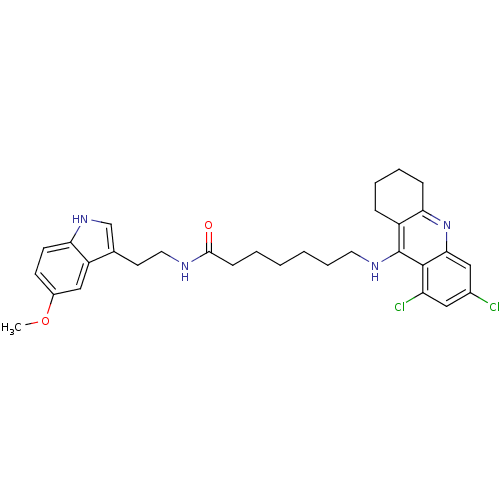
(7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...)Show SMILES COc1ccc2[nH]cc(CCNC(=O)CCCCCCNc3c4CCCCc4nc4cc(Cl)cc(Cl)c34)c2c1 Show InChI InChI=1S/C31H36Cl2N4O2/c1-39-22-11-12-26-24(18-22)20(19-36-26)13-15-34-29(38)10-4-2-3-7-14-35-31-23-8-5-6-9-27(23)37-28-17-21(32)16-25(33)30(28)31/h11-12,16-19,36H,2-10,13-15H2,1H3,(H,34,38)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50564213
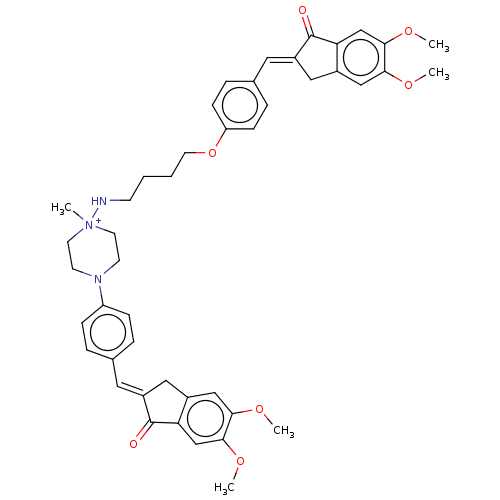
(CHEMBL4780352)Show SMILES [Br-].COc1cc2C\C(=C/c3ccc(OCCCCN[N+]4(C)CCN(CC4)c4ccc(\C=C5/Cc6cc(OC)c(OC)cc6C5=O)cc4)cc3)C(=O)c2cc1OC | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112140
BindingDB Entry DOI: 10.7270/Q2RV0SGV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9006
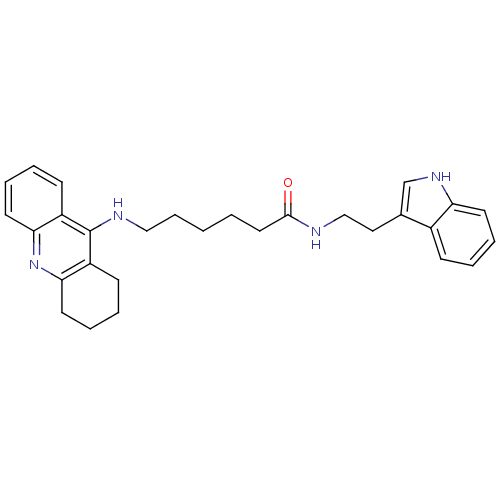
(6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...)Show SMILES O=C(CCCCCNc1c2CCCCc2nc2ccccc12)NCCc1c[nH]c2ccccc12 Show InChI InChI=1S/C29H34N4O/c34-28(30-19-17-21-20-32-25-13-6-3-10-22(21)25)16-2-1-9-18-31-29-23-11-4-7-14-26(23)33-27-15-8-5-12-24(27)29/h3-4,6-7,10-11,13-14,20,32H,1-2,5,8-9,12,15-19H2,(H,30,34)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9011
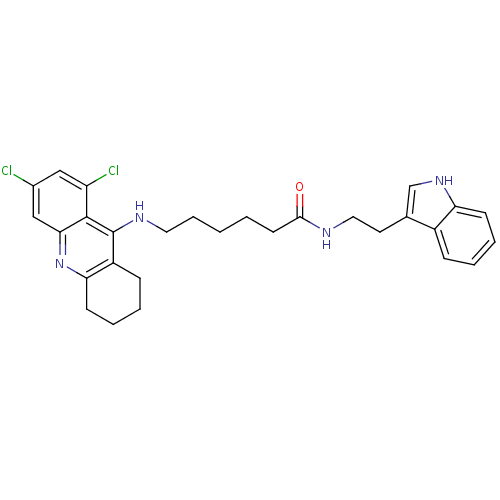
(6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...)Show SMILES Clc1cc(Cl)c2c(NCCCCCC(=O)NCCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C29H32Cl2N4O/c30-20-16-23(31)28-26(17-20)35-25-11-6-4-9-22(25)29(28)33-14-7-1-2-12-27(36)32-15-13-19-18-34-24-10-5-3-8-21(19)24/h3,5,8,10,16-18,34H,1-2,4,6-7,9,11-15H2,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510865

(CHEMBL4532770)Show SMILES COc1ccc2[nH]cc(CCNC(=O)CCCCCCCNc3c4CCCCc4nc4ccccc34)c2c1 Show InChI InChI=1S/C32H40N4O2/c1-38-24-16-17-28-27(21-24)23(22-35-28)18-20-33-31(37)15-5-3-2-4-10-19-34-32-25-11-6-8-13-29(25)36-30-14-9-7-12-26(30)32/h6,8,11,13,16-17,21-22,35H,2-5,7,9-10,12,14-15,18-20H2,1H3,(H,33,37)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9018
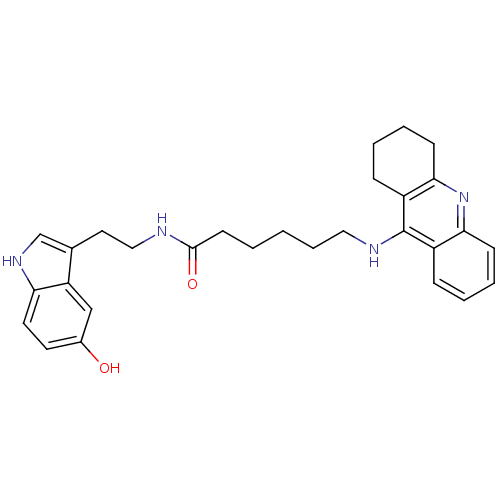
(6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...)Show SMILES Oc1ccc2[nH]cc(CCNC(=O)CCCCCNc3c4CCCCc4nc4ccccc34)c2c1 Show InChI InChI=1S/C29H34N4O2/c34-21-13-14-25-24(18-21)20(19-32-25)15-17-30-28(35)12-2-1-7-16-31-29-22-8-3-5-10-26(22)33-27-11-6-4-9-23(27)29/h3,5,8,10,13-14,18-19,32,34H,1-2,4,6-7,9,11-12,15-17H2,(H,30,35)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510867
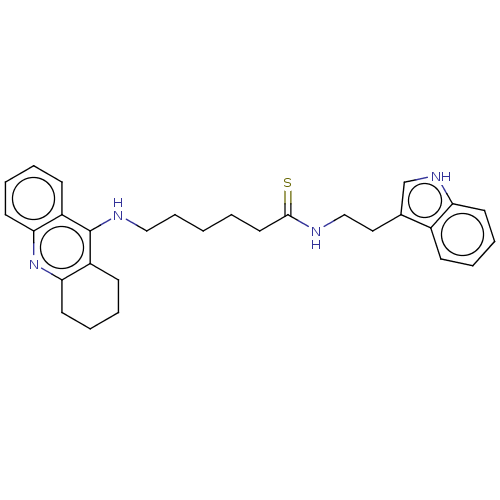
(CHEMBL4469239)Show SMILES S=C(CCCCCNc1c2CCCCc2nc2ccccc12)NCCc1c[nH]c2ccccc12 Show InChI InChI=1S/C29H34N4S/c34-28(30-19-17-21-20-32-25-13-6-3-10-22(21)25)16-2-1-9-18-31-29-23-11-4-7-14-26(23)33-27-15-8-5-12-24(27)29/h3-4,6-7,10-11,13-14,20,32H,1-2,5,8-9,12,15-19H2,(H,30,34)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510868

(CHEMBL4448350)Show SMILES Clc1cccc2c(NCCCCCC(=O)NCCc3c[nH]c4ccccc34)c3CCCCc3nc12 Show InChI InChI=1S/C29H33ClN4O/c30-24-12-8-11-23-28(22-10-4-6-14-26(22)34-29(23)24)32-17-7-1-2-15-27(35)31-18-16-20-19-33-25-13-5-3-9-21(20)25/h3,5,8-9,11-13,19,33H,1-2,4,6-7,10,14-18H2,(H,31,35)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510869

(CHEMBL4443426)Show SMILES O=C(CCCCNc1c2CCCCc2nc2ccccc12)NCCc1c[nH]c2ccccc12 Show InChI InChI=1S/C28H32N4O/c33-27(29-18-16-20-19-31-24-12-4-1-9-21(20)24)15-7-8-17-30-28-22-10-2-5-13-25(22)32-26-14-6-3-11-23(26)28/h1-2,4-5,9-10,12-13,19,31H,3,6-8,11,14-18H2,(H,29,33)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9009
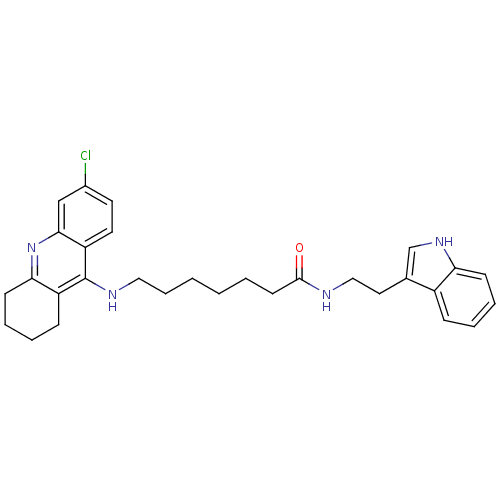
(7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...)Show SMILES Clc1ccc2c(NCCCCCCC(=O)NCCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C30H35ClN4O/c31-22-14-15-25-28(19-22)35-27-12-7-5-10-24(27)30(25)33-17-8-2-1-3-13-29(36)32-18-16-21-20-34-26-11-6-4-9-23(21)26/h4,6,9,11,14-15,19-20,34H,1-3,5,7-8,10,12-13,16-18H2,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510870

(CHEMBL4543251)Show SMILES Fc1ccc2nc3CCCCc3c(NCCCCCC(=O)NCCc3c[nH]c4ccccc34)c2c1 Show InChI InChI=1S/C29H33FN4O/c30-21-13-14-27-24(18-21)29(23-9-4-6-11-26(23)34-27)32-16-7-1-2-12-28(35)31-17-15-20-19-33-25-10-5-3-8-22(20)25/h3,5,8,10,13-14,18-19,33H,1-2,4,6-7,9,11-12,15-17H2,(H,31,35)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9016

(6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...)Show SMILES COc1ccc2[nH]cc(CCNC(=O)CCCCCNc3c4CCCCc4nc4cccc(Cl)c34)c2c1 Show InChI InChI=1S/C30H35ClN4O2/c1-37-21-13-14-25-23(18-21)20(19-34-25)15-17-32-28(36)12-3-2-6-16-33-30-22-8-4-5-10-26(22)35-27-11-7-9-24(31)29(27)30/h7,9,11,13-14,18-19,34H,2-6,8,10,12,15-17H2,1H3,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510837

(CHEMBL4467284)Show SMILES S=C(CCCCCCCNc1c2CCCCc2nc2ccccc12)NCCc1c[nH]c2ccccc12 Show InChI InChI=1S/C31H38N4S/c36-30(32-21-19-23-22-34-27-15-8-5-12-24(23)27)18-4-2-1-3-11-20-33-31-25-13-6-9-16-28(25)35-29-17-10-7-14-26(29)31/h5-6,8-9,12-13,15-16,22,34H,1-4,7,10-11,14,17-21H2,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9012
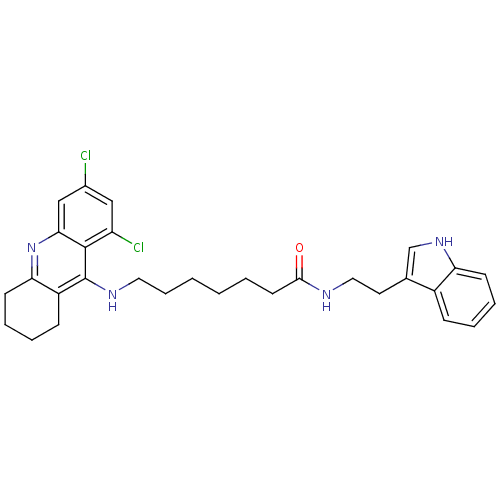
(7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...)Show SMILES Clc1cc(Cl)c2c(NCCCCCCC(=O)NCCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C30H34Cl2N4O/c31-21-17-24(32)29-27(18-21)36-26-12-7-5-10-23(26)30(29)34-15-8-2-1-3-13-28(37)33-16-14-20-19-35-25-11-6-4-9-22(20)25/h4,6,9,11,17-19,35H,1-3,5,7-8,10,12-16H2,(H,33,37)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9007
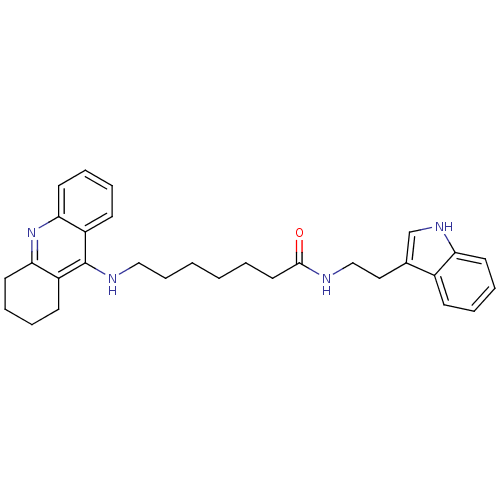
(7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...)Show SMILES O=C(CCCCCCNc1c2CCCCc2nc2ccccc12)NCCc1c[nH]c2ccccc12 Show InChI InChI=1S/C30H36N4O/c35-29(31-20-18-22-21-33-26-14-7-4-11-23(22)26)17-3-1-2-10-19-32-30-24-12-5-8-15-27(24)34-28-16-9-6-13-25(28)30/h4-5,7-8,11-12,14-15,21,33H,1-3,6,9-10,13,16-20H2,(H,31,35)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510845
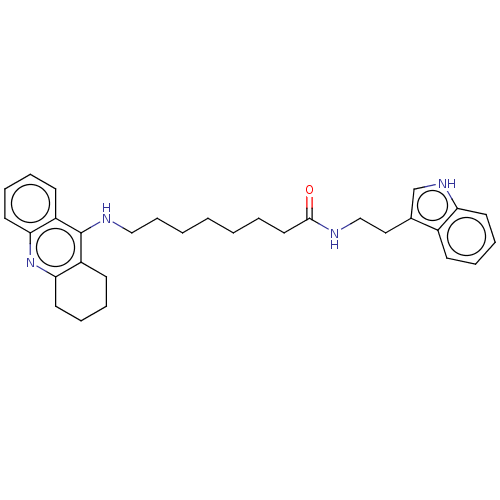
(CHEMBL4556734)Show SMILES O=C(CCCCCCCNc1c2CCCCc2nc2ccccc12)NCCc1c[nH]c2ccccc12 Show InChI InChI=1S/C31H38N4O/c36-30(32-21-19-23-22-34-27-15-8-5-12-24(23)27)18-4-2-1-3-11-20-33-31-25-13-6-9-16-28(25)35-29-17-10-7-14-26(29)31/h5-6,8-9,12-13,15-16,22,34H,1-4,7,10-11,14,17-21H2,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9008
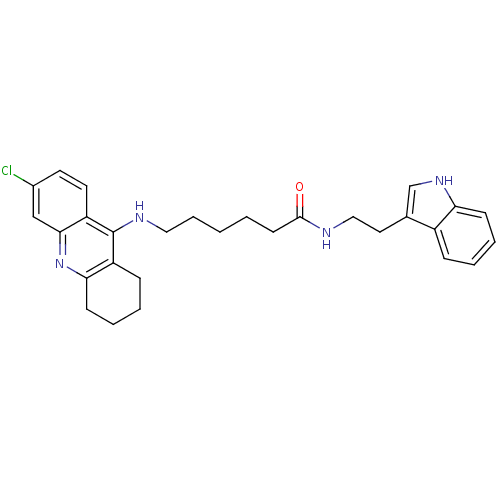
(6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...)Show SMILES Clc1ccc2c(NCCCCCC(=O)NCCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C29H33ClN4O/c30-21-13-14-24-27(18-21)34-26-11-6-4-9-23(26)29(24)32-16-7-1-2-12-28(35)31-17-15-20-19-33-25-10-5-3-8-22(20)25/h3,5,8,10,13-14,18-19,33H,1-2,4,6-7,9,11-12,15-17H2,(H,31,35)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510846

(CHEMBL4590945)Show SMILES Clc1cc(Cl)c2c(NCCCCCCCC(=O)NCCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C31H36Cl2N4O/c32-22-18-25(33)30-28(19-22)37-27-13-8-6-11-24(27)31(30)35-16-9-3-1-2-4-14-29(38)34-17-15-21-20-36-26-12-7-5-10-23(21)26/h5,7,10,12,18-20,36H,1-4,6,8-9,11,13-17H2,(H,34,38)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510847
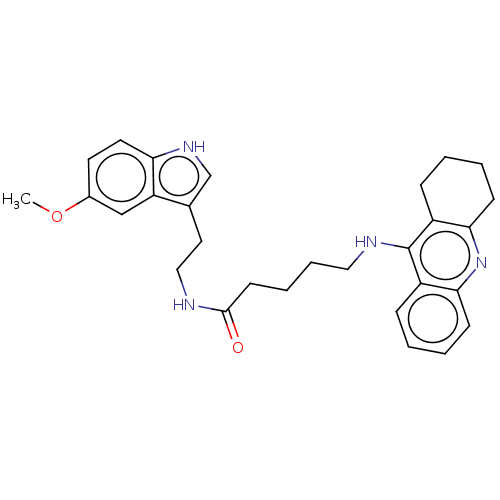
(CHEMBL4455677)Show SMILES COc1ccc2[nH]cc(CCNC(=O)CCCCNc3c4CCCCc4nc4ccccc34)c2c1 Show InChI InChI=1S/C29H34N4O2/c1-35-21-13-14-25-24(18-21)20(19-32-25)15-17-30-28(34)12-6-7-16-31-29-22-8-2-4-10-26(22)33-27-11-5-3-9-23(27)29/h2,4,8,10,13-14,18-19,32H,3,5-7,9,11-12,15-17H2,1H3,(H,30,34)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9013
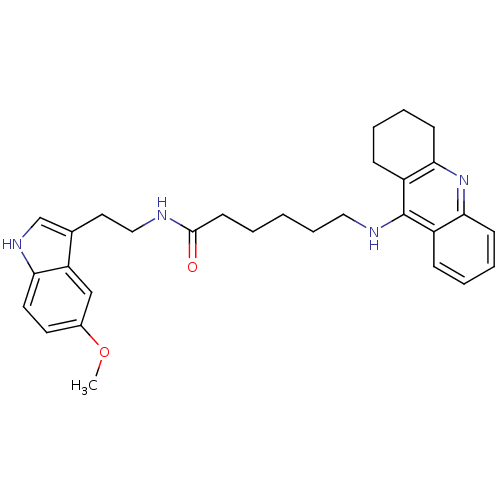
(6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...)Show SMILES COc1ccc2[nH]cc(CCNC(=O)CCCCCNc3c4CCCCc4nc4ccccc34)c2c1 Show InChI InChI=1S/C30H36N4O2/c1-36-22-14-15-26-25(19-22)21(20-33-26)16-18-31-29(35)13-3-2-8-17-32-30-23-9-4-6-11-27(23)34-28-12-7-5-10-24(28)30/h4,6,9,11,14-15,19-20,33H,2-3,5,7-8,10,12-13,16-18H2,1H3,(H,31,35)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9014
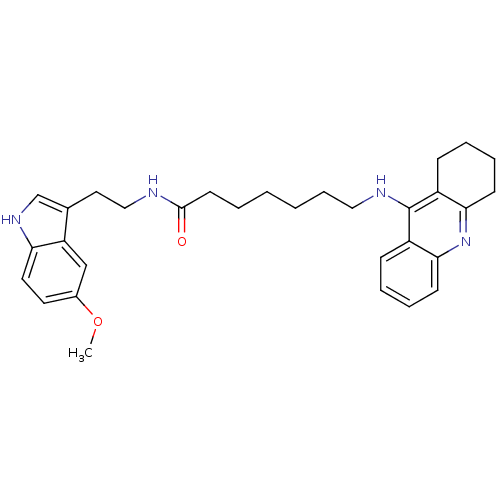
(7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic...)Show SMILES COc1ccc2[nH]cc(CCNC(=O)CCCCCCNc3c4CCCCc4nc4ccccc34)c2c1 Show InChI InChI=1S/C31H38N4O2/c1-37-23-15-16-27-26(20-23)22(21-34-27)17-19-32-30(36)14-4-2-3-9-18-33-31-24-10-5-7-12-28(24)35-29-13-8-6-11-25(29)31/h5,7,10,12,15-16,20-21,34H,2-4,6,8-9,11,13-14,17-19H2,1H3,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9015

(6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...)Show SMILES COc1ccc2[nH]cc(CCNC(=O)CCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c2c1 Show InChI InChI=1S/C30H35ClN4O2/c1-37-22-11-13-26-25(18-22)20(19-34-26)14-16-32-29(36)9-3-2-6-15-33-30-23-7-4-5-8-27(23)35-28-17-21(31)10-12-24(28)30/h10-13,17-19,34H,2-9,14-16H2,1H3,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510848

(CHEMBL4468781)Show SMILES COc1ccc2[nH]cc(CCNC(=O)CCCCCNc3c4CCCCc4nc4cc(Cl)cc(Cl)c34)c2c1 Show InChI InChI=1S/C30H34Cl2N4O2/c1-38-21-10-11-25-23(17-21)19(18-35-25)12-14-33-28(37)9-3-2-6-13-34-30-22-7-4-5-8-26(22)36-27-16-20(31)15-24(32)29(27)30/h10-11,15-18,35H,2-9,12-14H2,1H3,(H,33,37)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50510849

(CHEMBL4579667)Show SMILES Oc1ccc2[nH]cc(CCNC(=O)CCCCCCCNc3c4CCCCc4nc4ccccc34)c2c1 Show InChI InChI=1S/C31H38N4O2/c36-23-15-16-27-26(20-23)22(21-34-27)17-19-32-30(37)14-4-2-1-3-9-18-33-31-24-10-5-7-12-28(24)35-29-13-8-6-11-25(29)31/h5,7,10,12,15-16,20-21,34,36H,1-4,6,8-9,11,13-14,17-19H2,(H,32,37)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's method |
Bioorg Med Chem 27: 895-930 (2019)
Article DOI: 10.1016/j.bmc.2019.01.025
BindingDB Entry DOI: 10.7270/Q2N87F3N |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50049862
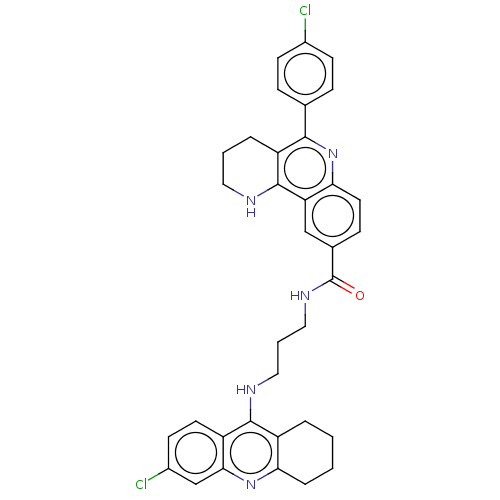
(CHEMBL3322232)Show SMILES Cl.Clc1ccc(cc1)-c1nc2ccc(cc2c2NCCCc12)C(=O)NCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H33Cl2N5O/c36-23-11-8-21(9-12-23)32-27-6-3-16-38-34(27)28-19-22(10-15-30(28)42-32)35(43)40-18-4-17-39-33-25-5-1-2-7-29(25)41-31-20-24(37)13-14-26(31)33/h8-15,19-20,38H,1-7,16-18H2,(H,39,41)(H,40,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate by spectrophotometric analysis |
Eur J Med Chem 84: 107-17 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.021
BindingDB Entry DOI: 10.7270/Q2571DNF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523376

(CHEMBL4589980)Show SMILES Clc1cccc2nc3CCCCc3c(NCCCCCCC(=O)NCCc3c[nH]c4ccccc34)c12 Show InChI InChI=1S/C30H35ClN4O/c31-24-12-9-15-27-29(24)30(23-11-5-7-14-26(23)35-27)33-18-8-2-1-3-16-28(36)32-19-17-21-20-34-25-13-6-4-10-22(21)25/h4,6,9-10,12-13,15,20,34H,1-3,5,7-8,11,14,16-19H2,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... |
Eur J Med Chem 168: 491-514 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.021
BindingDB Entry DOI: 10.7270/Q2RV0S3X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9012

(7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...)Show SMILES Clc1cc(Cl)c2c(NCCCCCCC(=O)NCCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C30H34Cl2N4O/c31-21-17-24(32)29-27(18-21)36-26-12-7-5-10-23(26)30(29)34-15-8-2-1-3-13-28(37)33-16-14-20-19-35-25-11-6-4-9-22(20)25/h4,6,9,11,17-19,35H,1-3,5,7-8,10,12-16H2,(H,33,37)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AchE |
J Med Chem 51: 347-72 (2008)
Article DOI: 10.1021/jm7009364
BindingDB Entry DOI: 10.7270/Q25B039W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9012

(7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylami...)Show SMILES Clc1cc(Cl)c2c(NCCCCCCC(=O)NCCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C30H34Cl2N4O/c31-21-17-24(32)29-27(18-21)36-26-12-7-5-10-23(26)30(29)34-15-8-2-1-3-13-28(37)33-16-14-20-19-35-25-11-6-4-9-22(20)25/h4,6,9,11,17-19,35H,1-3,5,7-8,10,12-16H2,(H,33,37)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Quimica Medica (CSIC)
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... |
J Med Chem 49: 459-62 (2006)
Article DOI: 10.1021/jm050746d
BindingDB Entry DOI: 10.7270/Q2VD6WN2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456700

(CHEMBL4205425)Show SMILES COc1ccccc1C(=O)N1Cc2cc(NC(=O)c3cccc(Cl)c3)ccc2CC1=O Show InChI InChI=1S/C24H19ClN2O4/c1-31-21-8-3-2-7-20(21)24(30)27-14-17-12-19(10-9-15(17)13-22(27)28)26-23(29)16-5-4-6-18(25)11-16/h2-12H,13-14H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456689
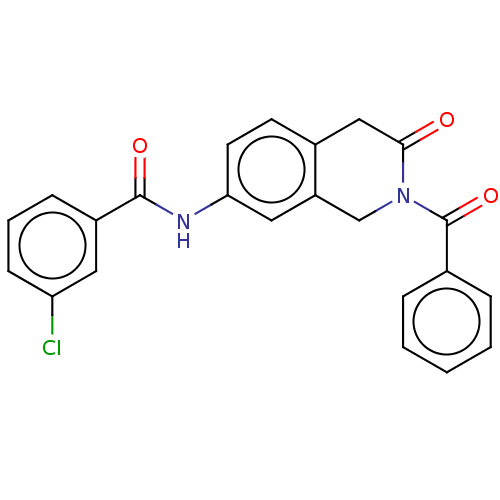
(CHEMBL4205954)Show SMILES Clc1cccc(c1)C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1ccccc1 Show InChI InChI=1S/C23H17ClN2O3/c24-19-8-4-7-17(11-19)22(28)25-20-10-9-16-13-21(27)26(14-18(16)12-20)23(29)15-5-2-1-3-6-15/h1-12H,13-14H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456691
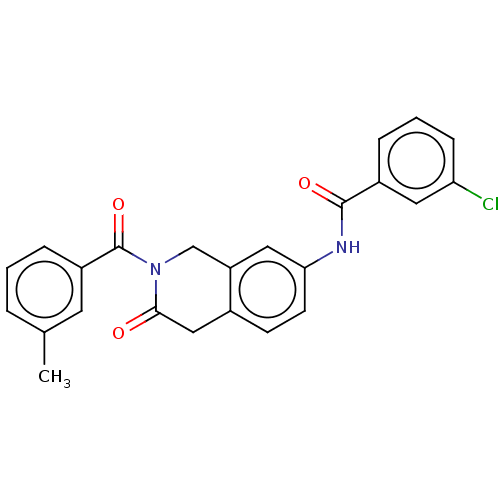
(CHEMBL4210316)Show SMILES Cc1cccc(c1)C(=O)N1Cc2cc(NC(=O)c3cccc(Cl)c3)ccc2CC1=O Show InChI InChI=1S/C24H19ClN2O3/c1-15-4-2-6-18(10-15)24(30)27-14-19-12-21(9-8-16(19)13-22(27)28)26-23(29)17-5-3-7-20(25)11-17/h2-12H,13-14H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456690

(CHEMBL4217663)Show SMILES Clc1cccc(c1)C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C23H16Cl2N2O3/c24-18-5-1-3-15(9-18)22(29)26-20-8-7-14-12-21(28)27(13-17(14)11-20)23(30)16-4-2-6-19(25)10-16/h1-11H,12-13H2,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456692

(CHEMBL4209803)Show SMILES Cc1cccc(c1)C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1cccc(C)c1 Show InChI InChI=1S/C25H22N2O3/c1-16-5-3-7-19(11-16)24(29)26-22-10-9-18-14-23(28)27(15-21(18)13-22)25(30)20-8-4-6-17(2)12-20/h3-13H,14-15H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456693
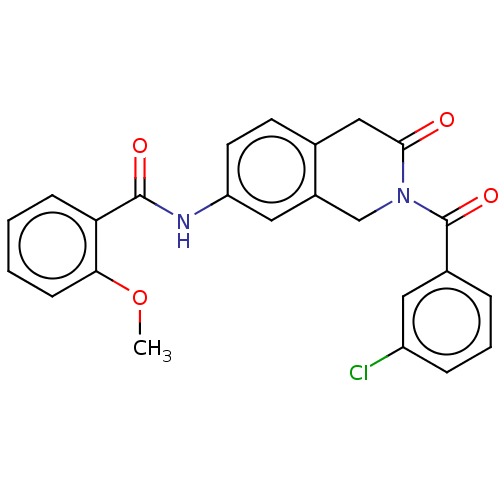
(CHEMBL4214430)Show SMILES COc1ccccc1C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C24H19ClN2O4/c1-31-21-8-3-2-7-20(21)23(29)26-19-10-9-15-13-22(28)27(14-17(15)12-19)24(30)16-5-4-6-18(25)11-16/h2-12H,13-14H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456699
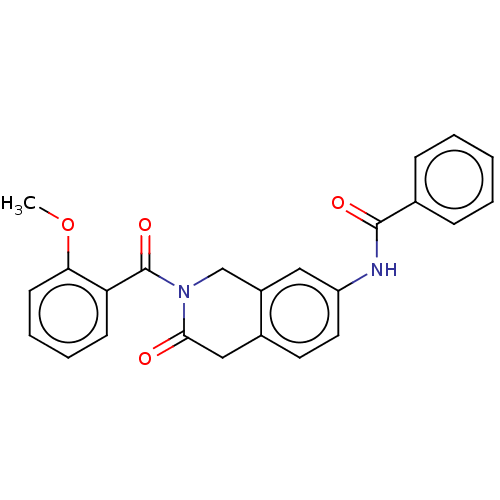
(CHEMBL4213253)Show SMILES COc1ccccc1C(=O)N1Cc2cc(NC(=O)c3ccccc3)ccc2CC1=O Show InChI InChI=1S/C24H20N2O4/c1-30-21-10-6-5-9-20(21)24(29)26-15-18-13-19(12-11-17(18)14-22(26)27)25-23(28)16-7-3-2-4-8-16/h2-13H,14-15H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9022
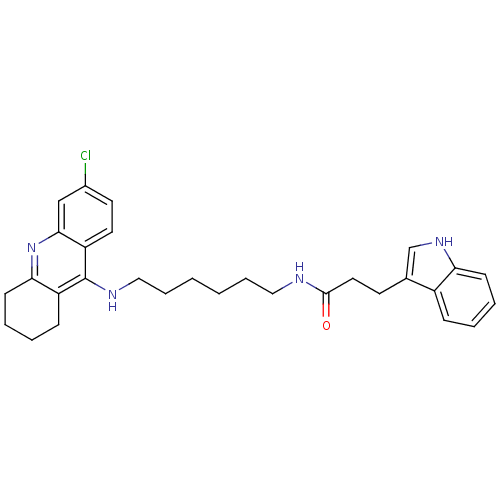
(CHEMBL225567 | Indole-Tacrine Heterodimer 5 | N-[5...)Show SMILES Clc1ccc2c(NCCCCCCNC(=O)CCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C30H35ClN4O/c31-22-14-15-25-28(19-22)35-27-12-6-4-10-24(27)30(25)33-18-8-2-1-7-17-32-29(36)16-13-21-20-34-26-11-5-3-9-23(21)26/h3,5,9,11,14-15,19-20,34H,1-2,4,6-8,10,12-13,16-18H2,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human AchE |
J Med Chem 51: 347-72 (2008)
Article DOI: 10.1021/jm7009364
BindingDB Entry DOI: 10.7270/Q25B039W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50318731

(3-(1H-indol-3-yl)-N-(6-(1,2,3,4-tetrahydroacridin-...)Show SMILES O=C(CCc1c[nH]c2ccccc12)NCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H36N4O/c35-29(18-17-22-21-33-26-14-6-3-11-23(22)26)31-19-9-1-2-10-20-32-30-24-12-4-7-15-27(24)34-28-16-8-5-13-25(28)30/h3-4,6-7,11-12,14-15,21,33H,1-2,5,8-10,13,16-20H2,(H,31,35)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of AChE |
Eur J Med Chem 45: 1167-72 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.038
BindingDB Entry DOI: 10.7270/Q25H7GFM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456696
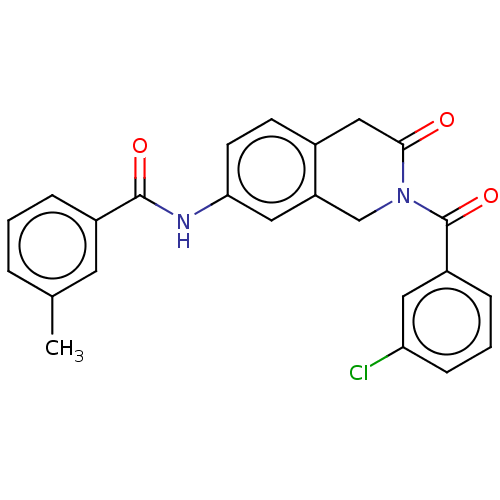
(CHEMBL4214707)Show SMILES Cc1cccc(c1)C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C24H19ClN2O3/c1-15-4-2-5-17(10-15)23(29)26-21-9-8-16-13-22(28)27(14-19(16)12-21)24(30)18-6-3-7-20(25)11-18/h2-12H,13-14H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456682
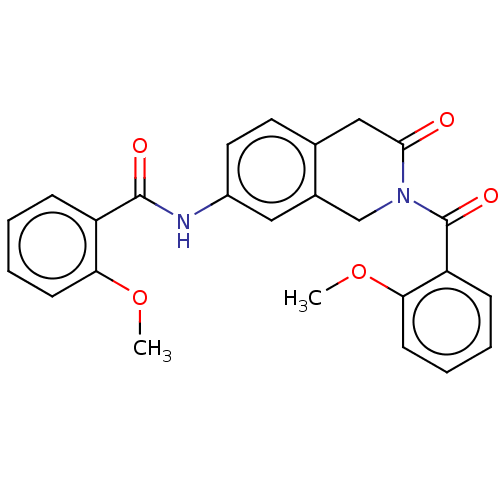
(CHEMBL4210041)Show SMILES COc1ccccc1C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1ccccc1OC Show InChI InChI=1S/C25H22N2O5/c1-31-21-9-5-3-7-19(21)24(29)26-18-12-11-16-14-23(28)27(15-17(16)13-18)25(30)20-8-4-6-10-22(20)32-2/h3-13H,14-15H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456688
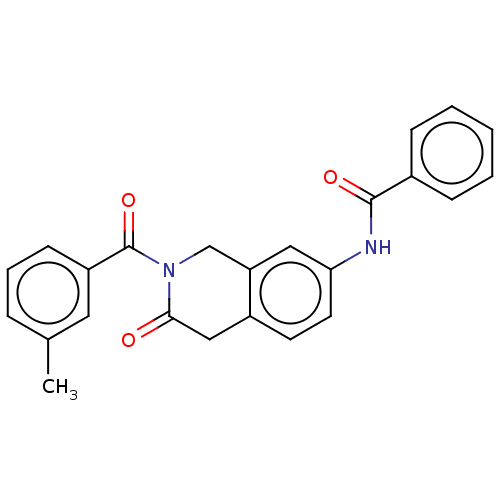
(CHEMBL4218191)Show SMILES Cc1cccc(c1)C(=O)N1Cc2cc(NC(=O)c3ccccc3)ccc2CC1=O Show InChI InChI=1S/C24H20N2O3/c1-16-6-5-9-19(12-16)24(29)26-15-20-13-21(11-10-18(20)14-22(26)27)25-23(28)17-7-3-2-4-8-17/h2-13H,14-15H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456694
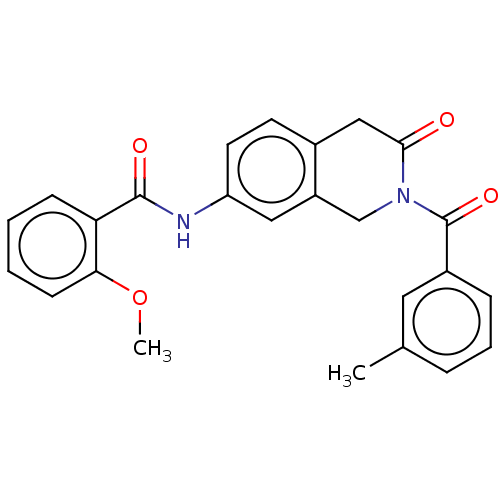
(CHEMBL4205144)Show SMILES COc1ccccc1C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1cccc(C)c1 Show InChI InChI=1S/C25H22N2O4/c1-16-6-5-7-18(12-16)25(30)27-15-19-13-20(11-10-17(19)14-23(27)28)26-24(29)21-8-3-4-9-22(21)31-2/h3-13H,14-15H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093588
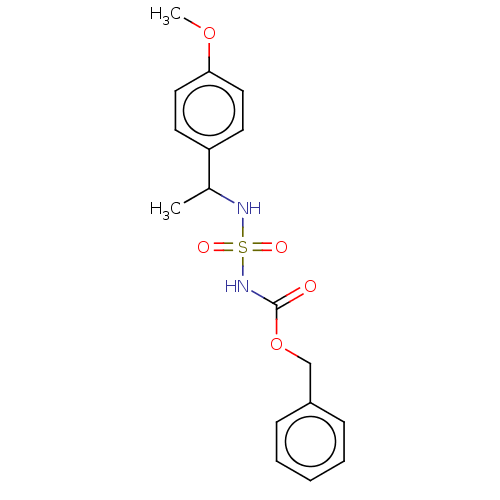
(CHEMBL3585776)Show InChI InChI=1S/C17H20N2O5S/c1-13(15-8-10-16(23-2)11-9-15)18-25(21,22)19-17(20)24-12-14-6-4-3-5-7-14/h3-11,13,18H,12H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093584
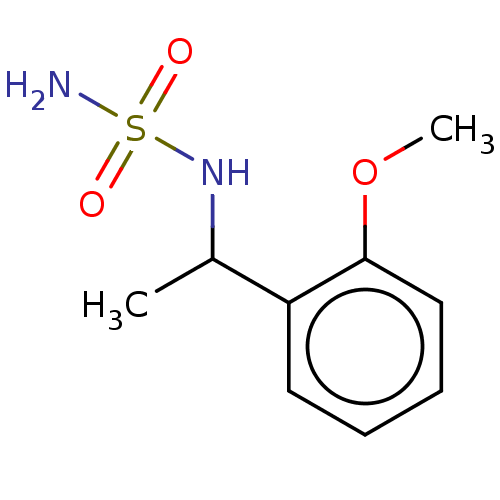
(CHEMBL3585780)Show InChI InChI=1S/C9H14N2O3S/c1-7(11-15(10,12)13)8-5-3-4-6-9(8)14-2/h3-7,11H,1-2H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093586
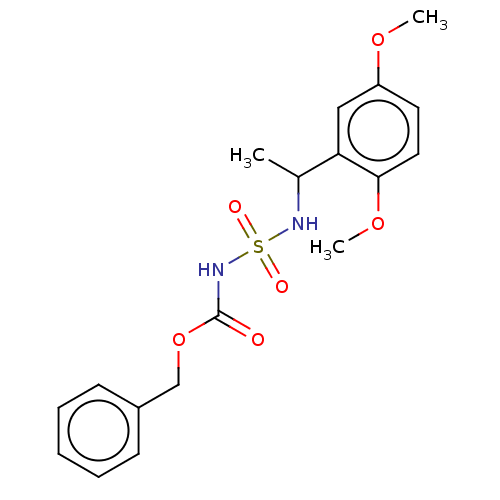
(CHEMBL3585778)Show SMILES COc1ccc(OC)c(c1)C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(16-11-15(24-2)9-10-17(16)25-3)19-27(22,23)20-18(21)26-12-14-7-5-4-6-8-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093587
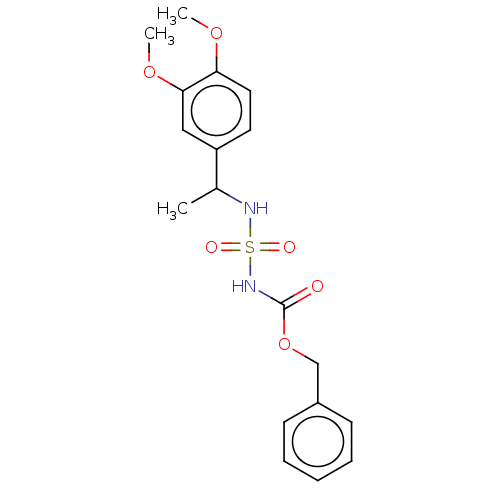
(CHEMBL3585777)Show SMILES COc1ccc(cc1OC)C(C)NS(=O)(=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C18H22N2O6S/c1-13(15-9-10-16(24-2)17(11-15)25-3)19-27(22,23)20-18(21)26-12-14-7-5-4-6-8-14/h4-11,13,19H,12H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093589
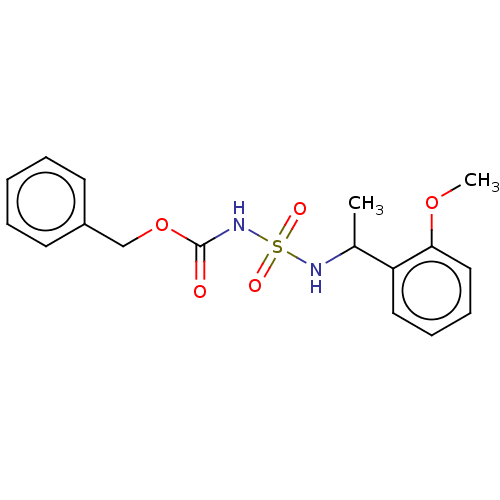
(CHEMBL3585775)Show InChI InChI=1S/C17H20N2O5S/c1-13(15-10-6-7-11-16(15)23-2)18-25(21,22)19-17(20)24-12-14-8-4-3-5-9-14/h3-11,13,18H,12H2,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093581
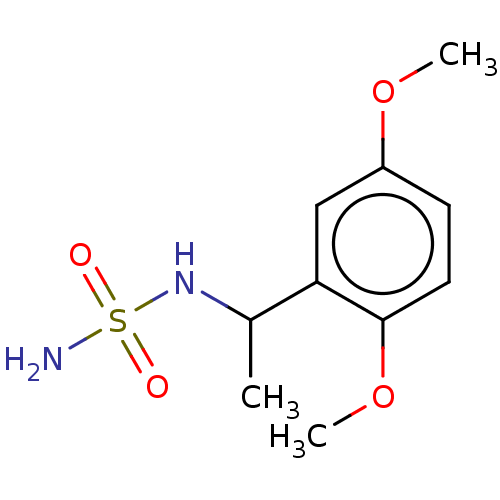
(CHEMBL3585783)Show InChI InChI=1S/C10H16N2O4S/c1-7(12-17(11,13)14)9-6-8(15-2)4-5-10(9)16-3/h4-7,12H,1-3H3,(H2,11,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
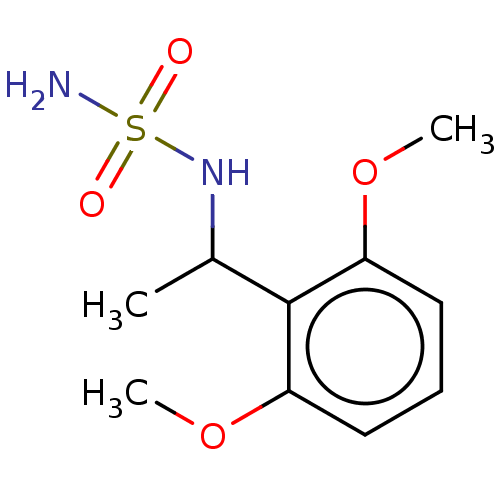
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093597
(CHEMBL3585784)Show InChI InChI=1S/C21H33NO2/c1-13(22-24)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4,15-19,23-24H,5-12H2,1-3H3/b22-13-/t15?,16?,17-,18?,19?,20?,21?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
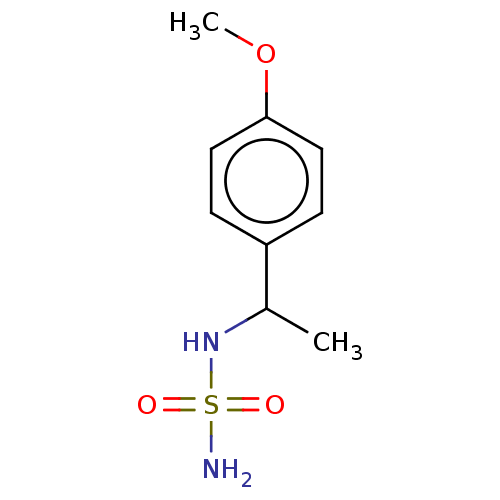
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093583
(CHEMBL3585781)Show InChI InChI=1S/C9H14N2O3S/c1-7(11-15(10,12)13)8-3-5-9(14-2)6-4-8/h3-7,11H,1-2H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50093582
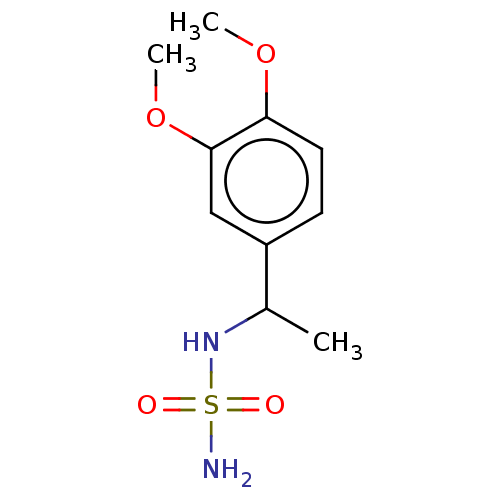
(CHEMBL3585782)Show InChI InChI=1S/C10H16N2O4S/c1-7(12-17(11,13)14)8-4-5-9(15-2)10(6-8)16-3/h4-7,12H,1-3H3,(H2,11,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University
Curated by ChEMBL
| Assay Description
Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysis |
Bioorg Med Chem 23: 3592-602 (2015)
Article DOI: 10.1016/j.bmc.2015.04.019
BindingDB Entry DOI: 10.7270/Q2J104XP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data