 Found 4533 hits of ic50 data for polymerid = 3620
Found 4533 hits of ic50 data for polymerid = 3620 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50218506

(3-{4-[(benzylmethylamino)methyl]phenyl}-6,7-dimeth...)Show SMILES COc1cc2occ(-c3ccc(CN(C)Cc4ccccc4)cc3)c(=O)c2cc1OC Show InChI InChI=1S/C26H25NO4/c1-27(15-18-7-5-4-6-8-18)16-19-9-11-20(12-10-19)22-17-31-23-14-25(30-3)24(29-2)13-21(23)26(22)28/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218509
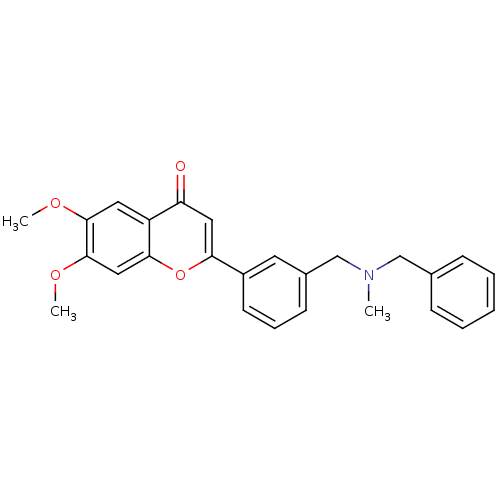
(2-{3-[(benzylmethylamino)methyl]phenyl}-6,7-dimeth...)Show SMILES COc1cc2oc(cc(=O)c2cc1OC)-c1cccc(CN(C)Cc2ccccc2)c1 Show InChI InChI=1S/C26H25NO4/c1-27(16-18-8-5-4-6-9-18)17-19-10-7-11-20(12-19)23-14-22(28)21-13-25(29-2)26(30-3)15-24(21)31-23/h4-15H,16-17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218525

(6,7-dimethoxy-3-(4-{[(4-methoxybenzyl)methylamino]...)Show SMILES COc1ccc(CN(C)Cc2ccc(cc2)-c2cc3cc(OC)c(OC)cc3oc2=O)cc1 Show InChI InChI=1S/C27H27NO5/c1-28(17-19-7-11-22(30-2)12-8-19)16-18-5-9-20(10-6-18)23-13-21-14-25(31-3)26(32-4)15-24(21)33-27(23)29/h5-15H,16-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218504

(3-{3-[(benzylmethylamino)methyl]phenyl}-6,7-dimeth...)Show SMILES COc1cc2occ(-c3cccc(CN(C)Cc4ccccc4)c3)c(=O)c2cc1OC Show InChI InChI=1S/C26H25NO4/c1-27(15-18-8-5-4-6-9-18)16-19-10-7-11-20(12-19)22-17-31-23-14-25(30-3)24(29-2)13-21(23)26(22)28/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218507

(6,7-dimethoxy-3-(4-{[(3-nitrobenzyl)methylamino]me...)Show SMILES COc1cc2cc(-c3ccc(CN(C)Cc4cccc(c4)[N+]([O-])=O)cc3)c(=O)oc2cc1OC Show InChI InChI=1S/C26H24N2O6/c1-27(16-18-5-4-6-21(11-18)28(30)31)15-17-7-9-19(10-8-17)22-12-20-13-24(32-2)25(33-3)14-23(20)34-26(22)29/h4-14H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218508
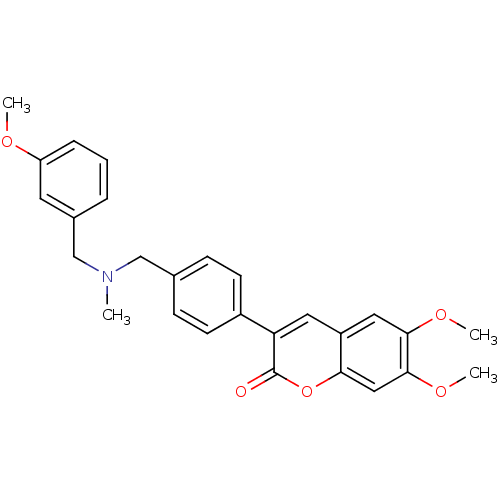
(6,7-dimethoxy-3-(4-{[(3-methoxybenzyl)methylamino]...)Show SMILES COc1cccc(CN(C)Cc2ccc(cc2)-c2cc3cc(OC)c(OC)cc3oc2=O)c1 Show InChI InChI=1S/C27H27NO5/c1-28(17-19-6-5-7-22(12-19)30-2)16-18-8-10-20(11-9-18)23-13-21-14-25(31-3)26(32-4)15-24(21)33-27(23)29/h5-15H,16-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218522

(6-amino-3-{4-[(benzylmethylamino)methyl]phenyl}-ch...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)-c1cc2cc(N)ccc2oc1=O Show InChI InChI=1S/C24H22N2O2/c1-26(15-17-5-3-2-4-6-17)16-18-7-9-19(10-8-18)22-14-20-13-21(25)11-12-23(20)28-24(22)27/h2-14H,15-16,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218519

(6,7-dimethoxy-3-(4-{[(2-nitrobenzyl)methylamino]me...)Show SMILES COc1cc2cc(-c3ccc(CN(C)Cc4ccccc4[N+]([O-])=O)cc3)c(=O)oc2cc1OC Show InChI InChI=1S/C26H24N2O6/c1-27(16-19-6-4-5-7-22(19)28(30)31)15-17-8-10-18(11-9-17)21-12-20-13-24(32-2)25(33-3)14-23(20)34-26(21)29/h4-14H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218511
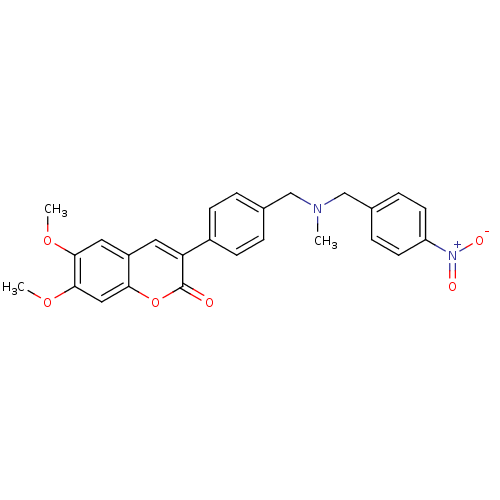
(6,7-dimethoxy-3-(4-{[(4-nitrobenzyl)methylamino]me...)Show SMILES COc1cc2cc(-c3ccc(CN(C)Cc4ccc(cc4)[N+]([O-])=O)cc3)c(=O)oc2cc1OC Show InChI InChI=1S/C26H24N2O6/c1-27(16-18-6-10-21(11-7-18)28(30)31)15-17-4-8-19(9-5-17)22-12-20-13-24(32-2)25(33-3)14-23(20)34-26(22)29/h4-14H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218524

(6-[(benzylmethylamino)methyl]-2,3-dimethoxyxanthen...)Show SMILES COc1cc2oc3cc(CN(C)Cc4ccccc4)ccc3c(=O)c2cc1OC Show InChI InChI=1S/C24H23NO4/c1-25(14-16-7-5-4-6-8-16)15-17-9-10-18-20(11-17)29-21-13-23(28-3)22(27-2)12-19(21)24(18)26/h4-13H,14-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218529

(6,7-dimethoxy-3-(4-{[(4-methylbenzyl)methylamino]m...)Show SMILES COc1cc2cc(-c3ccc(CN(C)Cc4ccc(C)cc4)cc3)c(=O)oc2cc1OC Show InChI InChI=1S/C27H27NO4/c1-18-5-7-19(8-6-18)16-28(2)17-20-9-11-21(12-10-20)23-13-22-14-25(30-3)26(31-4)15-24(22)32-27(23)29/h5-15H,16-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218526

(6,7-dimethoxy-3-(4-{[(3-methylbenzyl)methylamino]m...)Show SMILES COc1cc2cc(-c3ccc(CN(C)Cc4cccc(C)c4)cc3)c(=O)oc2cc1OC Show InChI InChI=1S/C27H27NO4/c1-18-6-5-7-20(12-18)17-28(2)16-19-8-10-21(11-9-19)23-13-22-14-25(30-3)26(31-4)15-24(22)32-27(23)29/h5-15H,16-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218516
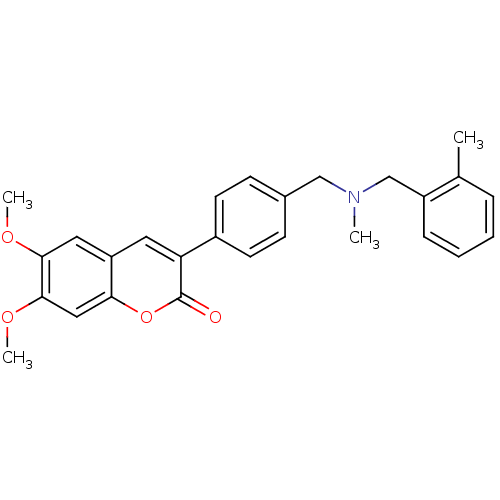
(6,7-dimethoxy-3-(4-{[(2-methylbenzyl)methylamino]m...)Show SMILES COc1cc2cc(-c3ccc(CN(C)Cc4ccccc4C)cc3)c(=O)oc2cc1OC Show InChI InChI=1S/C27H27NO4/c1-18-7-5-6-8-21(18)17-28(2)16-19-9-11-20(12-10-19)23-13-22-14-25(30-3)26(31-4)15-24(22)32-27(23)29/h5-15H,16-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218523
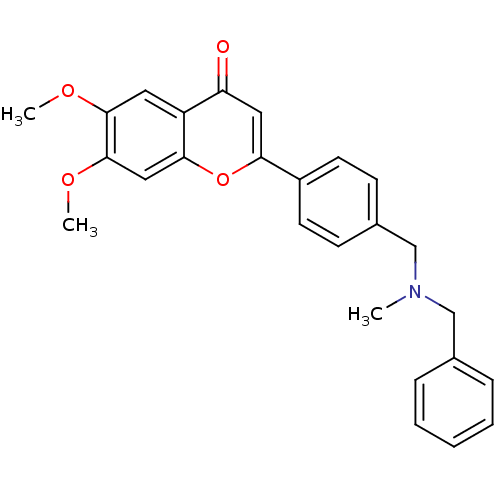
(2-{4-[(benzylmethylamino)methyl]phenyl}-6,7-dimeth...)Show SMILES COc1cc2oc(cc(=O)c2cc1OC)-c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C26H25NO4/c1-27(16-18-7-5-4-6-8-18)17-19-9-11-20(12-10-19)23-14-22(28)21-13-25(29-2)26(30-3)15-24(21)31-23/h4-15H,16-17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218503

(3-{3-[(benzylmethylamino)methyl]phenyl}-7-methoxyc...)Show SMILES COc1ccc2cc(-c3cccc(CN(C)Cc4ccccc4)c3)c(=O)oc2c1 Show InChI InChI=1S/C25H23NO3/c1-26(16-18-7-4-3-5-8-18)17-19-9-6-10-20(13-19)23-14-21-11-12-22(28-2)15-24(21)29-25(23)27/h3-15H,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218514

(2-{4-[(benzylmethylamino)methyl]phenyl}-7-methoxyc...)Show SMILES COc1ccc2c(c1)oc(cc2=O)-c1ccc(CN(C)Cc2ccccc2)cc1 Show InChI InChI=1S/C25H23NO3/c1-26(16-18-6-4-3-5-7-18)17-19-8-10-20(11-9-19)24-15-23(27)22-13-12-21(28-2)14-25(22)29-24/h3-15H,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218510

(3-{4-[(benzylmethylamino)methyl]phenyl}-6-nitrochr...)Show SMILES CN(Cc1ccccc1)Cc1ccc(cc1)-c1cc2cc(ccc2oc1=O)[N+]([O-])=O Show InChI InChI=1S/C24H20N2O4/c1-25(15-17-5-3-2-4-6-17)16-18-7-9-19(10-8-18)22-14-20-13-21(26(28)29)11-12-23(20)30-24(22)27/h2-14H,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50218521
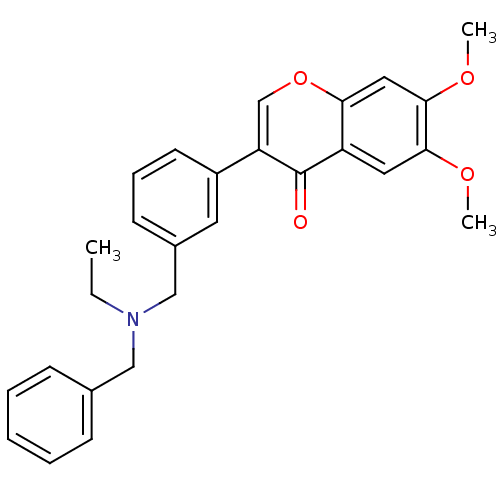
(3-{3-[(benzylethylamino)-methyl]-phenyl}-6,7-dimet...)Show SMILES CCN(Cc1ccccc1)Cc1cccc(c1)-c1coc2cc(OC)c(OC)cc2c1=O Show InChI InChI=1S/C27H27NO4/c1-4-28(16-19-9-6-5-7-10-19)17-20-11-8-12-21(13-20)23-18-32-24-15-26(31-3)25(30-2)14-22(24)27(23)29/h5-15,18H,4,16-17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BChE |
J Med Chem 50: 4250-4 (2007)
Article DOI: 10.1021/jm070100g
BindingDB Entry DOI: 10.7270/Q2QC036V |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50349922
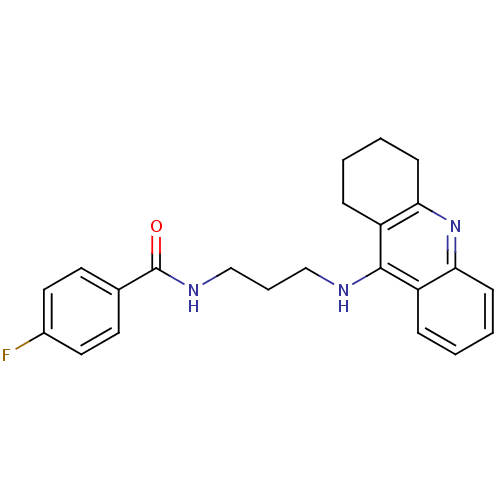
(CHEMBL1814730)Show InChI InChI=1S/C23H24FN3O/c24-17-12-10-16(11-13-17)23(28)26-15-5-14-25-22-18-6-1-3-8-20(18)27-21-9-4-2-7-19(21)22/h1,3,6,8,10-13H,2,4-5,7,9,14-15H2,(H,25,27)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0212 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE by Ellman's method |
Eur J Med Chem 46: 3250-7 (2011)
Article DOI: 10.1016/j.ejmech.2011.04.038
BindingDB Entry DOI: 10.7270/Q2J67H9J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0267 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE by Ellman's method |
Eur J Med Chem 46: 3250-7 (2011)
Article DOI: 10.1016/j.ejmech.2011.04.038
BindingDB Entry DOI: 10.7270/Q2J67H9J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM9027
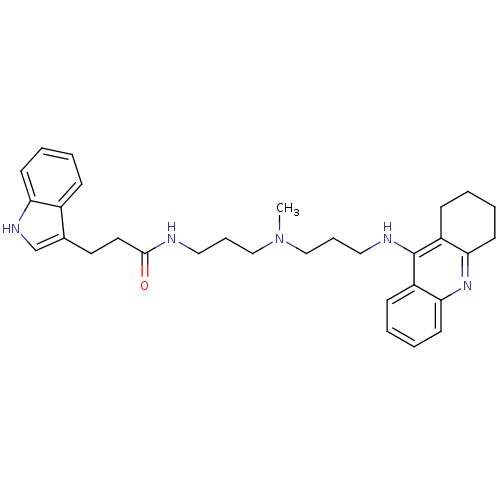
(3-(1H-indol-3-yl)-N-(3-{methyl[3-(1,2,3,4-tetrahyd...)Show SMILES CN(CCCNC(=O)CCc1c[nH]c2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H39N5O/c1-36(20-8-18-32-30(37)17-16-23-22-34-27-13-5-2-10-24(23)27)21-9-19-33-31-25-11-3-6-14-28(25)35-29-15-7-4-12-26(29)31/h2-3,5-6,10-11,13-14,22,34H,4,7-9,12,15-21H2,1H3,(H,32,37)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... |
J Med Chem 48: 7223-33 (2005)
Article DOI: 10.1021/jm0503289
BindingDB Entry DOI: 10.7270/Q2QN64Z5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587056

(CHEMBL5080685)Show SMILES COC(=O)c1c(CCCCCCCCNc2c3CCCCc3nc3ccccc23)cccc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0352 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50349923

(CHEMBL1814731)Show InChI InChI=1S/C24H26FN3O/c25-18-13-11-17(12-14-18)24(29)27-16-6-5-15-26-23-19-7-1-3-9-21(19)28-22-10-4-2-8-20(22)23/h1,3,7,9,11-14H,2,4-6,8,10,15-16H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0354 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE by Ellman's method |
Eur J Med Chem 46: 3250-7 (2011)
Article DOI: 10.1016/j.ejmech.2011.04.038
BindingDB Entry DOI: 10.7270/Q2J67H9J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50599186
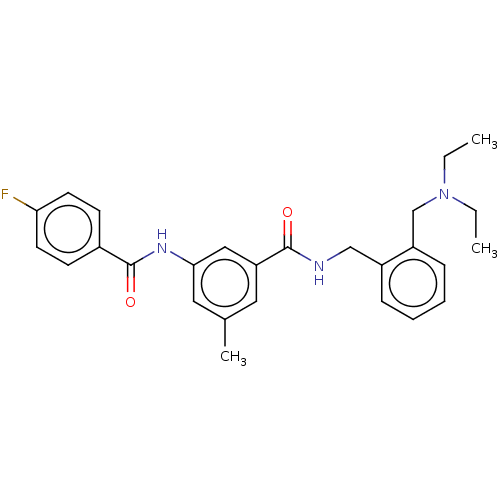
(CHEMBL5201089)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cc(C)cc(NC(=O)c2ccc(F)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380553
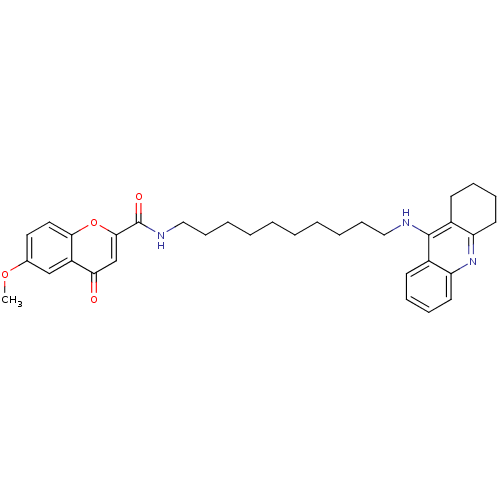
(CHEMBL2019034)Show SMILES COc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H41N3O4/c1-40-24-18-19-31-27(22-24)30(38)23-32(41-31)34(39)36-21-13-7-5-3-2-4-6-12-20-35-33-25-14-8-10-16-28(25)37-29-17-11-9-15-26(29)33/h8,10,14,16,18-19,22-23H,2-7,9,11-13,15,17,20-21H2,1H3,(H,35,37)(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50349921
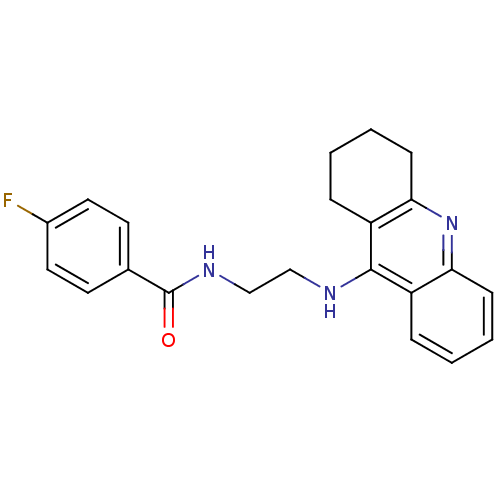
(CHEMBL1814729)Show InChI InChI=1S/C22H22FN3O/c23-16-11-9-15(10-12-16)22(27)25-14-13-24-21-17-5-1-3-7-19(17)26-20-8-4-2-6-18(20)21/h1,3,5,7,9-12H,2,4,6,8,13-14H2,(H,24,26)(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0449 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University
Curated by ChEMBL
| Assay Description
Inhibition of BChE by Ellman's method |
Eur J Med Chem 46: 3250-7 (2011)
Article DOI: 10.1016/j.ejmech.2011.04.038
BindingDB Entry DOI: 10.7270/Q2J67H9J |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50525979
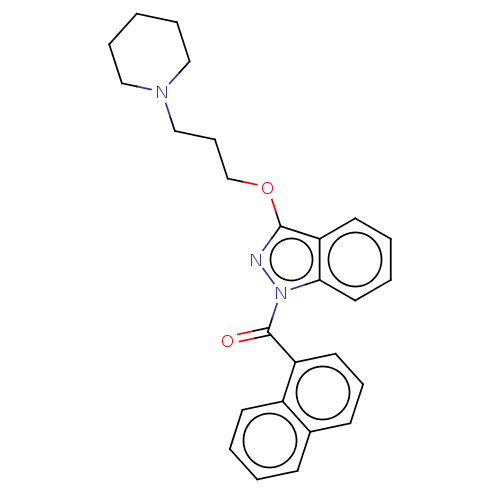
(CHEMBL4444855)Show SMILES O=C(c1cccc2ccccc12)n1nc(OCCCN2CCCCC2)c2ccccc12 Show InChI InChI=1S/C26H27N3O2/c30-26(22-14-8-11-20-10-2-3-12-21(20)22)29-24-15-5-4-13-23(24)25(27-29)31-19-9-18-28-16-6-1-7-17-28/h2-5,8,10-15H,1,6-7,9,16-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine as substrate by Ellman's assay |
Eur J Med Chem 166: 90-107 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.030
BindingDB Entry DOI: 10.7270/Q2VQ363K |
More data for this
Ligand-Target Pair | |
Cholinesterase
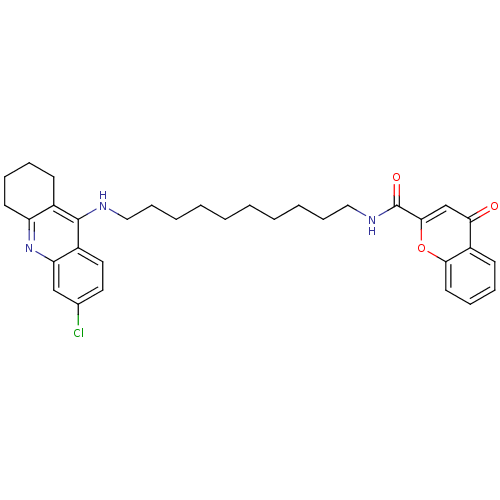
(Homo sapiens (Human)) | BDBM50380541
(CHEMBL2019032)Show SMILES Clc1ccc2c(NCCCCCCCCCCNC(=O)c3cc(=O)c4ccccc4o3)c3CCCCc3nc2c1 Show InChI InChI=1S/C33H38ClN3O3/c34-23-17-18-25-28(21-23)37-27-15-9-7-13-24(27)32(25)35-19-11-5-3-1-2-4-6-12-20-36-33(39)31-22-29(38)26-14-8-10-16-30(26)40-31/h8,10,14,16-18,21-22H,1-7,9,11-13,15,19-20H2,(H,35,37)(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
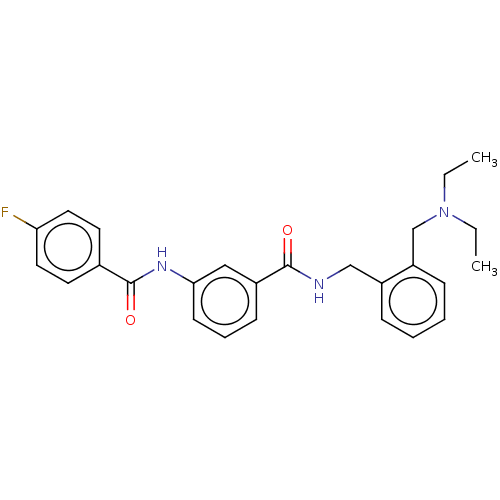
(Homo sapiens (Human)) | BDBM50599167
(CHEMBL5195228)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(F)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
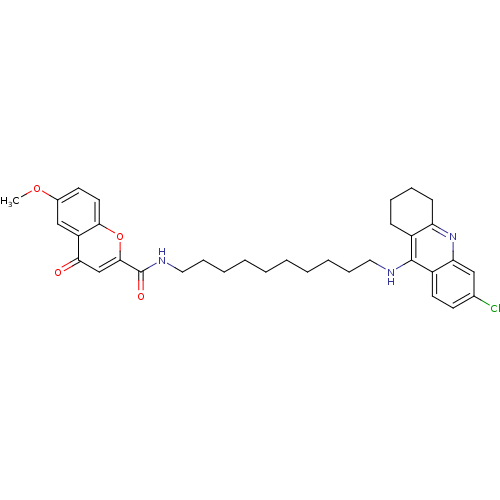
(Homo sapiens (Human)) | BDBM50380552
(CHEMBL2019035)Show SMILES COc1ccc2oc(cc(=O)c2c1)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H40ClN3O4/c1-41-24-15-17-31-27(21-24)30(39)22-32(42-31)34(40)37-19-11-7-5-3-2-4-6-10-18-36-33-25-12-8-9-13-28(25)38-29-20-23(35)14-16-26(29)33/h14-17,20-22H,2-13,18-19H2,1H3,(H,36,38)(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
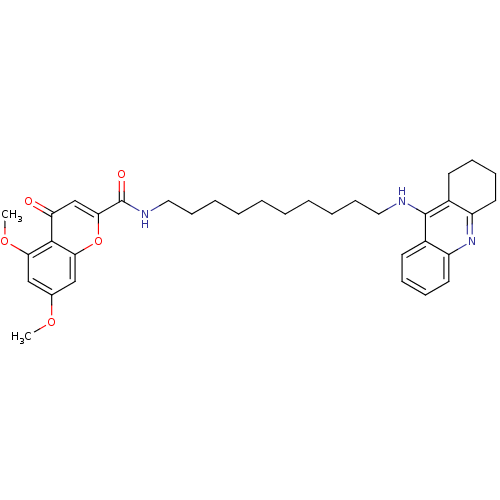
(Homo sapiens (Human)) | BDBM50380542
(CHEMBL2019037)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N3O5/c1-41-24-21-30(42-2)33-29(39)23-32(43-31(33)22-24)35(40)37-20-14-8-6-4-3-5-7-13-19-36-34-25-15-9-11-17-27(25)38-28-18-12-10-16-26(28)34/h9,11,15,17,21-23H,3-8,10,12-14,16,18-20H2,1-2H3,(H,36,38)(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM9023
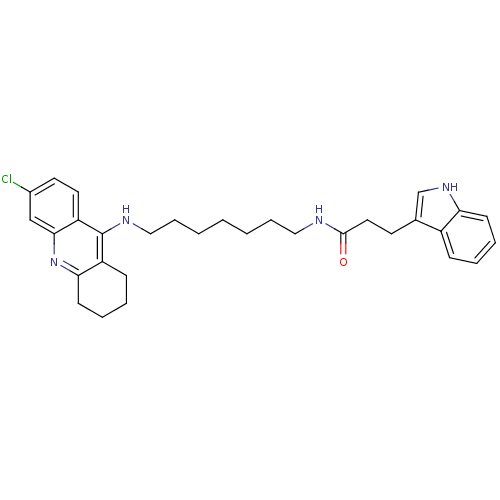
(CHEMBL225198 | Indole-Tacrine Heterodimer 6 | N-[7...)Show SMILES Clc1ccc2c(NCCCCCCCNC(=O)CCc3c[nH]c4ccccc34)c3CCCCc3nc2c1 Show InChI InChI=1S/C31H37ClN4O/c32-23-15-16-26-29(20-23)36-28-13-7-5-11-25(28)31(26)34-19-9-3-1-2-8-18-33-30(37)17-14-22-21-35-27-12-6-4-10-24(22)27/h4,6,10,12,15-16,20-21,35H,1-3,5,7-9,11,13-14,17-19H2,(H,33,37)(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... |
J Med Chem 48: 7223-33 (2005)
Article DOI: 10.1021/jm0503289
BindingDB Entry DOI: 10.7270/Q2QN64Z5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380551
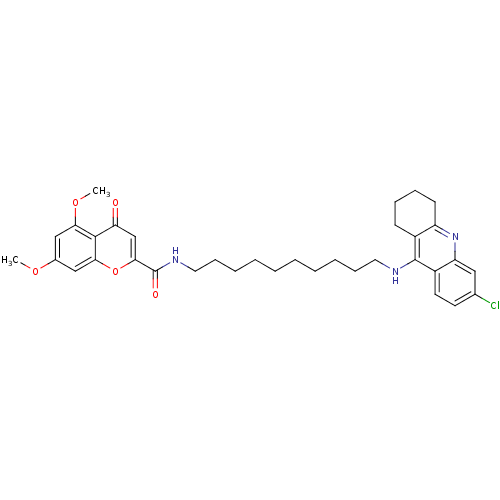
(CHEMBL2019038)Show SMILES COc1cc(OC)c2c(c1)oc(cc2=O)C(=O)NCCCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H42ClN3O5/c1-42-24-20-30(43-2)33-29(40)22-32(44-31(33)21-24)35(41)38-18-12-8-6-4-3-5-7-11-17-37-34-25-13-9-10-14-27(25)39-28-19-23(36)15-16-26(28)34/h15-16,19-22H,3-14,17-18H2,1-2H3,(H,37,39)(H,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50599173

(CHEMBL5200785)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(I)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50599172

(CHEMBL5181960)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(Br)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM13549
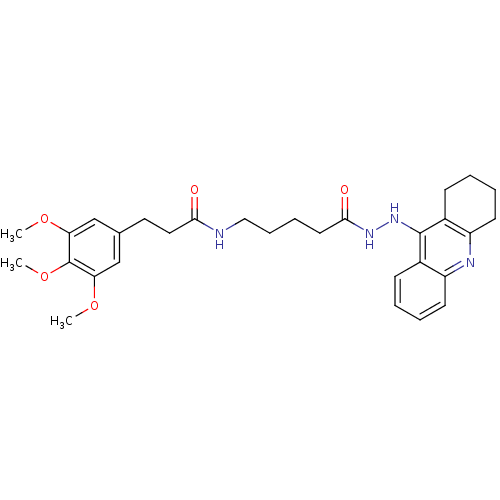
(3-(3,4,5-Trimethoxyphenyl)-N-(5-oxo-5-(2-(1,2,3,4-...)Show SMILES COc1cc(CCC(=O)NCCCCC(=O)NNc2c3CCCCc3nc3ccccc23)cc(OC)c1OC Show InChI InChI=1S/C30H38N4O5/c1-37-25-18-20(19-26(38-2)30(25)39-3)15-16-27(35)31-17-9-8-14-28(36)33-34-29-21-10-4-6-12-23(21)32-24-13-7-5-11-22(24)29/h4,6,10,12,18-19H,5,7-9,11,13-17H2,1-3H3,(H,31,35)(H,32,34)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.139 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... |
J Med Chem 49: 7540-4 (2006)
Article DOI: 10.1021/jm060742o
BindingDB Entry DOI: 10.7270/Q21Z42NF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM13550

(3-(3,4,5-Trimethoxyphenyl)-N-(6-oxo-6-(2-(1,2,3,4-...)Show SMILES COc1cc(CCC(=O)NCCCCCC(=O)NNc2c3CCCCc3nc3ccccc23)cc(OC)c1OC Show InChI InChI=1S/C31H40N4O5/c1-38-26-19-21(20-27(39-2)31(26)40-3)16-17-28(36)32-18-10-4-5-15-29(37)34-35-30-22-11-6-8-13-24(22)33-25-14-9-7-12-23(25)30/h6,8,11,13,19-20H,4-5,7,9-10,12,14-18H2,1-3H3,(H,32,36)(H,33,35)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.141 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... |
J Med Chem 49: 7540-4 (2006)
Article DOI: 10.1021/jm060742o
BindingDB Entry DOI: 10.7270/Q21Z42NF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303940
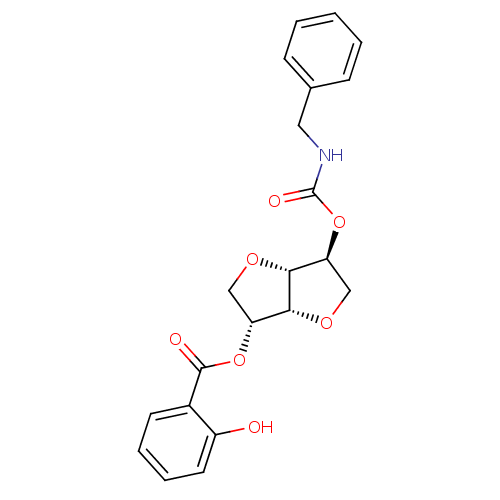
(2-(Benzylaminocarbonyloxy-)5-O-Salicyloyl-1,4:3,6-...)Show SMILES Oc1ccccc1C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H21NO7/c23-15-9-5-4-8-14(15)20(24)28-16-11-26-19-17(12-27-18(16)19)29-21(25)22-10-13-6-2-1-3-7-13/h1-9,16-19,23H,10-12H2,(H,22,25)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50380537
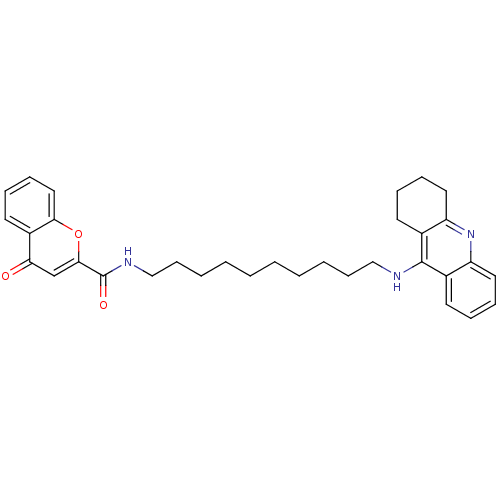
(CHEMBL2019030)Show SMILES O=C(NCCCCCCCCCCNc1c2CCCCc2nc2ccccc12)c1cc(=O)c2ccccc2o1 Show InChI InChI=1S/C33H39N3O3/c37-29-23-31(39-30-20-12-9-17-26(29)30)33(38)35-22-14-6-4-2-1-3-5-13-21-34-32-24-15-7-10-18-27(24)36-28-19-11-8-16-25(28)32/h7,9-10,12,15,17-18,20,23H,1-6,8,11,13-14,16,19,21-22H2,(H,34,36)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE by Ellman's method |
J Med Chem 55: 1303-17 (2012)
Article DOI: 10.1021/jm201460y
BindingDB Entry DOI: 10.7270/Q2M32WS2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50525990
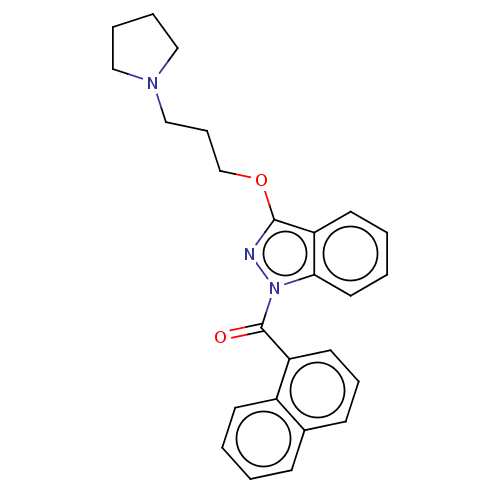
(CHEMBL4476861)Show SMILES O=C(c1cccc2ccccc12)n1nc(OCCCN2CCCC2)c2ccccc12 Show InChI InChI=1S/C25H25N3O2/c29-25(21-13-7-10-19-9-1-2-11-20(19)21)28-23-14-4-3-12-22(23)24(26-28)30-18-8-17-27-15-5-6-16-27/h1-4,7,9-14H,5-6,8,15-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine as substrate by Ellman's assay |
Eur J Med Chem 166: 90-107 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.030
BindingDB Entry DOI: 10.7270/Q2VQ363K |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50532207
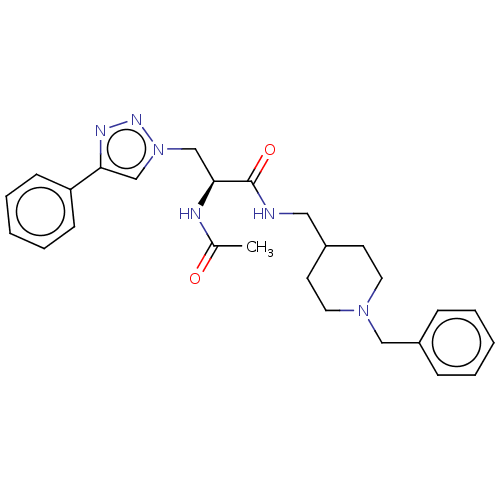
(CHEMBL4464964)Show SMILES CC(=O)N[C@@H](Cn1cc(nn1)-c1ccccc1)C(=O)NCC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C26H32N6O2/c1-20(33)28-25(19-32-18-24(29-30-32)23-10-6-3-7-11-23)26(34)27-16-21-12-14-31(15-13-21)17-22-8-4-2-5-9-22/h2-11,18,21,25H,12-17,19H2,1H3,(H,27,34)(H,28,33)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measure... |
Bioorg Med Chem 27: 931-943 (2019)
Article DOI: 10.1016/j.bmc.2018.12.030
BindingDB Entry DOI: 10.7270/Q2XP78D7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50587059

(CHEMBL5092094)Show SMILES COC(=O)c1c(O)cccc1CCCCCCCCNc1c2CCCCc2nc2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.177 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00048
BindingDB Entry DOI: 10.7270/Q24F1VNK |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50599191
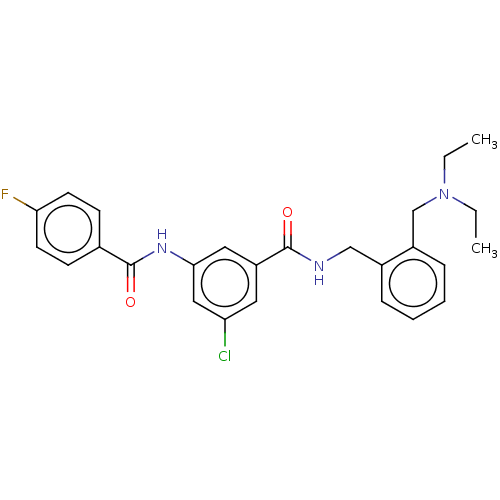
(CHEMBL5184728)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cc(Cl)cc(NC(=O)c2ccc(F)cc2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM13548

(3-(3,4,5-Trimethoxyphenyl)-N-(4-oxo-4-(2-(1,2,3,4-...)Show SMILES COc1cc(CCC(=O)NCCCC(=O)NNc2c3CCCCc3nc3ccccc23)cc(OC)c1OC Show InChI InChI=1S/C29H36N4O5/c1-36-24-17-19(18-25(37-2)29(24)38-3)14-15-26(34)30-16-8-13-27(35)32-33-28-20-9-4-6-11-22(20)31-23-12-7-5-10-21(23)28/h4,6,9,11,17-18H,5,7-8,10,12-16H2,1-3H3,(H,30,34)(H,31,33)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.226 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... |
J Med Chem 49: 7540-4 (2006)
Article DOI: 10.1021/jm060742o
BindingDB Entry DOI: 10.7270/Q21Z42NF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM132084
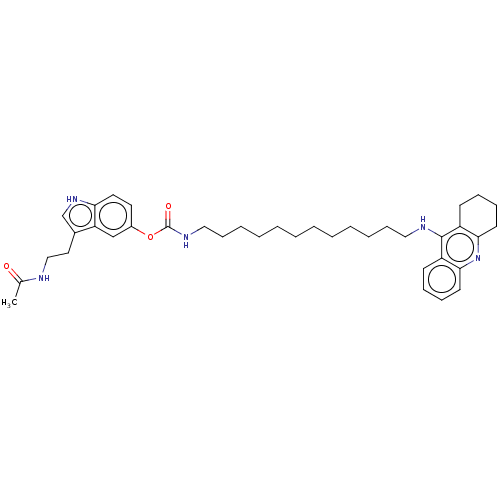
(US8841453, 15)Show SMILES CC(=O)NCCc1c[nH]c2ccc(OC(=O)NCCCCCCCCCCCCNc3c4CCCCc4nc4ccccc34)cc12 Show InChI InChI=1S/C38H51N5O3/c1-28(44)39-25-22-29-27-42-34-21-20-30(26-33(29)34)46-38(45)41-24-15-9-7-5-3-2-4-6-8-14-23-40-37-31-16-10-12-18-35(31)43-36-19-13-11-17-32(36)37/h10,12,16,18,20-21,26-27,42H,2-9,11,13-15,17,19,22-25H2,1H3,(H,39,44)(H,40,43)(H,41,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego
US Patent
| Assay Description
The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... |
US Patent US8841453 (2014)
BindingDB Entry DOI: 10.7270/Q2W957VS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM132071
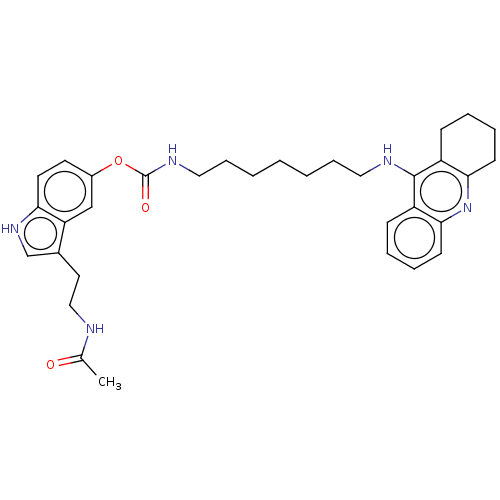
(US8841453, 1)Show SMILES CC(=O)NCCc1c[nH]c2ccc(OC(=O)NCCCCCCCNc3c4CCCCc4nc4ccccc34)cc12 Show InChI InChI=1S/C33H41N5O3/c1-23(39)34-20-17-24-22-37-29-16-15-25(21-28(24)29)41-33(40)36-19-10-4-2-3-9-18-35-32-26-11-5-7-13-30(26)38-31-14-8-6-12-27(31)32/h5,7,11,13,15-16,21-22,37H,2-4,6,8-10,12,14,17-20H2,1H3,(H,34,39)(H,35,38)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego
US Patent
| Assay Description
The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... |
US Patent US8841453 (2014)
BindingDB Entry DOI: 10.7270/Q2W957VS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50464942
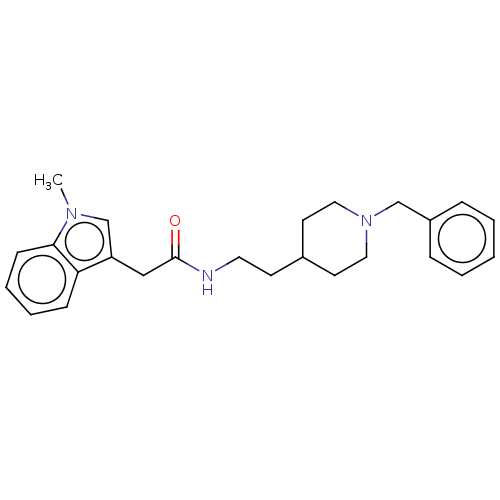
(CHEMBL4293015)Show SMILES Cn1cc(CC(=O)NCCC2CCN(Cc3ccccc3)CC2)c2ccccc12 Show InChI InChI=1S/C25H31N3O/c1-27-19-22(23-9-5-6-10-24(23)27)17-25(29)26-14-11-20-12-15-28(16-13-20)18-21-7-3-2-4-8-21/h2-10,19-20H,11-18H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human serum BuChE using butyrylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition measure... |
Eur J Med Chem 145: 431-444 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.007
BindingDB Entry DOI: 10.7270/Q2JQ13PD |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM132080

(US8841453, 11)Show SMILES CC(=O)NCCc1c[nH]c2ccc(OC(=O)NCCCCCCCCNc3c4CCCCc4nc4ccccc34)cc12 Show InChI InChI=1S/C34H43N5O3/c1-24(40)35-21-18-25-23-38-30-17-16-26(22-29(25)30)42-34(41)37-20-11-5-3-2-4-10-19-36-33-27-12-6-8-14-31(27)39-32-15-9-7-13-28(32)33/h6,8,12,14,16-17,22-23,38H,2-5,7,9-11,13,15,18-21H2,1H3,(H,35,40)(H,36,39)(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Uniwersytet Warszawski; Centrum Medyczne Ksztalcenia Podyplomowego
US Patent
| Assay Description
The biological activity of the compounds of general formula (I) towards the cholinesterase inhibition was evaluated using Ellman's method (Ellman, G.... |
US Patent US8841453 (2014)
BindingDB Entry DOI: 10.7270/Q2W957VS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50525991
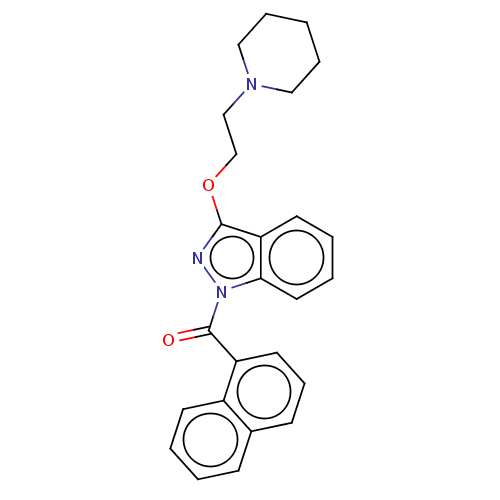
(CHEMBL4461027)Show SMILES O=C(c1cccc2ccccc12)n1nc(OCCN2CCCCC2)c2ccccc12 Show InChI InChI=1S/C25H25N3O2/c29-25(21-13-8-10-19-9-2-3-11-20(19)21)28-23-14-5-4-12-22(23)24(26-28)30-18-17-27-15-6-1-7-16-27/h2-5,8-14H,1,6-7,15-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using butyrylthiocholine as substrate by Ellman's assay |
Eur J Med Chem 166: 90-107 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.030
BindingDB Entry DOI: 10.7270/Q2VQ363K |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50599188

(CHEMBL5184944)Show SMILES CCN(CC)Cc1ccccc1CNC(=O)c1cccc(NC(=O)c2ccc(F)cc2)c1C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00944
BindingDB Entry DOI: 10.7270/Q2HX1HP4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data