

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM175970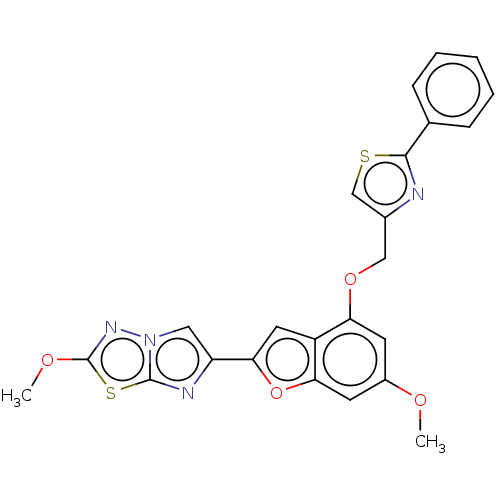 (US10047103, 3 | US9605024, Example 3 | US9688695, ...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at PAR4 (unknown origin) assessed as inhibition of activating peptide-induced receptor activation | Bioorg Med Chem Lett 26: 5481-5486 (2016) Article DOI: 10.1016/j.bmcl.2016.10.020 BindingDB Entry DOI: 10.7270/Q2P55QGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM175970 (US10047103, 3 | US9605024, Example 3 | US9688695, ...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Antagonist activity at PAR4 (unknown origin) assessed as inhibition of gamma-thrombin-induced receptor activation | Bioorg Med Chem Lett 26: 5481-5486 (2016) Article DOI: 10.1016/j.bmcl.2016.10.020 BindingDB Entry DOI: 10.7270/Q2P55QGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176023 (US10047103, 56 | US9688695, 56) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286492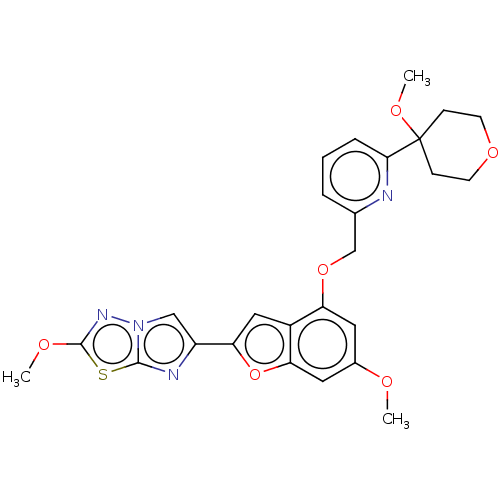 (2-Methoxy-6-(6-methoxy-4-((6-(4-methoxytetrahydro-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286477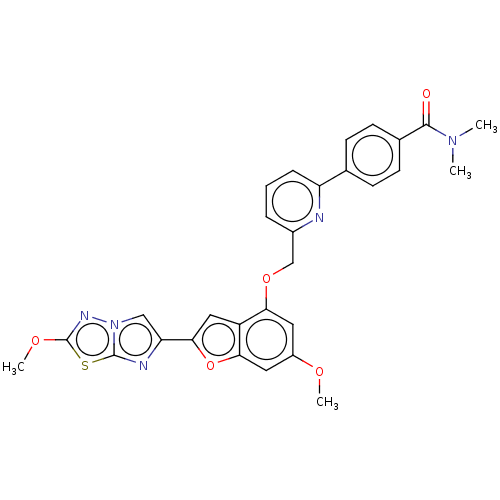 (4-(6-(((6-Methoxy-2-(2-methoxyimidazo[2,1-b][1,3,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286479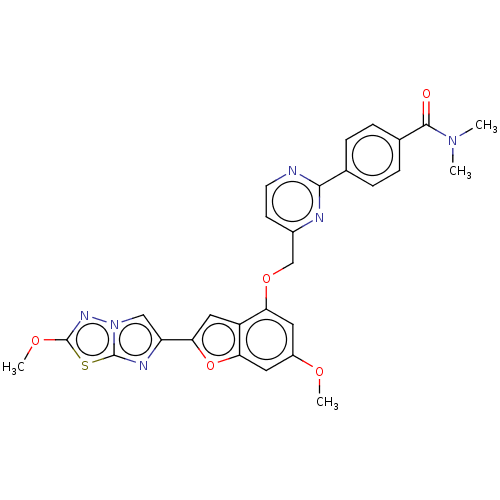 (US9518064, Example 98) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176053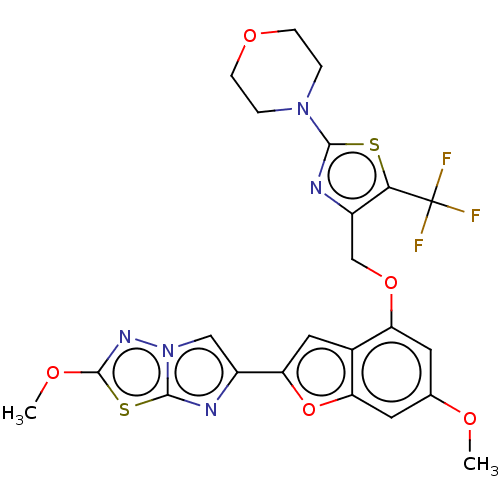 (US10047103, 86 | US9688695, 86) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM175970 (US10047103, 3 | US9605024, Example 3 | US9688695, ...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176082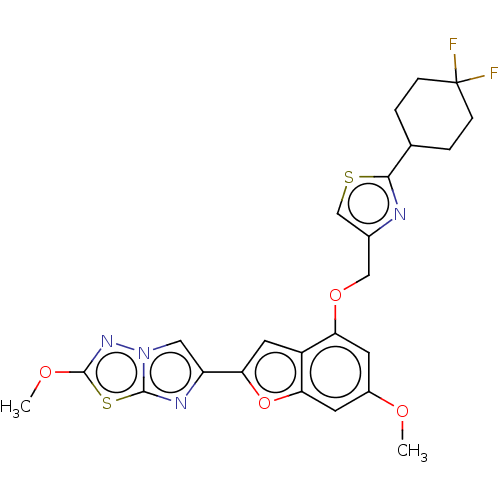 (US10047103, 115 | US9688695, 115) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176039 (US10047103, 72 | US9688695, 72) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286495 ((S)-2-(1-Fluoroethyl)-6-(4-((6-(4-fluorotetrahydro...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM364891 (US9862730, Example 349) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Houston | Assay Description The FLIPR assay is an exemplary in vitro assay for measuring the activity of the PAR4 antagonists of the present invention. In this assay, intracellu... | J Med Chem 48: 6661-70 (2005) BindingDB Entry DOI: 10.7270/Q2X92DKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286494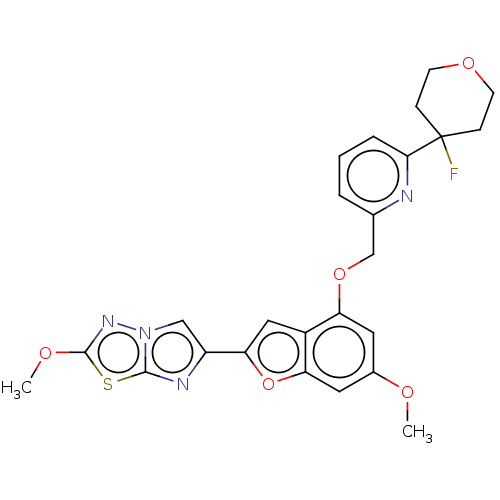 (6-(4-((6-(4-Fluorotetrahydro-2H-pyran-4-yl)pyridin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286478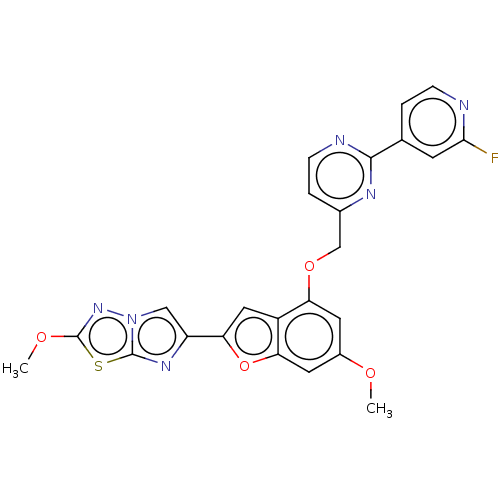 (6-(4-((2-(2-fluoropyridin-4-yl)pyrimidin-4-yl)meth...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286421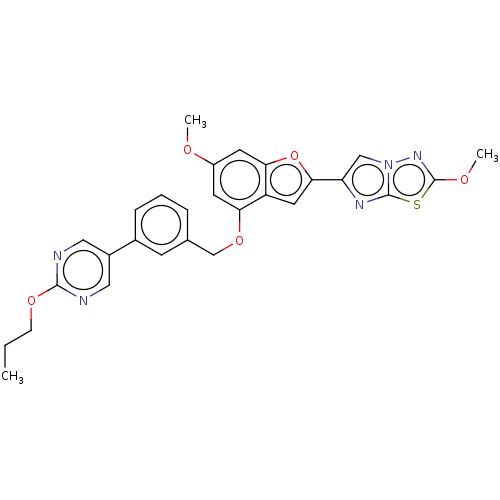 (US9518064, Example 37) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286493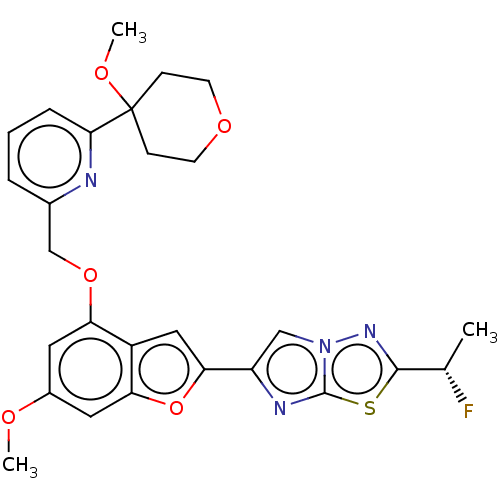 ((S)-2-(1-Fluoroethyl)-6-(6-methoxy-4-((6-(4-methox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286420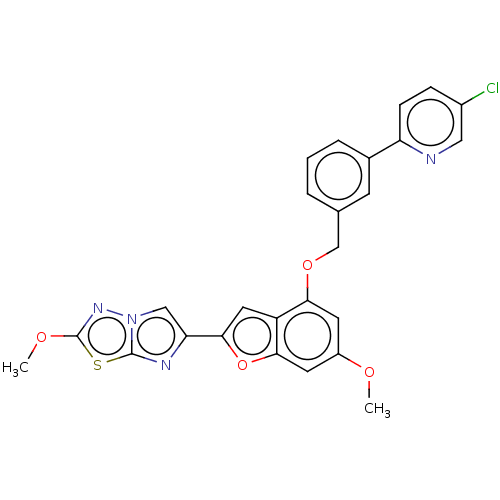 (US9518064, Example 36) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286464 (6-(4-((3-Fluoro-5-(2-methoxypyrimidin-5-yl)benzyl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50600788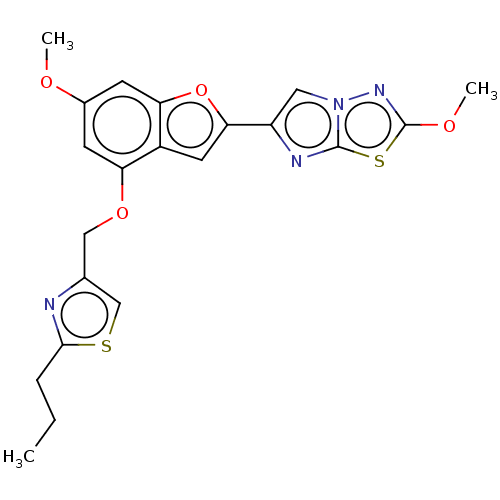 (CHEMBL5206065) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176003 (US10047103, 36 | US9688695, 36) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176061 (US10047103, 94 | US9688695, 94) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50600792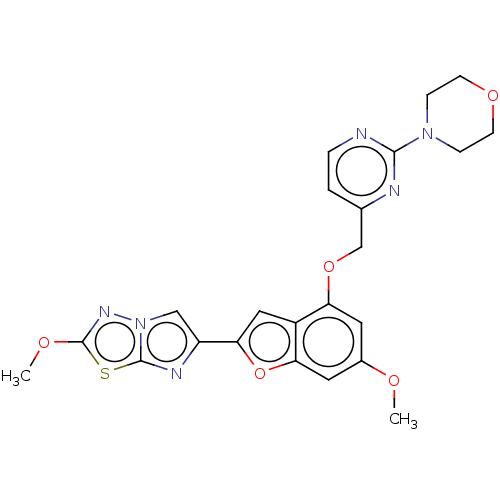 (CHEMBL5189522) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM175988 (US10047103, 21 | US9688695, 21) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176007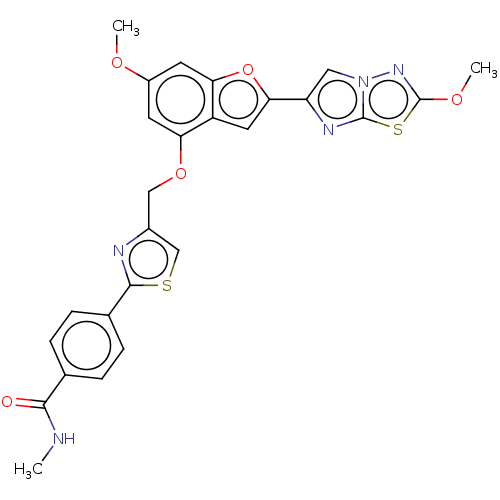 (US10047103, 40 | US9688695, 40) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286491 ((S)-4-(6-(((2-(2-(1-Fluoroethyl)imidazo[2,1-b][1,3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286490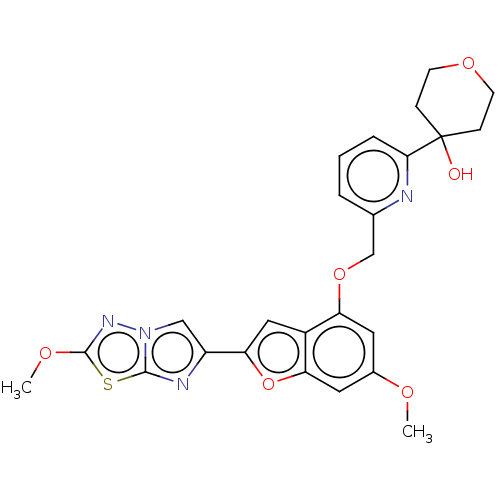 (4-(6-(((6-Methoxy-2-(2-methoxyimidazo[2,1-b][1,3,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176033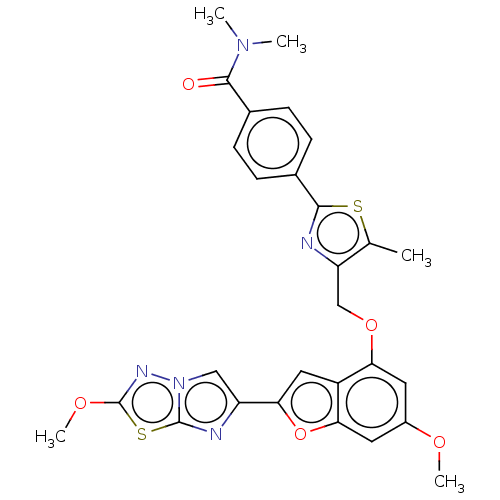 (US10047103, 66 | US9688695, 66) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PAR4 expressed in HEK293 cells assessed as Ala-(L-4-F-Phe)-Pro-Gly-Trp-Leu-Val-Lys-Asn-Gly-induced inhibition of calcium mobiliza... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112893 BindingDB Entry DOI: 10.7270/Q2028W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM454734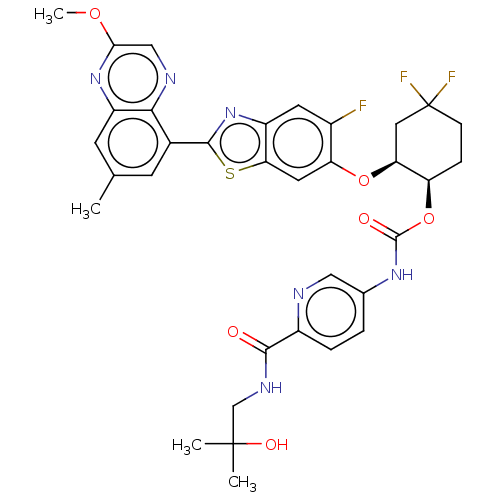 (US10730868, Ex. No. 2) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FLIPR-based calcium mobilization assay in HEK293 cells was used to measure PAR4 antagonism agonism, and selectivity against PAR1. The activity of the... | US Patent US10730868 (2020) BindingDB Entry DOI: 10.7270/Q2125WQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286424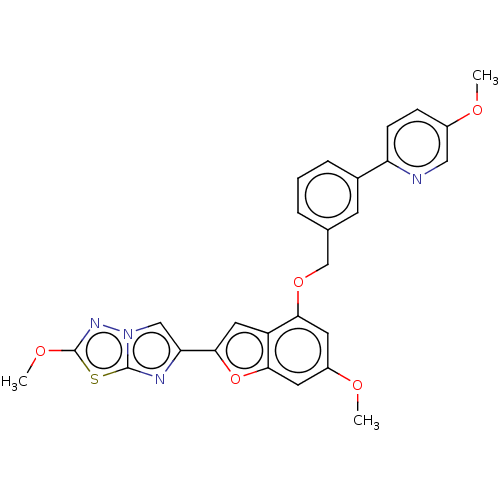 (US9518064, Example 40) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286418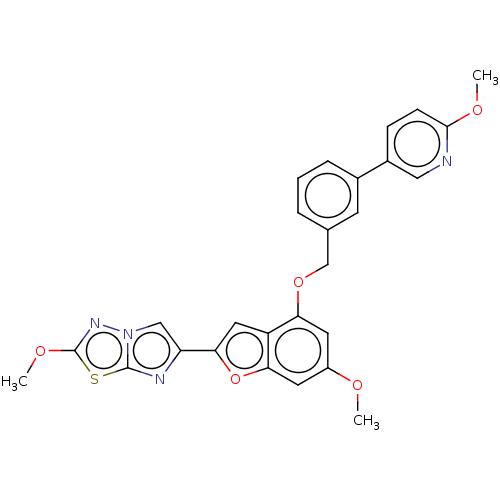 (US9518064, Example 34) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286419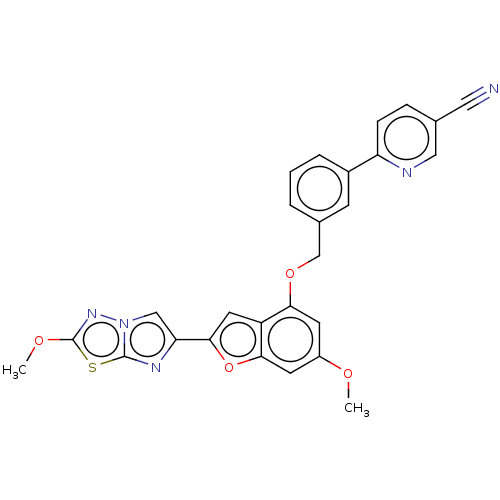 (US9518064, Example 35) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM454806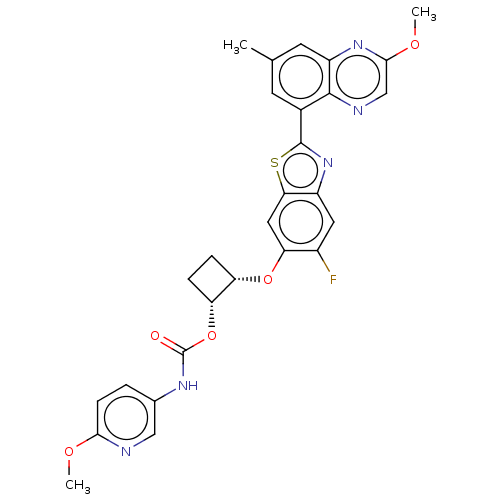 (US10730868, Ex. No. 73) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description FLIPR-based calcium mobilization assay in HEK293 cells was used to measure PAR4 antagonism agonism, and selectivity against PAR1. The activity of the... | US Patent US10730868 (2020) BindingDB Entry DOI: 10.7270/Q2125WQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286453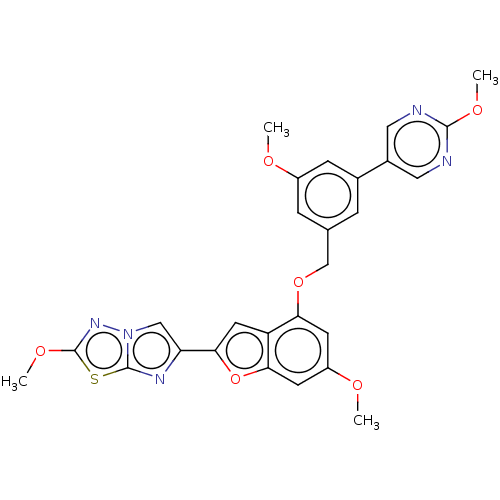 (US9518064, Example 71) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176026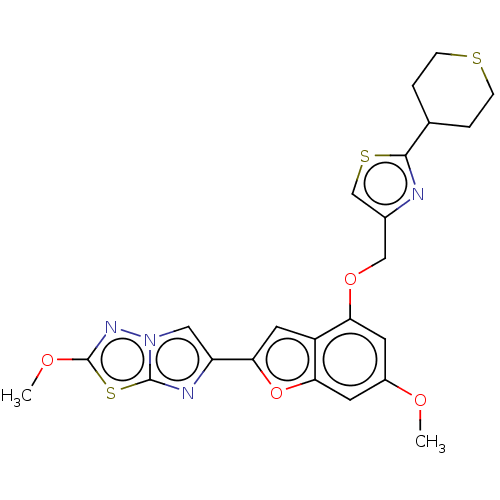 (US10047103, 59 | US9688695, 59) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176019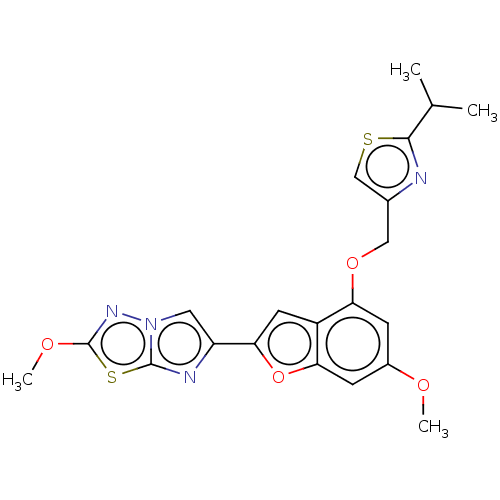 (US10047103, 52 | US9688695, 52) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286418 (US9518064, Example 34) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286419 (US9518064, Example 35) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286424 (US9518064, Example 40) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50578824 (CHEMBL4860533) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human PAR4 expressed in Ga15-HEK293 cells assessed as reduction in PAR4 AP AYPGKF-NH2-induced cytosolic calcium incubated for ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113764 BindingDB Entry DOI: 10.7270/Q2028WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286423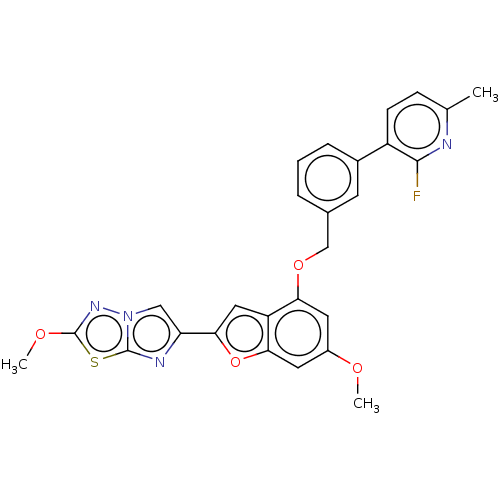 (US9518064, Example 39) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176061 (US10047103, 94 | US9688695, 94) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PAR4 expressed in HEK293 cells assessed as Ala-(L-4-F-Phe)-Pro-Gly-Trp-Leu-Val-Lys-Asn-Gly-induced inhibition of calcium mobiliza... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112893 BindingDB Entry DOI: 10.7270/Q2028W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286436 (US9518064, Example 52) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286467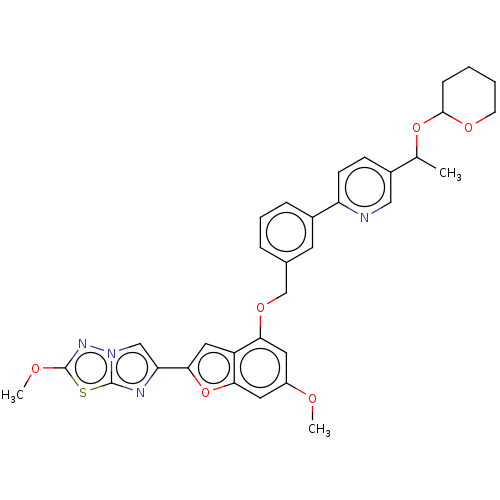 (2-Methoxy-6-(6-methoxy-4-((3-(5-(1-((tetrahydro-2H...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286449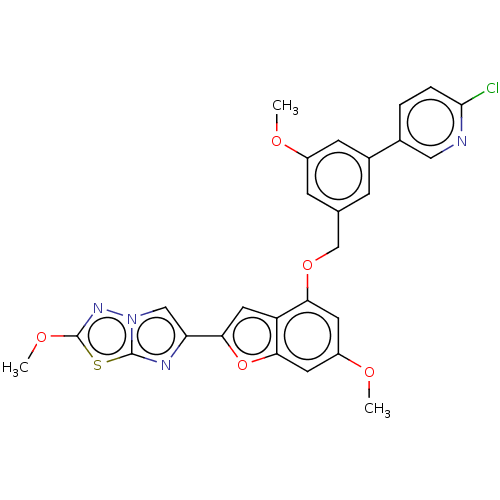 (6-(4-((3-(6-Chloropyridin-3-yl)-5-methoxybenzyl)ox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM286416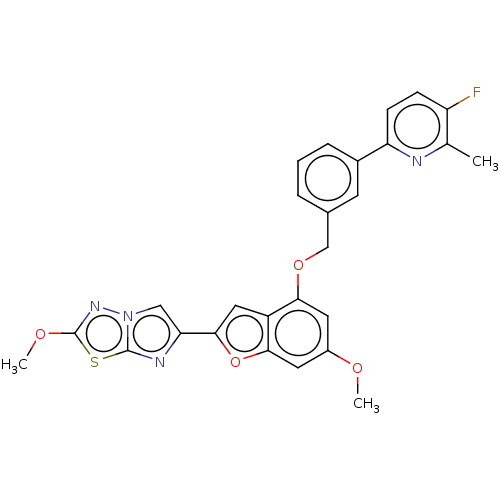 (US9518064, Example 32) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company; Universite De Montreal US Patent | Assay Description The activity of the PAR4 antagonists of the present invention were tested in PAR4 expressing cells by monitoring H-Ala-Phe(4-F)-Pro-Gly-Trp-Leu-Val-L... | US Patent US9518064 (2016) BindingDB Entry DOI: 10.7270/Q2TT4SZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM364941 (US9862730, Example 399) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Houston | Assay Description The FLIPR assay is an exemplary in vitro assay for measuring the activity of the PAR4 antagonists of the present invention. In this assay, intracellu... | J Med Chem 48: 6661-70 (2005) BindingDB Entry DOI: 10.7270/Q2X92DKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50600791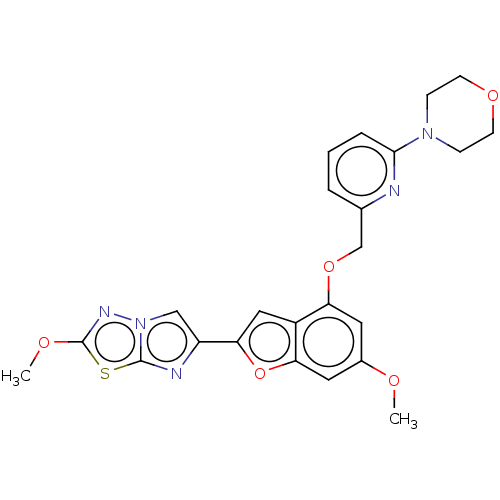 (CHEMBL5177223) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176059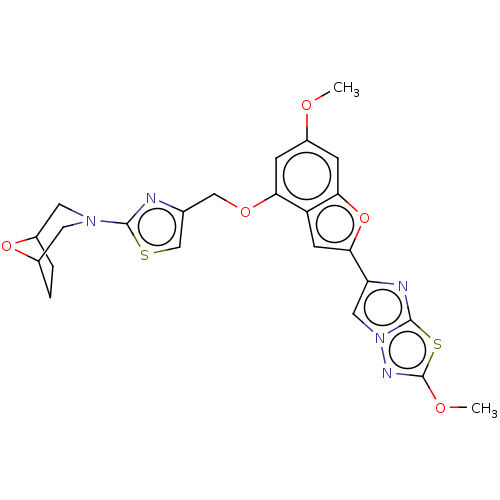 (US10047103, 92 | US9688695, 92) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176258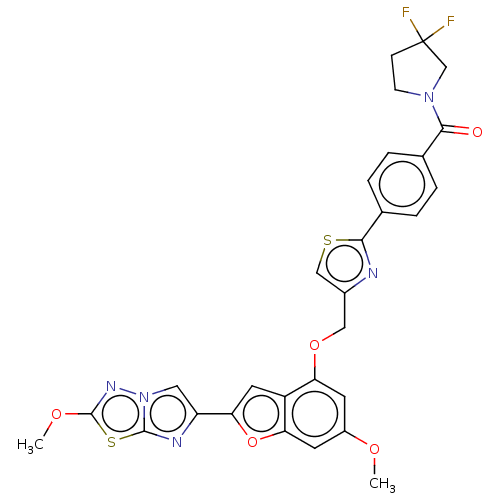 (US10047103, 291 | US9688695, 291) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM176063 (US10047103, 96 | US9688695, 96) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00359 BindingDB Entry DOI: 10.7270/Q2VH5SWM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 933 total ) | Next | Last >> |