

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446394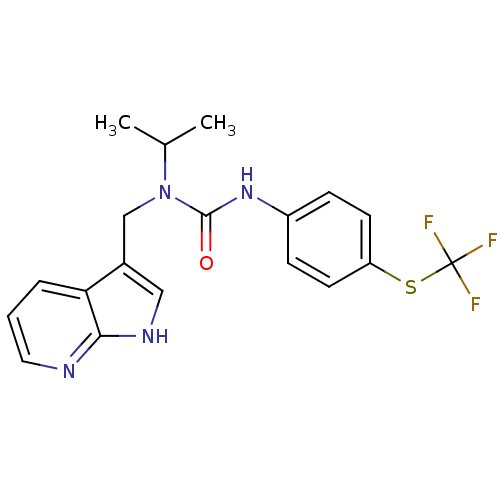 (CHEMBL3109646 | US9181261, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of TrkA (unknown origin) using fluorescently labeled peptide substrate after 3 hrs | ACS Med Chem Lett 5: 8-9 (2014) Article DOI: 10.1021/ml4005073 BindingDB Entry DOI: 10.7270/Q2B56M6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446394 (CHEMBL3109646 | US9181261, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an... | US Patent US9181261 (2015) BindingDB Entry DOI: 10.7270/Q24F1PJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446392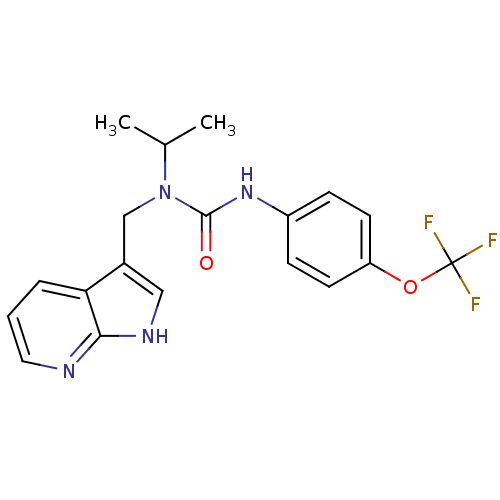 (CHEMBL3109645 | US9181261, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of TrkA (unknown origin) using fluorescently labeled peptide substrate after 3 hrs | ACS Med Chem Lett 5: 8-9 (2014) Article DOI: 10.1021/ml4005073 BindingDB Entry DOI: 10.7270/Q2B56M6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446392 (CHEMBL3109645 | US9181261, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 11.3 | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an... | US Patent US9181261 (2015) BindingDB Entry DOI: 10.7270/Q24F1PJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM191135 (US9181261, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 19.7 | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an... | US Patent US9181261 (2015) BindingDB Entry DOI: 10.7270/Q24F1PJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM191136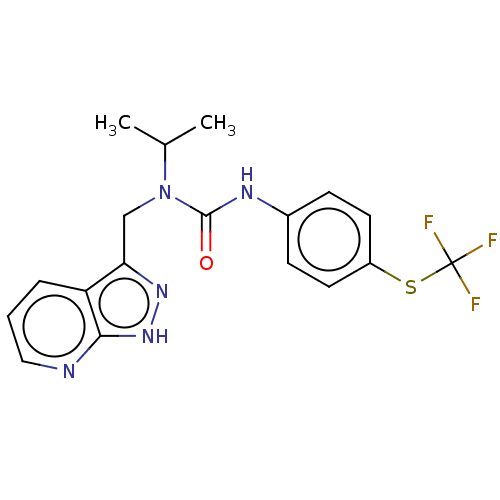 (US9181261, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 24.9 | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an... | US Patent US9181261 (2015) BindingDB Entry DOI: 10.7270/Q24F1PJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50565738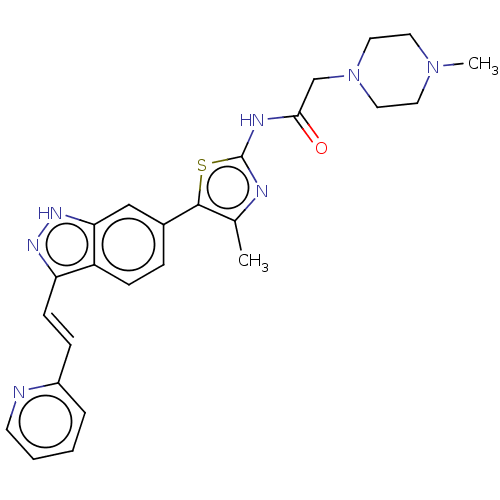 (CHEMBL4794404) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TEL fused wild type TRKA (unknown origin) transfected in mouse BaF3 cells assessed as reduction in phosphorylation at Y490 residue afte... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112744 BindingDB Entry DOI: 10.7270/Q20V8HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50565738 (CHEMBL4794404) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | <30 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKA in human KM-12-LUC cells assessed as reduction in AKT phosphorylation at T308 residue after 2 hrs by Western blot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112744 BindingDB Entry DOI: 10.7270/Q20V8HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50565738 (CHEMBL4794404) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | <30 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKA in human KM-12-LUC cells assessed as reduction in ERK1/2 phosphorylation at T202/Y204 residues after 2 hrs by Western blot analysi... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112744 BindingDB Entry DOI: 10.7270/Q20V8HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50565738 (CHEMBL4794404) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | <30 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKA in human KM-12-LUC cells assessed as reduction in AKT phosphorylation at S473 residue after 2 hrs by Western blot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112744 BindingDB Entry DOI: 10.7270/Q20V8HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM191137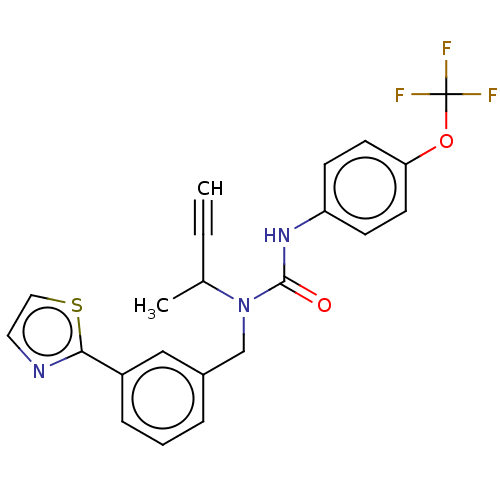 (US9181261, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 48 | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an... | US Patent US9181261 (2015) BindingDB Entry DOI: 10.7270/Q24F1PJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50578295 (CHEMBL4861572) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human full length TRKA at inactive state expressed in rat PC-12 cells assessed as induction of kinase conformational change at 40... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00483 BindingDB Entry DOI: 10.7270/Q2G73JJ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50565738 (CHEMBL4794404) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of LMNA fused TRKA G667C mutant (unknown origin) transfected in mouse BaF3 cells assessed as reduction in phosphorylation at Y490 residue ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112744 BindingDB Entry DOI: 10.7270/Q20V8HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50073587 (CHEMBL3408947 | US10358436, Example A185 | US20230...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of TRKA (unknown origin) | Eur J Med Chem 95: 35-40 (2015) Article DOI: 10.1016/j.ejmech.2015.03.020 BindingDB Entry DOI: 10.7270/Q2NG4SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50073587 (CHEMBL3408947 | US10358436, Example A185 | US20230...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of TRKA (unknown origin) by cell-based assay | J Med Chem 58: 147-69 (2015) Article DOI: 10.1021/jm5005336 BindingDB Entry DOI: 10.7270/Q2M0475V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM191138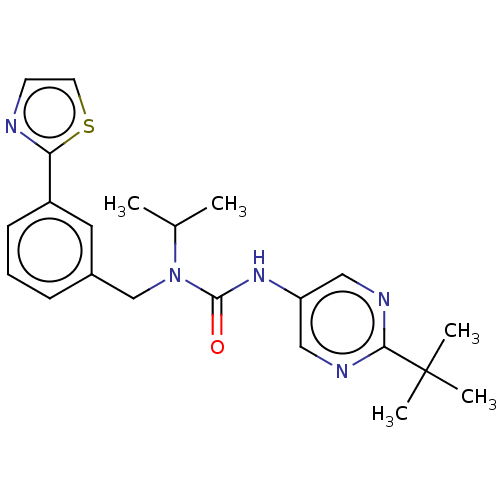 (US9181261, 15) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 102 | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an... | US Patent US9181261 (2015) BindingDB Entry DOI: 10.7270/Q24F1PJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50022677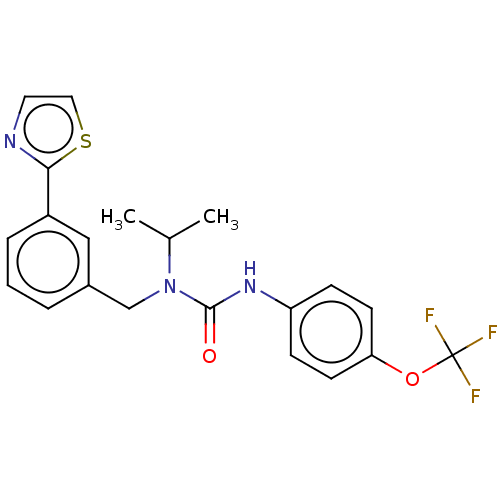 (CHEMBL3298270 | US9181261, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 119 | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an... | US Patent US9181261 (2015) BindingDB Entry DOI: 10.7270/Q24F1PJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50565738 (CHEMBL4794404) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of LMNA fused TRKA G595R mutant (unknown origin) transfected in mouse BaF3 cells assessed as reduction in phosphorylation at Y490 residue ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112744 BindingDB Entry DOI: 10.7270/Q20V8HJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM191139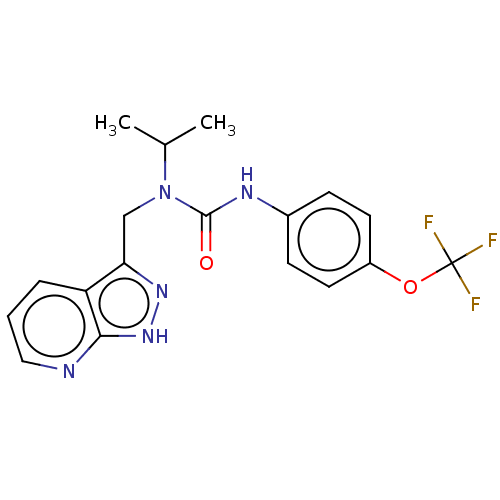 (US9181261, 20) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 141 | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an... | US Patent US9181261 (2015) BindingDB Entry DOI: 10.7270/Q24F1PJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505017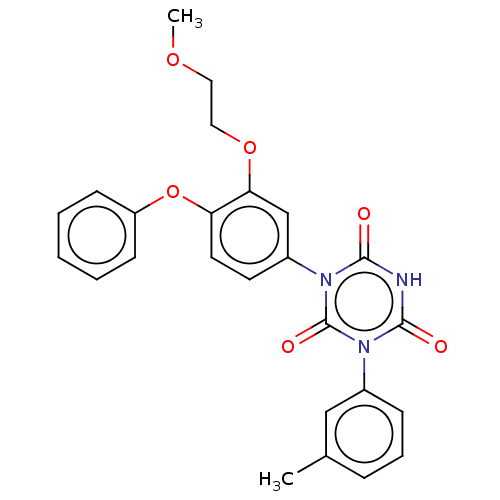 (CHEMBL4462161) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505015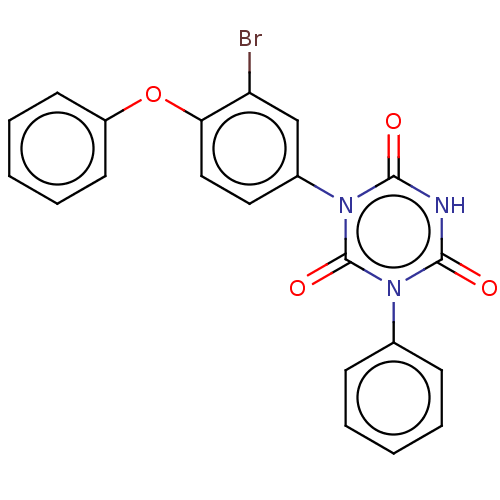 (CHEMBL4538772) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505010 (CHEMBL4455143) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505006 (CHEMBL4483402) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505011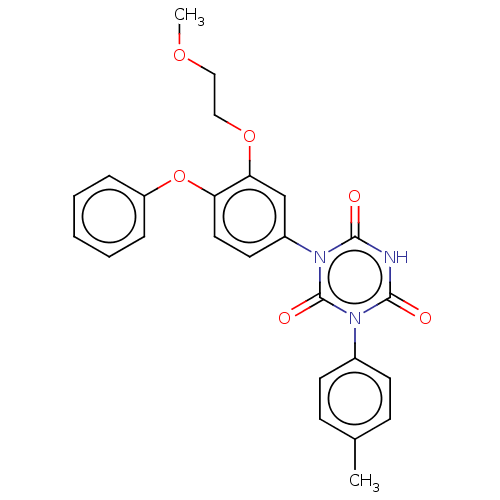 (CHEMBL4529332) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505018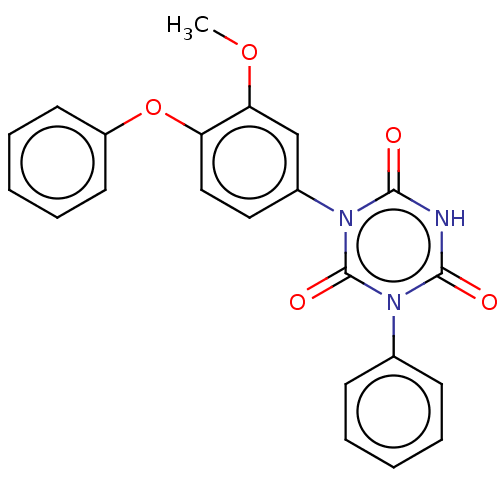 (CHEMBL4464173) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505007 (CHEMBL4464037) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505012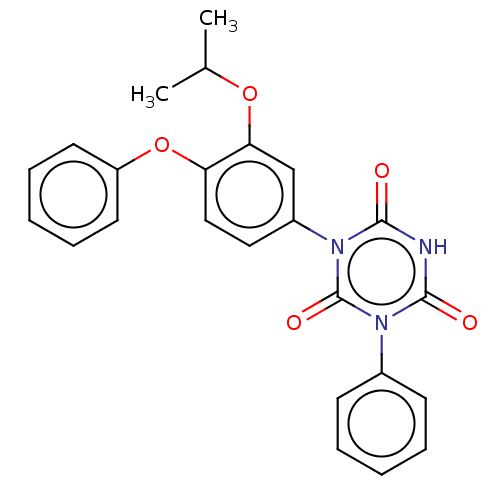 (CHEMBL4472787) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505016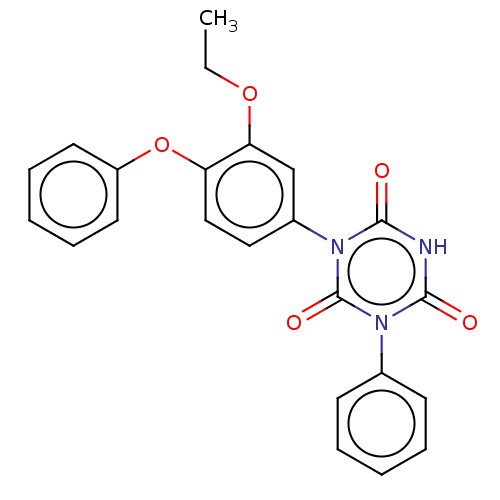 (CHEMBL4527488) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505005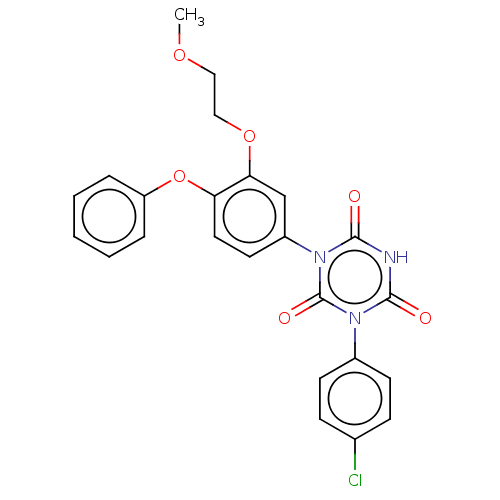 (CHEMBL4455810) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505013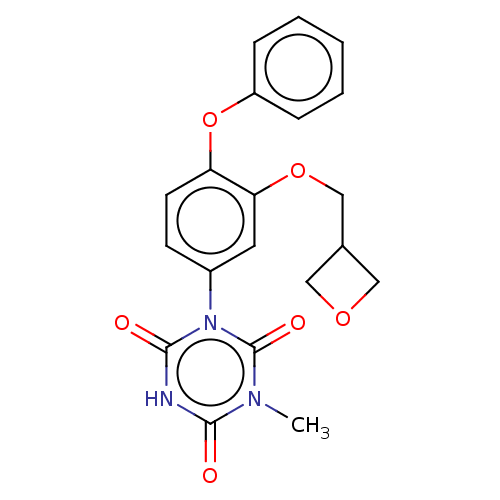 (CHEMBL4574315) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505019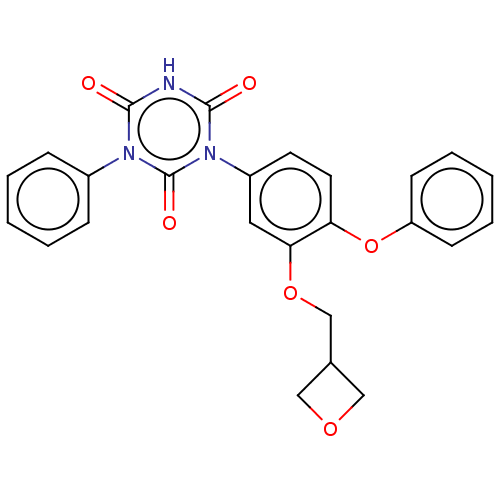 (CHEMBL4465865) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50508282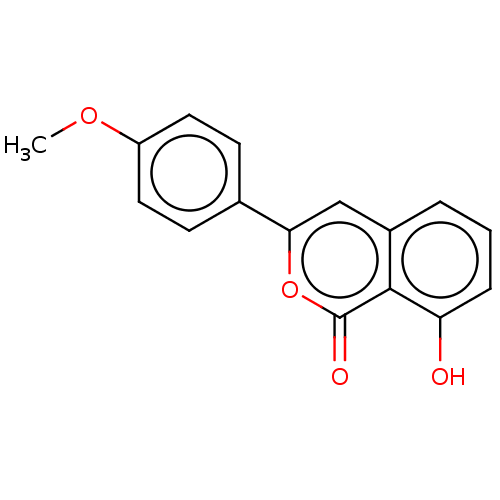 (CHEMBL4471556) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 667 | n/a | n/a | n/a | n/a |
Indian Institute of Technology Madras Curated by ChEMBL | Assay Description Agonist activity at RFP-fused TrkA receptor (unknown origin) expressed in HEK293T cells after 6 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 29: 585-590 (2019) Article DOI: 10.1016/j.bmcl.2018.12.057 BindingDB Entry DOI: 10.7270/Q2542RVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505014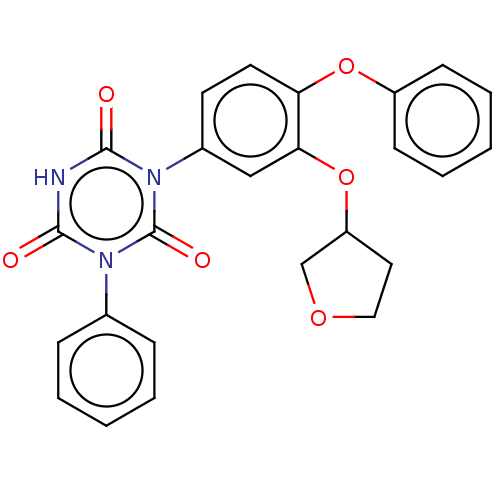 (CHEMBL4467829) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 780 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505008 (CHEMBL4518577) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 830 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446393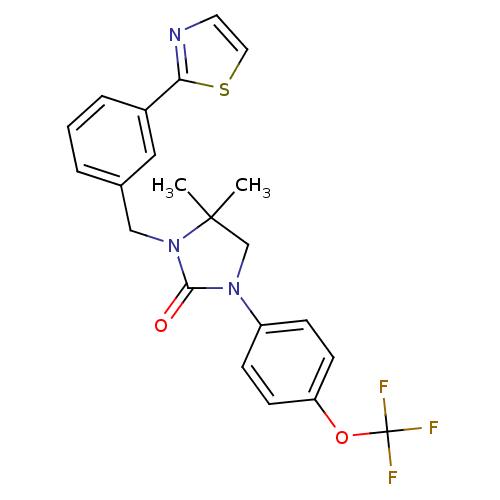 (CHEMBL3109644 | US9181261, 74) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of TrkA (unknown origin) using fluorescently labeled peptide substrate after 3 hrs | ACS Med Chem Lett 5: 8-9 (2014) Article DOI: 10.1021/ml4005073 BindingDB Entry DOI: 10.7270/Q2B56M6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446393 (CHEMBL3109644 | US9181261, 74) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an... | US Patent US9181261 (2015) BindingDB Entry DOI: 10.7270/Q24F1PJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50505009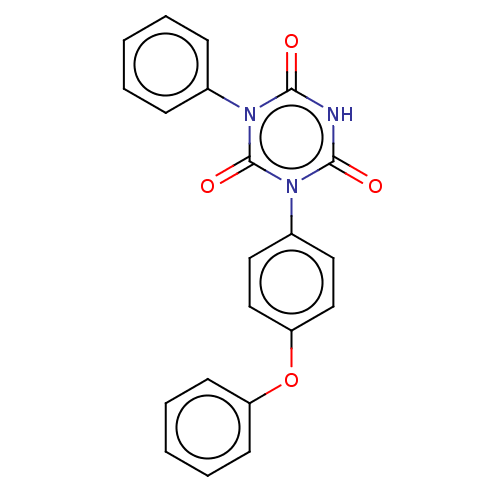 (CHEMBL4542212) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Agonist activity at TrkA (unknown origin) in expressed in U2OS cells incubated for 3 hrs and measured after 60 hrs in presence of NGF by fluorescence... | ACS Med Chem Lett 10: 1590-1591 (2019) Article DOI: 10.1021/acsmedchemlett.9b00506 BindingDB Entry DOI: 10.7270/Q2GM8BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50242740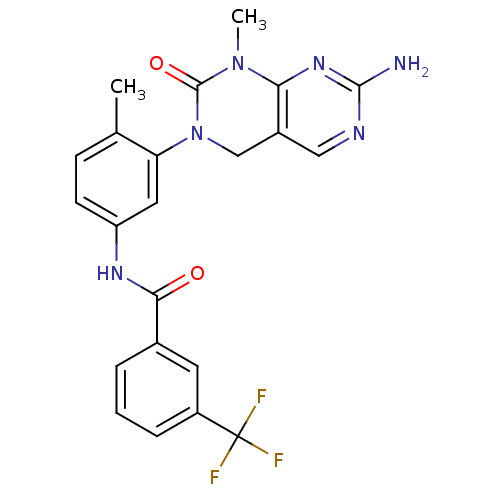 (CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of NTRK1 | Proc Natl Acad Sci USA 104: 19936-41 (2007) Article DOI: 10.1073/pnas.0707498104 BindingDB Entry DOI: 10.7270/Q24X58QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50551644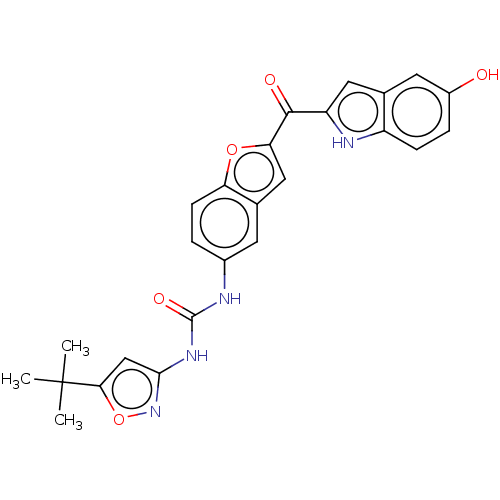 (CHEMBL4745937) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human NTRK1 incubated for 30 mins by Kinobead based assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM191140 (US9181261, 152) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 8.08E+3 | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an... | US Patent US9181261 (2015) BindingDB Entry DOI: 10.7270/Q24F1PJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50578295 (CHEMBL4861572) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human full length TRKA at active state expressed in rat PC-12 cells assessed as induction of kinase conformational change at 52 d... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00483 BindingDB Entry DOI: 10.7270/Q2G73JJ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50020712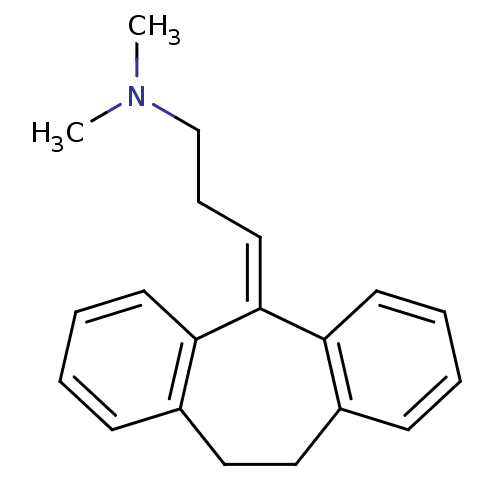 (10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a |
Southmead Hospital Curated by ChEMBL | Assay Description Antagonist activity at full length glycosylated human TrkA expressed in HEKN3S cells cells assessed as reduction in NGF-induced ERK 42/44 phosphoryla... | J Med Chem 58: 767-77 (2015) Article DOI: 10.1021/jm501307e BindingDB Entry DOI: 10.7270/Q25B045H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||