

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Activation of human ERalpha expressed in human U2-OS cells by luciferase reporter gene assay relative to untreated control | J Med Chem 55: 9607-18 (2012) Article DOI: 10.1021/jm300860j BindingDB Entry DOI: 10.7270/Q2R212H2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20625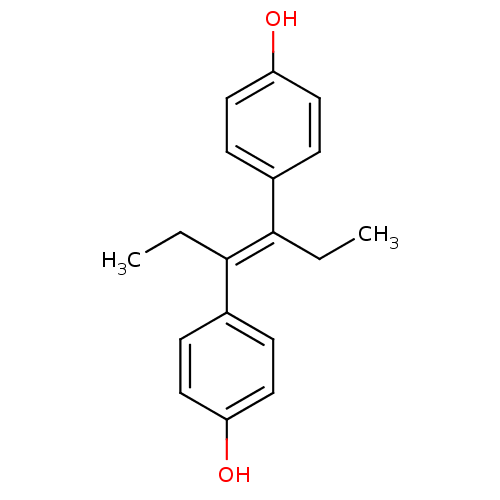 (4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro agonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells at 10 pM | J Med Chem 44: 1654-7 (2001) BindingDB Entry DOI: 10.7270/Q2F47NDX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]17-beta-estradiol from human Estrogen receptor alpha | Bioorg Med Chem Lett 10: 147-51 (2000) BindingDB Entry DOI: 10.7270/Q2JH3MQR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.00840 | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Agonist activity at human ERalpha in human MCF7 cells assessed as progesterone receptor endogenous genes activation after 24 hrs by qPCR | J Med Chem 58: 1184-94 (2015) Article DOI: 10.1021/jm501829f BindingDB Entry DOI: 10.7270/Q2XK8H8P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Effect on ERalpha in MCF7 cell line transfected with ER responsive luciferase reporter | Bioorg Med Chem Lett 15: 5562-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.010 BindingDB Entry DOI: 10.7270/Q2N879CK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50590844 (CHEMBL5177342) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114658 BindingDB Entry DOI: 10.7270/Q2NP28CD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50121317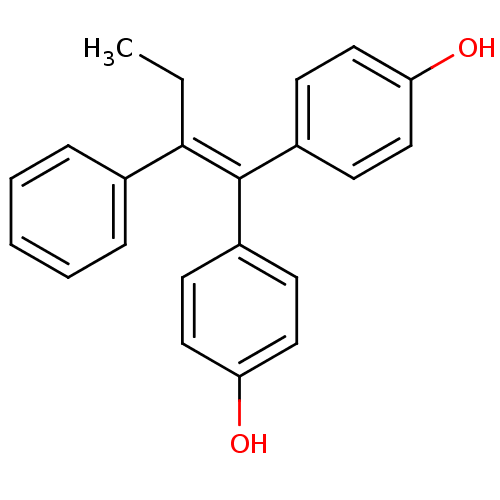 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a |
Georgetown University Curated by ChEMBL | Assay Description Agonist activity at ER in human MCF7:WS8 cells assessed as increase in cell growth by measuring DNA level after 7 days by fluorescence analysis | J Med Chem 57: 4569-83 (2014) Article DOI: 10.1021/jm500569h BindingDB Entry DOI: 10.7270/Q2H996R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Transcriptional potency (EC50) at Human estrogen receptor alpha | J Med Chem 44: 4230-51 (2001) BindingDB Entry DOI: 10.7270/Q289155W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Transcriptional activation of estrogen receptor alpha in U2OS cell using estrogen-regulated luciferase reporter gene plasmid upon incubation for 20-3... | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | 0.0200 | n/a | n/a | 7.4 | 22 |
Novartis Pharmaceuticals | Assay Description Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPA... | J Med Chem 45: 1399-401 (2002) Article DOI: 10.1021/jm015577l BindingDB Entry DOI: 10.7270/Q27M0665 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in HEK293 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 24: 5265-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.053 BindingDB Entry DOI: 10.7270/Q2S46TKZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50309557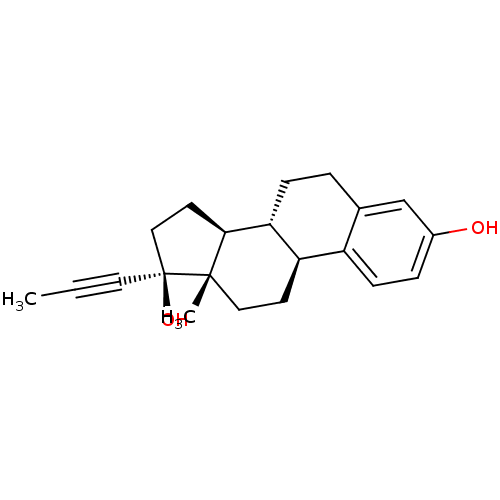 (CHEMBL592868 | estrogen) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Estrogenic activity at ERalpha in human Ishikawa cells assessed as stimulation of alkaline phosphatase activity after 4 days by microplate scanning s... | Bioorg Med Chem 18: 809-21 (2010) Article DOI: 10.1016/j.bmc.2009.11.046 BindingDB Entry DOI: 10.7270/Q28K797G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM412253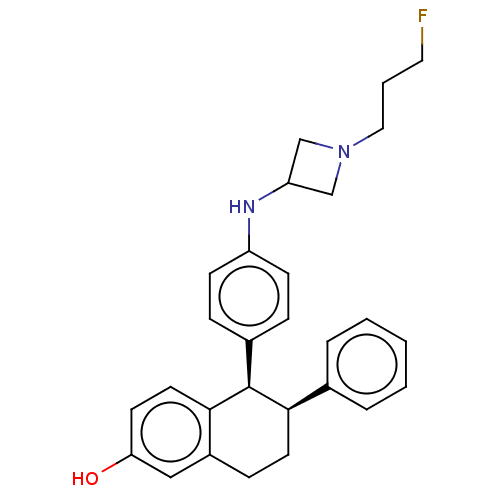 ((5R,6S)-5-(4-((1-(3- fluoropropyl)azetidin- 3-yl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The relative efficacies of Formula I compounds as inhibitors of an enzyme activity (or other biological activity) can be established by determining t... | US Patent US10399939 (2019) BindingDB Entry DOI: 10.7270/Q2G73H4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Estrogenic activity at ERalpha in human Ishikawa cells assessed as induction of alkaline phosphatase activity using p-nitrophenol as substrate treate... | J Nat Prod 81: 966-975 (2018) Article DOI: 10.1021/acs.jnatprod.7b01070 BindingDB Entry DOI: 10.7270/Q2DV1NKT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM412241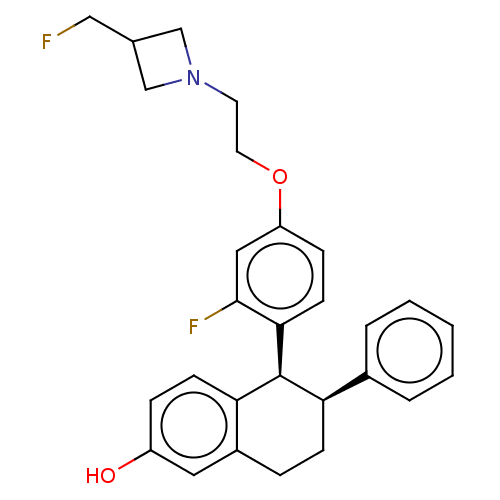 ((5S,6S)-5-(2-fluoro-4- (2-(3- (fluoromethyl)azetid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The relative efficacies of Formula I compounds as inhibitors of an enzyme activity (or other biological activity) can be established by determining t... | US Patent US10399939 (2019) BindingDB Entry DOI: 10.7270/Q2G73H4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM412245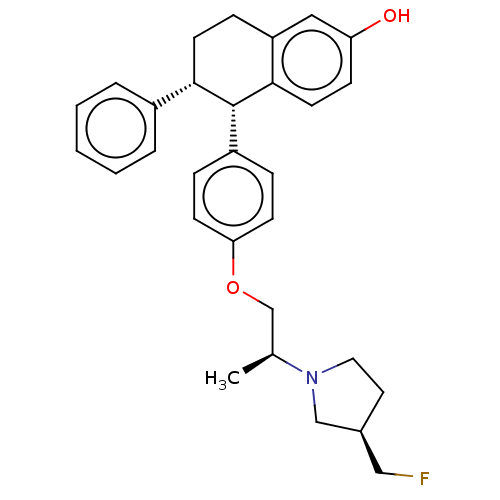 ((5S,6R)-5-(4-((S)-2- ((R)-3- (fluoromethyl)pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The relative efficacies of Formula I compounds as inhibitors of an enzyme activity (or other biological activity) can be established by determining t... | US Patent US10399939 (2019) BindingDB Entry DOI: 10.7270/Q2G73H4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM412255 (4-((1S,2R)-1-(4-(2-(3- (fluoromethyl)azetidin- 1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0380 | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The relative efficacies of Formula I compounds as inhibitors of an enzyme activity (or other biological activity) can be established by determining t... | US Patent US10399939 (2019) BindingDB Entry DOI: 10.7270/Q2G73H4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM412225 ((1S,2S)-1-[2,6- difluoro-4-[2-[3- (fluoromethyl)az...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The relative efficacies of Formula I compounds as inhibitors of an enzyme activity (or other biological activity) can be established by determining t... | US Patent US10399939 (2019) BindingDB Entry DOI: 10.7270/Q2G73H4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at ERalpha expressed in HEK cells co-expressing beta-lactamase after 18 hrs by beta-lactamase reporter gene assay | Bioorg Med Chem Lett 21: 5680-3 (2011) Article DOI: 10.1016/j.bmcl.2011.08.041 BindingDB Entry DOI: 10.7270/Q2JS9QTC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM412248 ((5S,6S)-5-(6-(2-(3- (fluoromethyl)azetidin- 1-yl)e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The relative efficacies of Formula I compounds as inhibitors of an enzyme activity (or other biological activity) can be established by determining t... | US Patent US10399939 (2019) BindingDB Entry DOI: 10.7270/Q2G73H4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Estrogenic activity at estrogen receptor in human Ishikawa Var-1 cells assessed as stimulation of alkaline phosphatase activity measured by metabolis... | J Nat Prod 67: 1829-32 (2004) Article DOI: 10.1021/np040069a BindingDB Entry DOI: 10.7270/Q2SN09TS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Transcriptional activation of ERalpha receptor expressed in CHO-K1 cells after 24 hrs by luciferase reporter gene assay | Eur J Med Chem 54: 188-96 (2012) Article DOI: 10.1016/j.ejmech.2012.04.041 BindingDB Entry DOI: 10.7270/Q2445NJ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50173661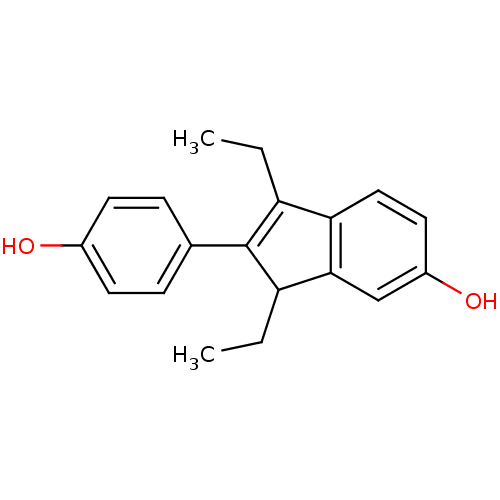 (1,3-Diethyl-2-(4-hydroxy-phenyl)-3H-inden-5-ol | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Transcriptional activation of estrogen receptor alpha in U2OS cell using estrogen-regulated luciferase reporter gene plasmid upon incubation for 20-3... | J Med Chem 48: 5989-6003 (2005) Article DOI: 10.1021/jm050226i BindingDB Entry DOI: 10.7270/Q2B857P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20625 (4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | 0.0600 | n/a | n/a | 7.4 | 22 |
Novartis Pharmaceuticals | Assay Description Radioligand binding assay was performed by using 96-well microtiterplates containing ER, 17beta-estradiol, and the test compound to be tested and SPA... | J Med Chem 45: 1399-401 (2002) Article DOI: 10.1021/jm015577l BindingDB Entry DOI: 10.7270/Q27M0665 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50106646 ((S)-3-(4-Hydroxy-phenyl)-2-((R)-4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Transcriptional potency (EC50) at Human estrogen receptor alpha | J Med Chem 44: 4230-51 (2001) BindingDB Entry DOI: 10.7270/Q289155W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50233216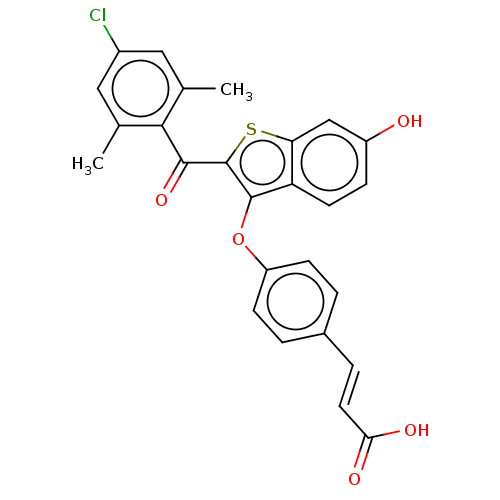 (CHEMBL4081807) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
University of Illinois College of Pharmacy Curated by ChEMBL | Assay Description Induction of ERalpha degradation in tamoxifen-sensitive human MCF7:WS8 cells after 24 hrs by CellTag 700 staining based In-cell western assay | J Med Chem 60: 1325-1342 (2017) Article DOI: 10.1021/acs.jmedchem.6b01355 BindingDB Entry DOI: 10.7270/Q29P33WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Agonist effect on transcriptional activation in T47D cells expressing estrogen receptor alpha | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a |
Free University of Berlin Curated by ChEMBL | Assay Description Concentration required to activate luciferase expression in MCF-7-2a cells | J Med Chem 47: 915-27 (2004) Article DOI: 10.1021/jm0309809 BindingDB Entry DOI: 10.7270/Q2BP041Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Agonist activity at human full-length ERalpha receptor expressed in human HEC1 cells co-expressing (ERE)2-pS2-luc gene assessed as transcriptional ac... | Eur J Med Chem 46: 2453-62 (2011) Article DOI: 10.1016/j.ejmech.2011.03.030 BindingDB Entry DOI: 10.7270/Q2ZC8362 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a |
the University of Tokyo Curated by ChEMBL | Assay Description Selective estrogen receptor down-regulator activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as induction of ERalph... | Bioorg Med Chem 27: 1952-1961 (2019) Article DOI: 10.1016/j.bmc.2019.03.042 BindingDB Entry DOI: 10.7270/Q24171GJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM412235 ((5S,6R)-5-(4-(2-(3- (fluoromethyl)pyrrolidin- 1- y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The relative efficacies of Formula I compounds as inhibitors of an enzyme activity (or other biological activity) can be established by determining t... | US Patent US10399939 (2019) BindingDB Entry DOI: 10.7270/Q2G73H4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50580473 (CHEMBL5094275) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
TBA | Assay Description Induction of degradation of ERalpha in human MCF7 cells incubated for 4 hrs in presence of anti-ERalpha rabbit monoclonal antibodies measured after 4... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00280 BindingDB Entry DOI: 10.7270/Q29Z98RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50005603 (CHEMBL3234630) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University) Curated by ChEMBL | Assay Description Agonist activity at ERalpha (unknown origin) expressed in human HepG2 cells assessed as transcriptional activation after 24 hrs by ERE-luciferase rep... | J Med Chem 57: 3532-45 (2014) Article DOI: 10.1021/jm500268j BindingDB Entry DOI: 10.7270/Q2MS3V9N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50398935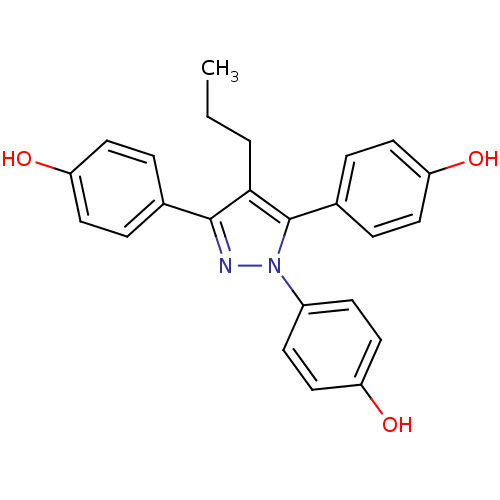 (PROPYLPYRAZOLETRIOL) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Activation of human ERalpha expressed in human U2-OS cells by luciferase reporter gene assay relative to untreated control | J Med Chem 55: 9607-18 (2012) Article DOI: 10.1021/jm300860j BindingDB Entry DOI: 10.7270/Q2R212H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Activation of ERalpha in human MCF7/2a cells by luciferase reporter gene assay relative to untreated control | J Med Chem 55: 9607-18 (2012) Article DOI: 10.1021/jm300860j BindingDB Entry DOI: 10.7270/Q2R212H2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at ERalpha expressed in human HEC1 cells assessed as transcriptional potency after 24 hrs by luciferase-beta galactosidase reporter ... | Eur J Med Chem 44: 3412-24 (2009) Article DOI: 10.1016/j.ejmech.2009.02.006 BindingDB Entry DOI: 10.7270/Q2NK3F37 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50099587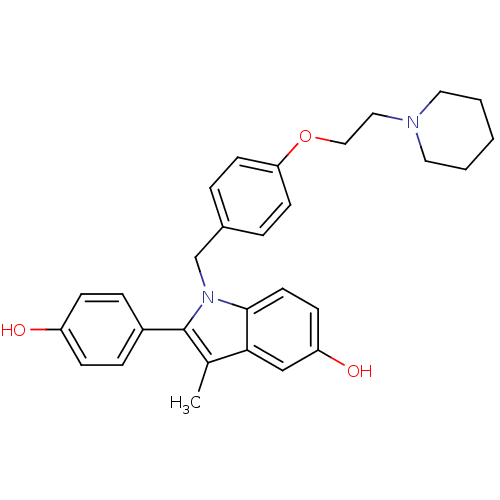 (2-(4-Hydroxy-phenyl)-3-methyl-1-[4-(2-piperidin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals Curated by ChEMBL | Assay Description Induction of estrogen receptor-alpha degradation in human MCF7 cells after 4 hrs by in-cell western assay | J Med Chem 58: 4888-904 (2015) Article DOI: 10.1021/acs.jmedchem.5b00054 BindingDB Entry DOI: 10.7270/Q29W0H7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM412251 ((5R,6S)-5-(4-((1-(3- fluoropropyl)azetidin- 3-yl)o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | n/a | n/a | 0.107 | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The relative efficacies of Formula I compounds as inhibitors of an enzyme activity (or other biological activity) can be established by determining t... | US Patent US10399939 (2019) BindingDB Entry DOI: 10.7270/Q2G73H4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.107 | n/a | n/a | n/a | n/a |
Concordia University Wisconsin Curated by ChEMBL | Assay Description Agonist activity at GAL4 DNA binding domain fused full-length chimeric estrogen receptor alpha (unknown origin) by FRET-based assay | J Med Chem 61: 4720-4738 (2018) Article DOI: 10.1021/acs.jmedchem.7b01601 BindingDB Entry DOI: 10.7270/Q2TH8R3Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
Nagasaki University Curated by ChEMBL | Assay Description Agonist activity at recombinant human GAL4-fused ERalpha expressed in HEk293 cells by luciferase reporter gene assay | Bioorg Med Chem 26: 1638-1642 (2018) Article DOI: 10.1016/j.bmc.2018.02.010 BindingDB Entry DOI: 10.7270/Q24170Q8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50297518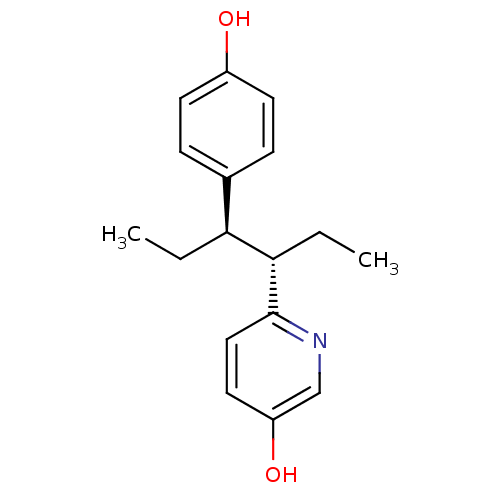 (CHEMBL556650 | trans-6-[1-ethyl-2-(4-hydroxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20625 (4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
the University of Tokyo Curated by ChEMBL | Assay Description Selective estrogen receptor down-regulator activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as induction of ERalph... | Bioorg Med Chem 27: 1952-1961 (2019) Article DOI: 10.1016/j.bmc.2019.03.042 BindingDB Entry DOI: 10.7270/Q24171GJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50297516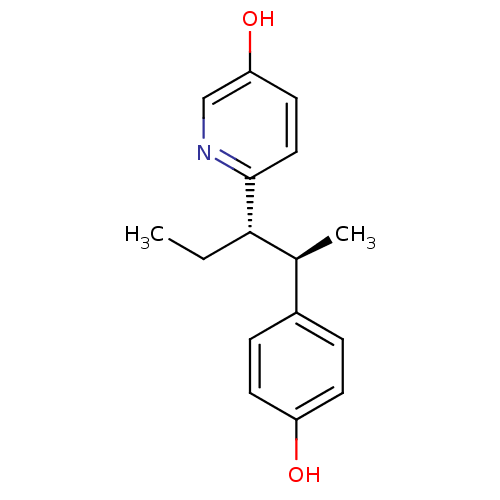 (CHEMBL538148 | trans-6-[1-ethyl-2-(4-hydroxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HEC1 cells cotransfected with 2ERE-pS2-Luc assessed as relative transcriptional potency by luciferase reporter... | Eur J Med Chem 44: 3560-70 (2009) Article DOI: 10.1016/j.ejmech.2009.03.013 BindingDB Entry DOI: 10.7270/Q21Z44G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50398932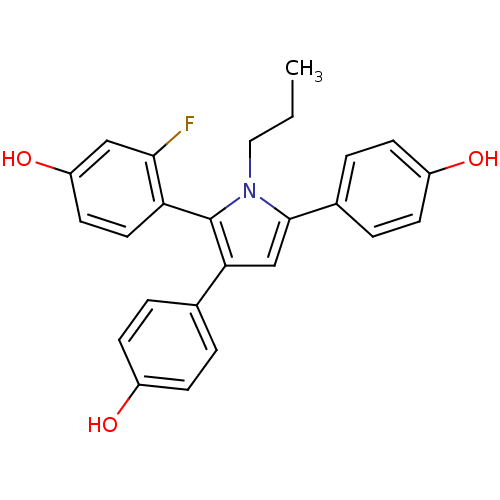 (CHEMBL2178797) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin Curated by ChEMBL | Assay Description Activation of human ERalpha expressed in human U2-OS cells by luciferase reporter gene assay relative to untreated control | J Med Chem 55: 9607-18 (2012) Article DOI: 10.1021/jm300860j BindingDB Entry DOI: 10.7270/Q2R212H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.123 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in HEC-1 cells coexpressing beta-gal assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
the University of Tokyo Curated by ChEMBL | Assay Description Agonist activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as induction of ER-alpha-mediated transcriptional activit... | Bioorg Med Chem 27: 1952-1961 (2019) Article DOI: 10.1016/j.bmc.2019.03.042 BindingDB Entry DOI: 10.7270/Q24171GJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50383076 (CHEMBL2031518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
Wuhan University Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in HepG2 cells after 24 hrs by luciferase reporter gene assay | J Med Chem 55: 2324-41 (2012) Article DOI: 10.1021/jm201556r BindingDB Entry DOI: 10.7270/Q2VT1T4R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50018262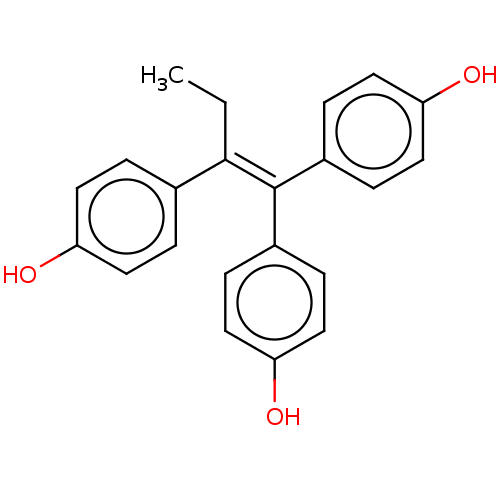 (CHEMBL37775) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Georgetown University Curated by ChEMBL | Assay Description Agonist activity at ER in human MCF7:WS8 cells assessed as increase in cell growth by measuring DNA level after 7 days by fluorescence analysis | J Med Chem 57: 4569-83 (2014) Article DOI: 10.1021/jm500569h BindingDB Entry DOI: 10.7270/Q2H996R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
University of Illinois Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in U2OS cells coexpressing pCMX-hSRC3 assessed as luciferase activity after 24 hrs by reporter gene assay | J Med Chem 55: 528-37 (2012) Article DOI: 10.1021/jm201436k BindingDB Entry DOI: 10.7270/Q2PN963M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Agonist activity at human ERalpha expressed in human HEC1 cells assessed as transcriptional activation after 24 hrs by ERE-luciferase reporter gene t... | J Med Chem 58: 1184-94 (2015) Article DOI: 10.1021/jm501829f BindingDB Entry DOI: 10.7270/Q2XK8H8P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 1316 total ) | Next | Last >> |